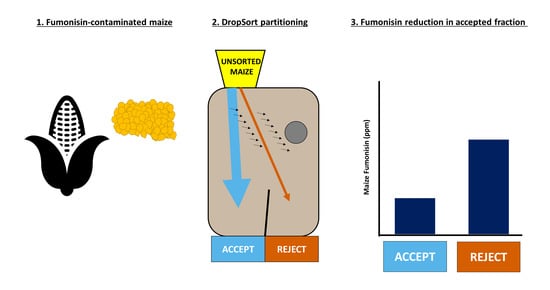
Validation and Application of a Low-Cost Sorting Device for Fumonisin Reduction in Maize
Abstract
:1. Introduction
2. Results
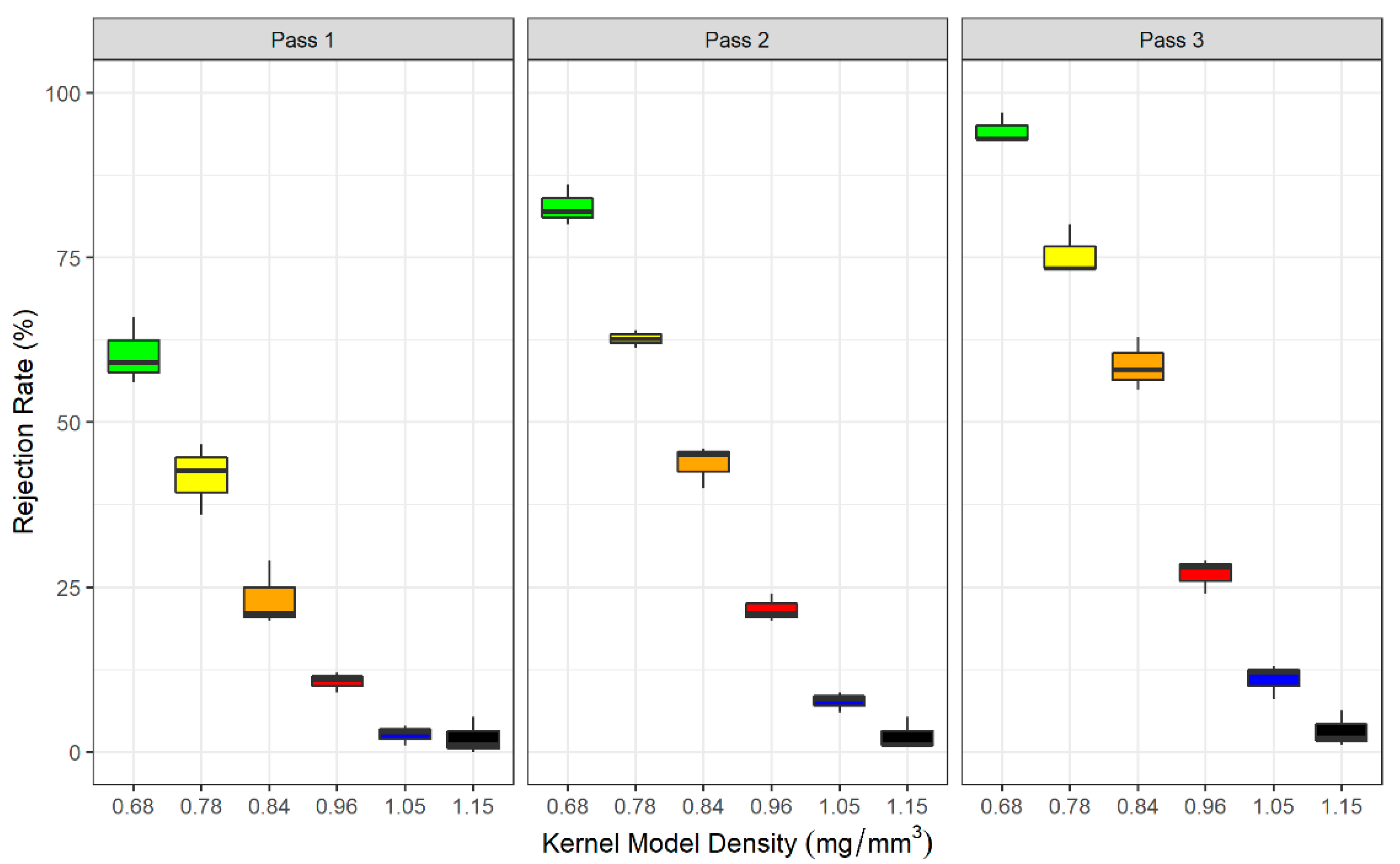
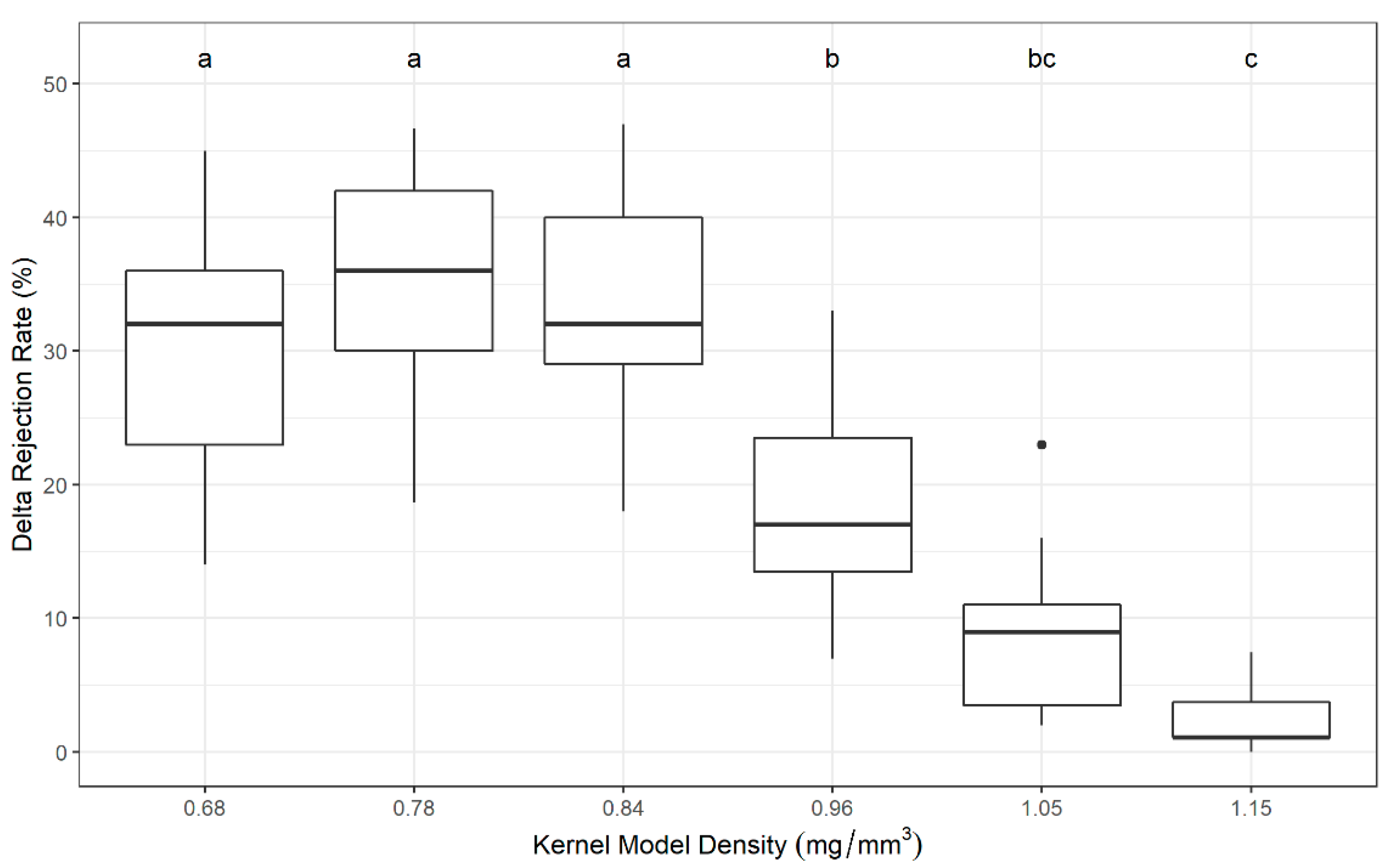
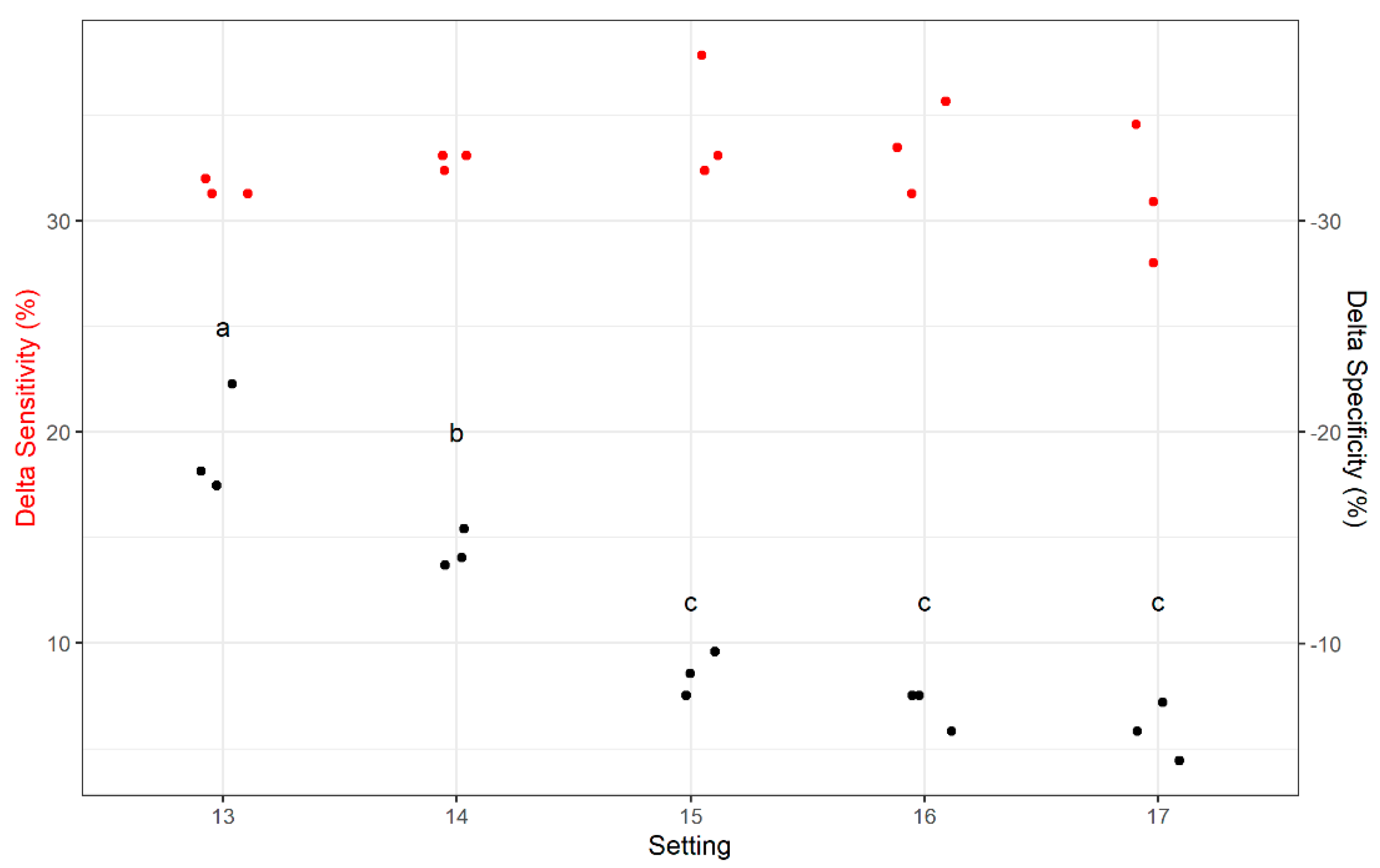
2.1. Plastic Kernel Sorting
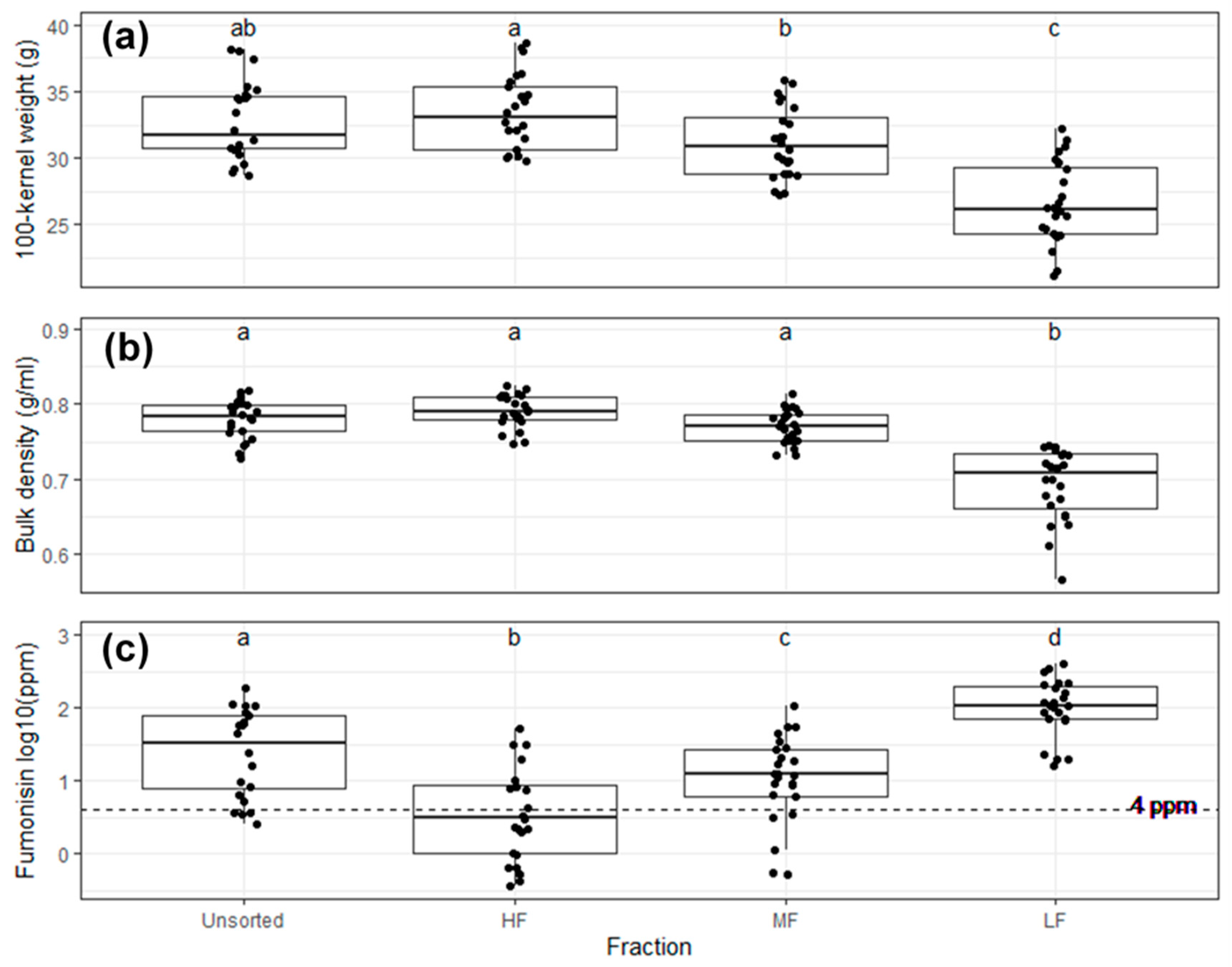
2.2. Bulk Maize Sorting
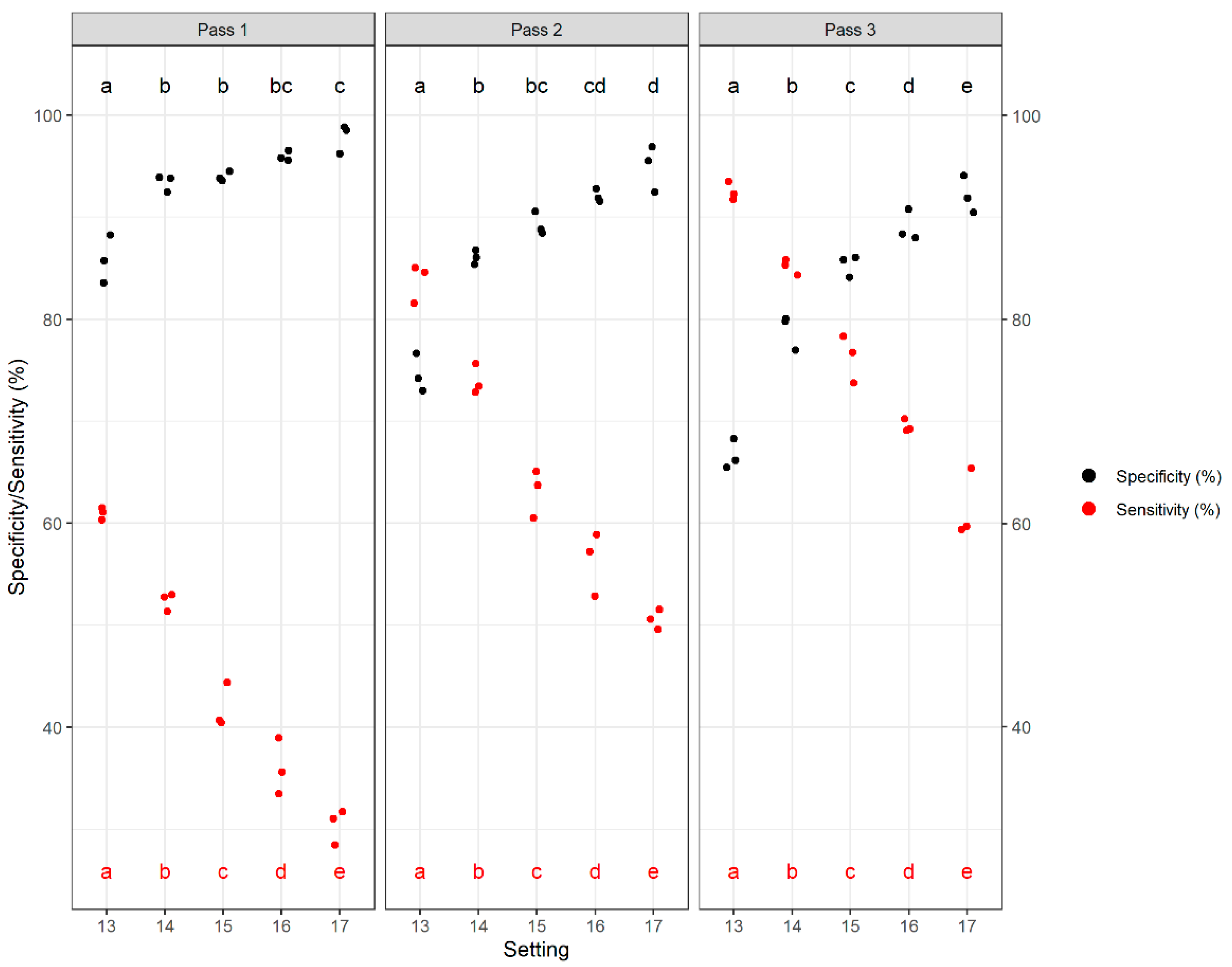
2.3. Single Kernel Analysis
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. DropSort Device
5.2. Plastic Kernel Printing
5.3. Plastic Kernel Sorting
5.4. Texas Maize
5.5. Single Kernel Analysis
5.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munkvold, G.P.; Carlton, W.M. Influence of inoculation method on systemic Fusarium moniliforme infection of maize plants grown from infected seeds. Plant Dis. 1997, 81, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Munkvold, G.P. Epidemiology of Fusarium Diseases and their Mycotoxins in Maize Ears. Eur. J. Plant Pathol. 2003, 109, 705–713. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rheeder, J.P.; Marasas, W.F.O.; Thiel, P.G.; Sydenham, E.W.; Shephard, G.S.; van Schalkwyk, D.J. Fusarium moniliforme and fumonisins in corn in relation to human esophageal cancer in Transkei. Phytopathology 1992, 82, 353–357. [Google Scholar] [CrossRef]
- Missmer, S.A.; Suarez, L.; Felkner, M.; Wang, E.; Merrill, A.H.; Rothman, K.J.; Hendricks, K.A. Exposure to fumonisins and the occurence of neutral tube defects along the Texas-Mexico border. Environ. Health Perspect. 2006, 114, 237–241. [Google Scholar] [CrossRef]
- Marasas, W.F.O.; Riley, R.T.; Hendricks, K.A.; Stevens, V.L.; Sadler, T.W.; Gelineau-van Waes, J.; Missmer, S.A.; Cabrera, J.; Torres, O.; Gelderblom, W.C.A.; et al. Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: A potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. J. Nutr. 2004, 134, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Kimanya, M.E.; De Meulenaer, B.; Roberfroid, D.; Lachat, C.; Kolsteren, P. Fumonisin exposure through maize in complementary foods is inversely associated with linear growth of infants in Tanzania. Mol. Nutr. Food Res. 2010, 54, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Shirima, C.P.; Kimanya, M.E.; Routledge, M.N.; Srey, C.; Kinabo, J.L.; Humpf, H.U.; Wild, C.P.; Tu, Y.K.; Gong, Y.Y. A prospective study of growth and biomarkers of exposure to aflatoxin and fumonisin during early childhood in Tanzania. Environ. Health Perspect. 2015, 123, 173–178. [Google Scholar] [CrossRef] [Green Version]
- USDA. FGIS FDA Contamination Levels. In Mycotoxin Handbook; USDA: Washington, DC, USA, 2015; pp. 53–58. ISBN 9781107671812. [Google Scholar]
- Codex Alimentarius Commission. Codex Alimentarius International Food Standards: General Standard for Contaminants and Toxins in Food and Feed; Codex Alimentarius Commission: Roma, Italy, 2019. [Google Scholar]
- Shephard, G.S. Aflatoxin and food safety: Recent African perspectives. J. Toxicol. Toxin Rev. 2003, 22, 267–286. [Google Scholar] [CrossRef]
- Hell, K.; Fandohan, P.; Bandyopadhyay, R.; Kiewnick, S.; Sikora, R. Cotty Pre- and Postharvest Management of Aflatoxin in Maize: An African Perspective. In Mycotoxins: Detection Methods, Management, Public Health and Agricultural Trade; Leslie, J.F., Bandyopadhyay, R., Visconti, A., Eds.; CABI: Wallingford, UK, 2008; pp. 219–229. [Google Scholar]
- Matumba, L.; Van Poucke, C.; Njumbe Ediage, E.; De Saeger, S. Keeping mycotoxins away from the food: Does the existence of regulations have any impact in Africa? Crit. Rev. Food Sci. Nutr. 2017, 57, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Shephard, G.S.; Burger, H.M.; Rheeder, J.P.; Alberts, J.F.; Gelderblom, W.C.A. The effectiveness of regulatory maximum levels for fumonisin mycotoxins in commercial and subsistence maize crops in South Africa. Food Control 2019, 97, 77–80. [Google Scholar] [CrossRef]
- Doko, M.B.; Canet, C.; Brown, N.; Sydenham, E.W.; Mpuchane, S.; Siame, B.A. Natural Co-occurrence of Fumonisins and Zearalenone in Cereals and Cereal-Based Foods from Eastern and Southern Africa. J. Agric. Food Chem. 1996, 44, 3240–3243. [Google Scholar] [CrossRef]
- Hove, M.; De Boevre, M.; Lachat, C.; Jacxsens, L.; Nyanga, L.K.; De Saeger, S. Occurrence and risk assessment of mycotoxins in subsistence farmed maize from Zimbabwe. Food Control 2016, 69, 36–44. [Google Scholar] [CrossRef]
- Nikièma, P.N.; Worrillow, L.; Traoré, A.S.; Wild, C.P.; Turner, P.C. Fumonisin contamination of maize in Burkina Faso, West Africa. Food Addit. Contam. 2004, 21, 865–870. [Google Scholar] [CrossRef]
- Kimanya, M.E.; De Meulenaer, B.; Tiisekwa, B.; Ndomondo-Sigonda, M.; Devlieghere, F.; Van Camp, J.; Kolsteren, P. Co-occurrence of fumonisins with aflatoxins in home-stored maize for human consumption in rural villages of Tanzania. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2008, 25, 1353–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamala, A.; Ortiz, J.; Kimanya, M.; Haesaert, G.; Donoso, S.; Tiisekwa, B.; De Meulenaer, B. Multiple mycotoxin co-occurrence in maize grown in three agro-ecological zones of Tanzania. Food Control 2015, 54, 208–215. [Google Scholar] [CrossRef]
- Wild, C.P.; Miller, D.J.; Groopman, J.D. (Eds.) Mycotoxin Control in Low- and Middle-Income Countries; International Agency for Research on Cancer: Geneva, Switzerland, 2008; ISBN 9789283225102. [Google Scholar]
- Alberts, J.F.; Lilly, M.; Rheeder, J.P.; Burger, H.-M.; Shephard, G.S.; Gelderblom, W.C.A. Technological and community-based methods to reduce mycotoxin exposure. Food Control 2017, 73, 101–109. [Google Scholar] [CrossRef]
- Alberts, J.F.; Davids, I.; Moll, W.D.; Schatzmayr, G.; Burger, H.M.; Shephard, G.S.; Gelderblom, W.C.A. Enzymatic detoxification of the fumonisin mycotoxins during dry milling of maize. Food Control 2021, 123, 107726. [Google Scholar] [CrossRef]
- FAO. Manual on the application of the HACCP system in mycotoxin prevention and control. FAO Food Nutr. Pap. 73 2001, 124. [Google Scholar]
- Van Der Westhuizen, L.; Shephard, G.S.; Rheeder, J.P.; Burger, H.-M.; Gelderblom, W.C.A.; Wild, C.P.; Gong, Y.Y. Simple intervention method to reduce fumonisin exposure in a subsistence maize-farming community in South Africa. Food Addit. Contam. 2010, 27, 1582–1588. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Manandhar, G.; Plattner, R.D.; Maragos, C.M.; Shrestha, K.; McCormick, S.P. Occurrence of Fusarium Species and Mycotoxins in Nepalese Maize and Wheat and the Effect of Traditional Processing Methods on Mycotoxin Levels. J. Agric. Food Chem. 2000, 48, 1377–1383. [Google Scholar] [CrossRef]
- Fandohan, P.; Zoumenou, D.; Hounhouigan, D.J.; Marasas, W.F.O.; Wingfield, M.J.; Hell, K. Fate of aflatoxins and fumonisins during the processing of maize into food products in Benin. Int. J. Food Microbiol. 2005, 98, 249–259. [Google Scholar] [CrossRef]
- Afolabi, C.G.; Bandyopadhyay, R.; Leslie, J.F.; Ekpo, E.J.A. Effect of Sorting on Incidence and Occurrence of Fumonisins and Fusarium verticillioides on Maize from Nigeria. J. Food Prot. 2006, 69, 2019–2023. [Google Scholar] [CrossRef]
- Van Der Westhuizen, L.; Shephard, G.S.; Rheeder, J.P.; Burger, H.-M.; Gelderblom, W.C.A.; Wild, C.P.; Gong, Y.Y. Optimising sorting and washing of home-grown maize to reduce fumonisin contamination under laboratory-controlled conditions. Food Control 2011, 22, 396–400. [Google Scholar] [CrossRef]
- Mutiga, S.K.; Were, V.; Hoffmann, V.; Harvey, J.W.; Milgroom, M.G.; Nelson, R.J. Extent and drivers of mycotoxin contamination: Inferences from a survey of Kenyan maize mills. Phytopathology 2014, 104, 1221–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matumba, L.; Van Poucke, C.; Njumbe Ediage, E.; Jacobs, B.; De Saeger, S.; Van Poucke, C. Effectiveness of hand sorting, flotation/washing, dehulling and combinations thereof on the decontamination of mycotoxin-contaminated white maize. Food Addit. Contam. Part A 2015, 32, 960–969. [Google Scholar] [CrossRef]
- Fandohan, P.; Hell, K.; Marasas, W.F.O. Food processing to reduce mycotoxins in Africa. In Mycotoxins: Detection Methods, Management, Public Health and Agricultural Trade; Leslie, J.F., Bandyopadhyay, R., Visconti, A., Eds.; CABI: Wallingford, UK, 2008; pp. 309–316. ISBN 9781845930820. [Google Scholar]
- Alberts, J.; Rheeder, J.; Gelderblom, W.; Shephard, G.; Burger, H.M. Rural subsistence maize farming in South Africa: Risk assessment and intervention models for reduction of exposure to fumonisin mycotoxins. Toxins 2019, 11, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breuer, T.; Brenneis, K.; Fortenbacher, D. Mechanisation-a catalyst for rural development in sub-Saharan Africa. Rural 21 2015, 49, 16–19. [Google Scholar]
- Komatsu, H.; Malapit, H.J.L.; Theis, S. Does women’s time in domestic work and agriculture affect women’s and children’s dietary diversity? Evidence from Bangladesh, Nepal, Cambodia, Ghana, and Mozambique. Food Policy 2018, 79, 256–270. [Google Scholar] [CrossRef]
- Pascale, M.; Logrieco, A.F.; Graeber, M.; Hirschberger, M.; Reichel, M.; Lippolis, V.; De Girolamo, A.; Lattanzio, V.M.; Slettengren, K. Aflatoxin Reduction in Maize by Industrial-Scale Cleaning Solutions. Toxins 2020, 12, 331. [Google Scholar] [CrossRef]
- Tkachuk, R.; Dexter, J.; Tipples, K.; Nowicki, T. Removal by specific gravity table of tombstone kernels and associated trichothecenes from wheat infected with Fusarium head blight. Cereal Chem. 1991, 68, 428–431. [Google Scholar]
- Brera, C.; Debegnach, F.; Grossi, S.; Miraglia, M. Effect of industrial processing on the distribution of fumonisin B1 in dry milling corn fractions. J. Food Prot. 2004, 67, 1261–1266. [Google Scholar] [CrossRef]
- Pietri, A.; Zanetti, M.; Bertuzzi, T. Distribution of aflatoxins and fumonisins in dry-milled maize fractions. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2009, 26, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Tibola, C.S.; Fernandes, J.M.C.; Guarienti, E.M. Effect of cleaning, sorting and milling processes in wheat mycotoxin content. Food Control 2016, 60, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Stroshine, R.L.; Ileleji, K. Differences in kernel shape, size, and density between healthy kernels and mold discolored kernels and their relationship to reduction in aflatoxin levels in a sample of shelled corn. Appl. Eng. Agric. Vol. 2017, 33, 421–431. [Google Scholar] [CrossRef]
- Stasiewicz, M.J.; Falade, T.D.O.; Mutuma, M.; Mutiga, S.K.; Harvey, J.J.W.; Fox, G.; Pearson, T.C.; Muthomi, J.W.; Nelson, R.J. Multi-spectral kernel sorting to reduce aflatoxins and fumonisins in Kenyan maize. Food Control 2017, 78, 203–214. [Google Scholar] [CrossRef] [Green Version]
- Murphy, P.A.; Rice, L.G.; Ross, P.F. Fumonisin B1, B2, and B3 content of Iowa, Wisconsin, and Illinois corn and corn screenings. J. Agric. Food Chem. 1993, 41, 263–266. [Google Scholar] [CrossRef]
- Mogensen, J.M.; Sørensen, S.M.; Sulyok, M.; Van Der Westhuizen, L.; Shephard, G.S.; Frisvad, J.C.; Thrane, U.; Krska, R.; Nielsen, K.F. Single-kernel analysis of fumonisins and other fungal metabolites in maize from South African subsistence farmers. Food Addit. Contam. Part A 2011, 28, 1724–1734. [Google Scholar] [CrossRef] [Green Version]
- Morales, L.; Zila, C.T.; Moreta Mejía, D.E.; Montoya Arbelaez, M.; Balint-Kurti, P.J.; Holland, J.B.; Nelson, R.J. Diverse Components of Resistance to Fusarium verticillioides Infection and Fumonisin Contamination in Four Maize Recombinant Inbred Families. Toxins 2019, 11, 86. [Google Scholar] [CrossRef] [Green Version]
- Butoto, E.; Marino, T.P.; Holland, J.B. Effects of artificial inoculation on trait correlations with resistance to Fusarium ear rot and fumonisin contamination in maize. Crop Sci. 2021, 61, 1–30. [Google Scholar] [CrossRef]
- Aoun, M.; Stafstrom, W.; Priest, P.; Fuchs, J.; Windham, G.L.; Williams, W.P.; Nelson, R.J. Low-cost grain sorting technologies to reduce mycotoxin contamination in maize and groundnut. Food Control 2020, 118, 107363. [Google Scholar] [CrossRef] [PubMed]
- Ngure, F.M.; Ngure, C.; Achieng, G.; Munga, F.; Moran, Z.; Stafstrom, W.; Nelson, R.J. Mycotoxins contamination of market maize and the potential of density sorting in reducing exposure in unregulated food systems in Kenya Abstract. World Mycotoxin J. 2021, 14, 165–178. [Google Scholar] [CrossRef]
- R Core Development Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Kassambara, A. ggcorrplot: Visualization of a Correlation Matrix Using “ggplot2”; Springer: New York, NY, USA, 2019. [Google Scholar]




| Spearman Correlations | Unsorted 100-Kernel Weight | Unsorted Bulk Density | HF 100-Kernel Weight | HF Bulk Density | MF 100-Kernel Weight | MF Bulk Density | LF 100-Kernel Weight | LF Bulk Density |
|---|---|---|---|---|---|---|---|---|
| Unsorted Fumonisin log10(ppm) | −0.24 ns | −0.08 ns | −0.34 ns | −0.12 ns | −0.24 ns | −0.13 ns | −0.51 * | −0.22 ns |
| Mean 100-Kernel Weight (g) | Mean Bulk Density (g/mL) | Fumonisin (ppm) | |
|---|---|---|---|
| Unsorted | 37.38 | 0.77 | 23.47 |
| HF | 38.60 | 0.78 | 0.65 |
| MF | 34.88 | 0.77 | 55.20 |
| LF | 25.64 | 0.65 | 305.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stafstrom, W.; Wushensky, J.; Fuchs, J.; Xu, W.; Ezera, N.; Nelson, R.J. Validation and Application of a Low-Cost Sorting Device for Fumonisin Reduction in Maize. Toxins 2021, 13, 652. https://doi.org/10.3390/toxins13090652
Stafstrom W, Wushensky J, Fuchs J, Xu W, Ezera N, Nelson RJ. Validation and Application of a Low-Cost Sorting Device for Fumonisin Reduction in Maize. Toxins. 2021; 13(9):652. https://doi.org/10.3390/toxins13090652
Chicago/Turabian StyleStafstrom, William, Julie Wushensky, John Fuchs, Wenwei Xu, Nnenna Ezera, and Rebecca J. Nelson. 2021. "Validation and Application of a Low-Cost Sorting Device for Fumonisin Reduction in Maize" Toxins 13, no. 9: 652. https://doi.org/10.3390/toxins13090652
APA StyleStafstrom, W., Wushensky, J., Fuchs, J., Xu, W., Ezera, N., & Nelson, R. J. (2021). Validation and Application of a Low-Cost Sorting Device for Fumonisin Reduction in Maize. Toxins, 13(9), 652. https://doi.org/10.3390/toxins13090652