Molecular Characterization and Functional Analysis of the Nattectin-like Toxin from the Venomous Fish Thalassophryne maculosa
Abstract
:1. Introduction
2. Results
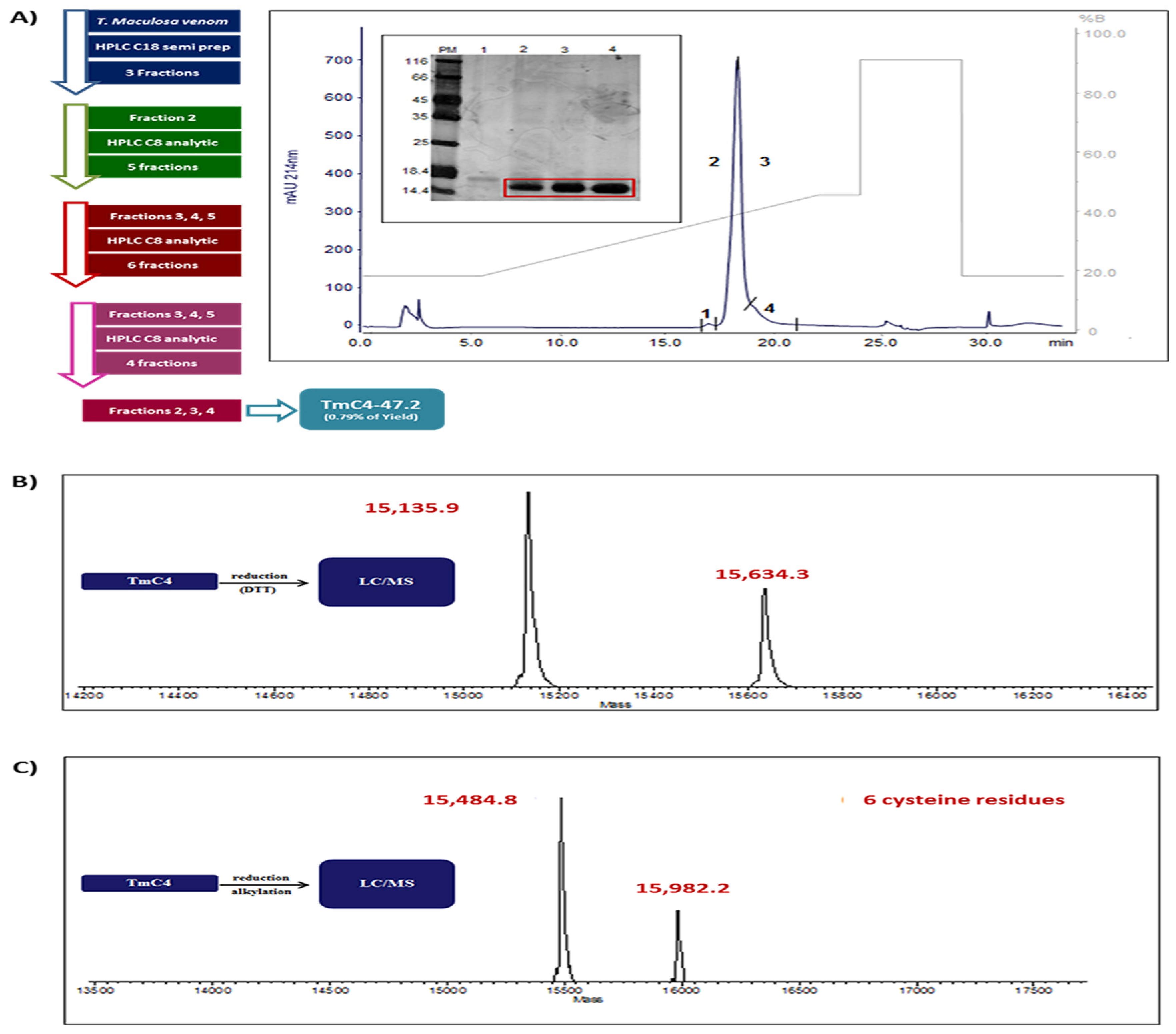
2.1. Purification of TmC4-47.2 Toxin from Thalassophryne maculosa Venom
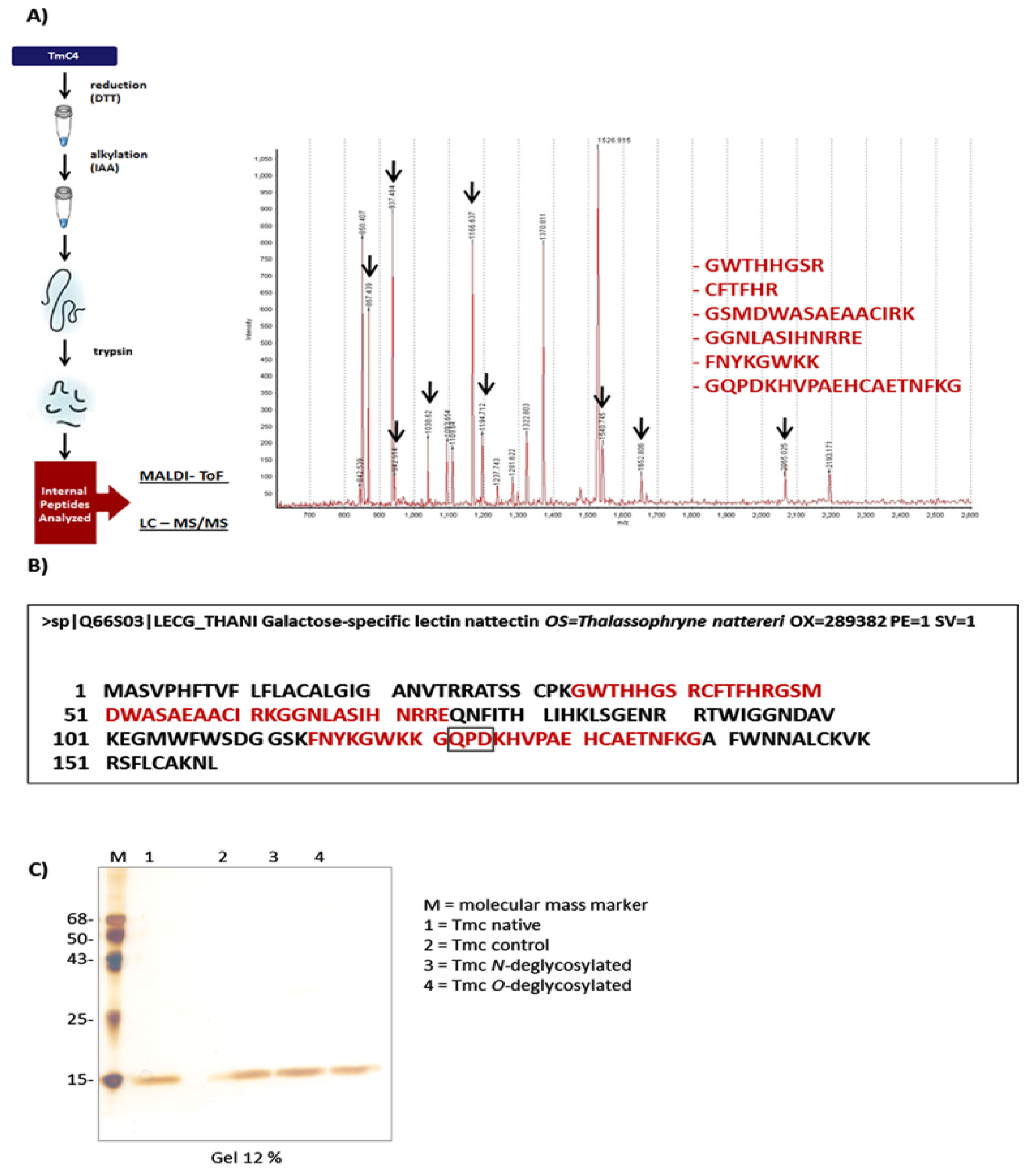
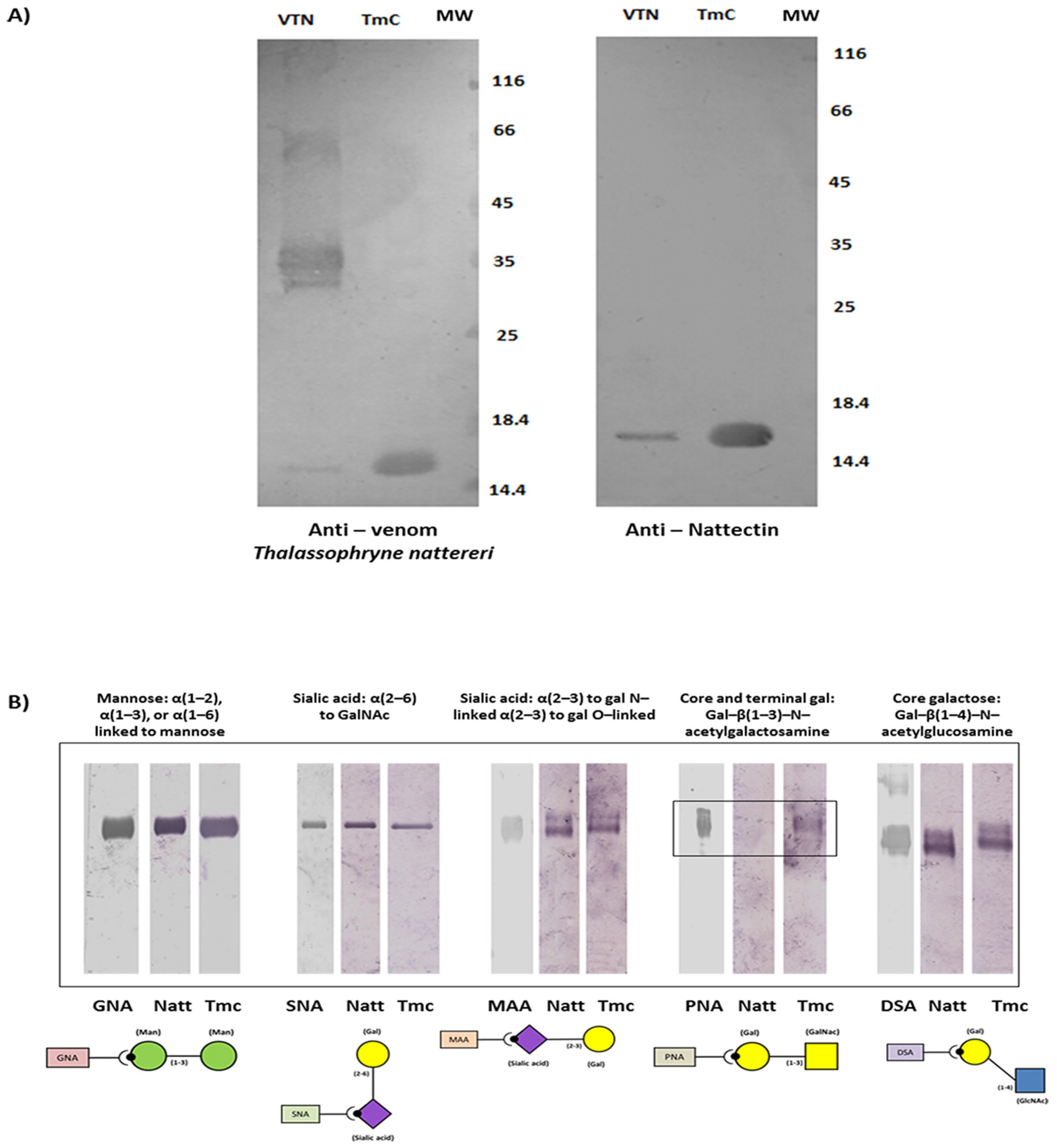
2.2. TmC4-47.2 Is a Galactose-Binding Nattectin-Like Lectin
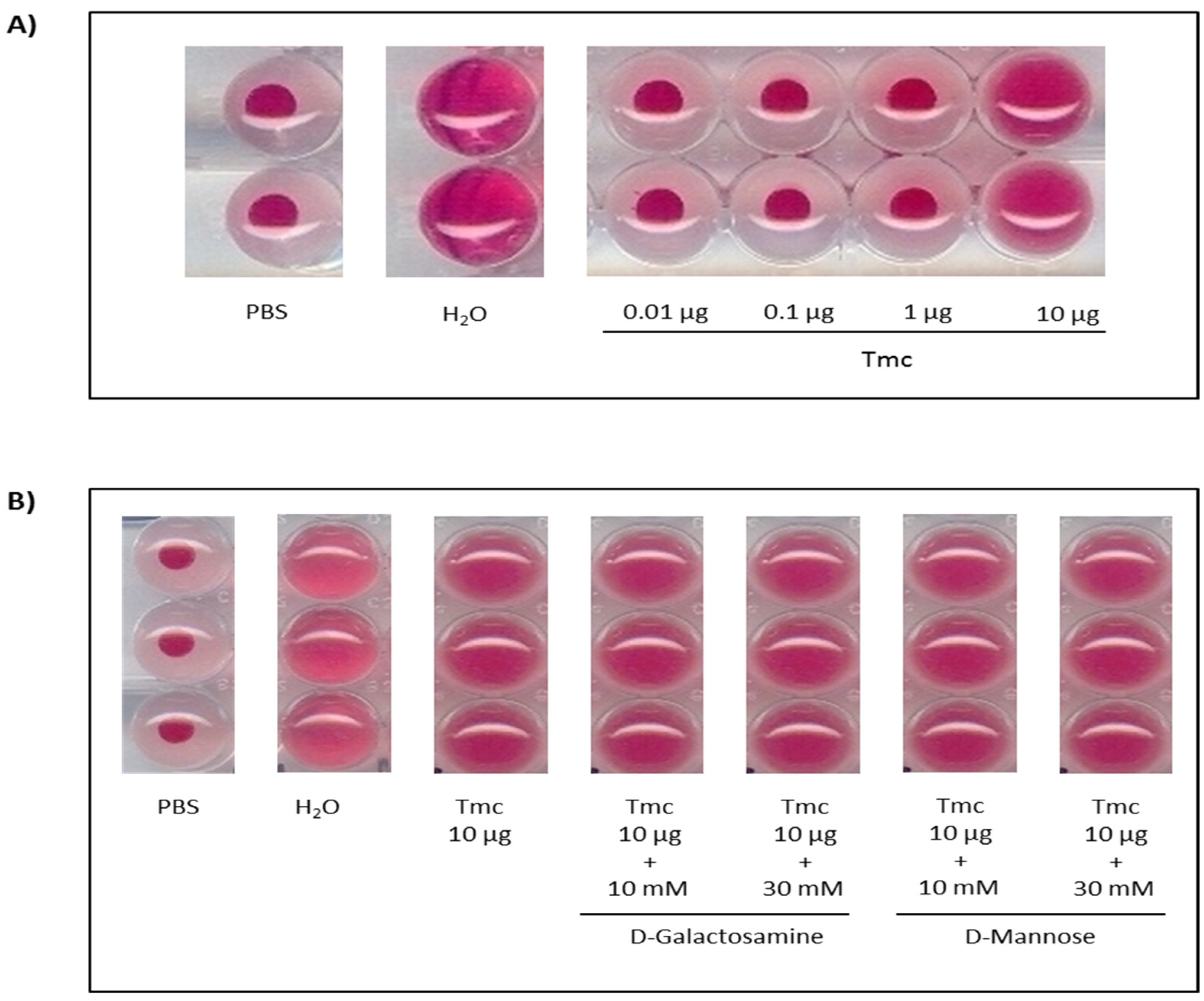
2.3. Hemagglutinating and Antimicrobial Activities of T. maculosa Nattectin-Like Toxin
2.4. TmC4-47.2 Toxin-Induced Alterations in the Microcirculation
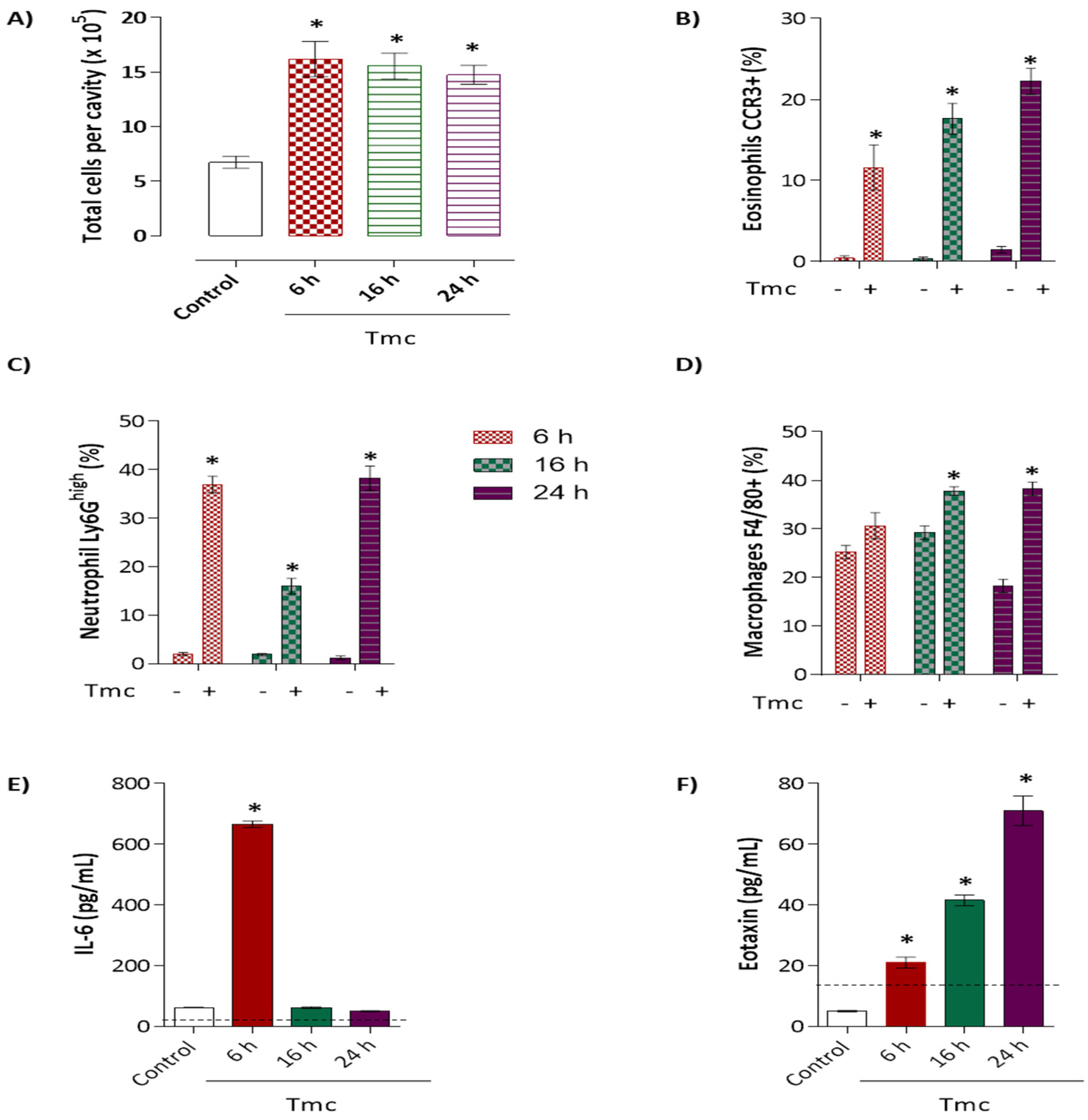
2.5. Induction of Acute Inflammation by Nattectin-Like Protein
3. Discussion
4. Materials and Methods
4.1. Thalassophryne maculosa Venom and Isolation of TmC4-47.2 Toxin
4.2. TmC4-47.2 Toxin Mass Determination
4.3. Reduction, Alkylation, and Digestion of TmC4-47.2 Toxin
4.4. Enzymatic Deglycosylation
4.5. Immunoblotting
4.6. Determination of TmC4-47.2 Toxin Binding Specificity to Carbohydrates
4.7. Hemagglutinating and Antimicrobial Activities
4.8. In Vivo Experimental Protocol for Intravital Microscopy
4.9. Acute Inflammation Induced by TmC4-47.2
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ng, T.B.; Cheung, R.C.F.; Wing Ng, C.C.; Fang, E.f.; Wong, J.H. A review of fish lectins. Curr. Protein Pept. Sci. 2015, 16, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Vasta, G.R.; Nita-Lazar, M.; Giomarelli, B.; Ahmed, H.; Du, S.; Cammarata, M.; Parrinello, N.; Bianchet, M.A.; Amzel, L.M. Structural and functional diversity of the lectin repertoire in teleost fish: Relevance to innate and adaptive immunity. Dev. Comp. Immunol. 2011, 35, 1388–1399. [Google Scholar] [CrossRef] [PubMed]
- Brinchmann, M.F.; Patel, D.M.; Pinto, N.; Iversen, M.H. Functional aspects of fish mucosal lectins-interaction with non-self. Molecules 2018, 23, 1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes-Ferreira, M.; Magalhães, G.S.; Fernandez, J.H.; Junqueira-de-Azevedo, I.L.; Le Ho, P.; Lima, C.; Valente, R.H.; Moura-da-Silva, A.M. Structural and biological characterization of Nattectin, a new C-type lectin from the venomous fish. Thalass. Nattereri Biochim. 2011, 93, 971–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes-Ferreira, M.; Barbaro, K.C.; Cardoso, D.F.; Moura-da-Silva, A.M.; Mota, I. Thalassophryne nattereri fish venom: Biological and biochemical characterization and serum neutralization of its toxic activities. Toxicon 1998, 36, 405–410. [Google Scholar] [CrossRef]
- Lopes-Ferreira, M.; Moura-da-Silva, A.M.; Mota, I.; Takehara, H.A. Neutralization of Thalassophryne nattereri (niquim) fish venom by an experimental antivenom. Toxicon 2000, 38, 1149–1156. [Google Scholar] [CrossRef]
- Almeida, V.G.; Rocha, C.M. Registro de acidentes com peixes peçonhentos e/ou venenosos. Rev. Soc. Bras. Toxicol 1989, 2, 49–51. [Google Scholar]
- Haddad Junior, V.; Pardal, P.P.; Cardoso, J.L.; Martins, I.A. The venomous toadfish Thalassophryne nattereri (niquim or miquim): Report of 43 injuries provoked in fishermen of Salinopolis (Para state) and Aracaju (Sergipe state), Brazil. Rev. Do Inst. De Med. Trop. De São Paulo 2003, 45, 221–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faco, P.E.; Bezerra, G.P.; Barbosa, P.S.; Martins, A.M.; Guimaraes, J.A.; Ferreira, M.L.; Monteiro, H.S. Epidemiology of the injuries caused by Thalassophryne nattereri (niquim) in Ceara State (1992–2002). Rev. Soc. Bras. Med. Trop. 2005, 38, 479–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, L.A.; Lopes-Ferreira, M. Clinical and experimental studies regarding poisoning caused by a fish Thalassophryne nattereri (Niquim). An. Bras. De Dermatol. 2000, 75, 435–443. [Google Scholar]
- Lopes-Ferreira, M.; Nunez, J.; Rucavado, A.; Farsky, S.H.; Lomonte, B.; Ângulo, Y.; Moura Da Silva, A.M.; Gutierrez, J.M. Skeletal muscle necrosis and regeneration after injection of Thalassophryne nattereri (niquim) fish venom in mice. Int. J. Exp. Pathol. 2001, 82, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Ferreira, M.; Moura-Da-Silva, A.M.; Piran-Soares, A.A.; Ângulo, Y.; Lomonte, B.; Gutierrez, J.M.; Farsky, S.H. Hemostatic effects induced by Thalassophryne nattereri fish venom: A model of endothelium-mediated blood flow impairment. Toxicon 2002, 40, 1141–1147. [Google Scholar] [CrossRef]
- Lopes-Ferreira, M.; Emim, J.A.; Oliveira, V.; Puzer, L.; Cezari, M.H.; Araujo, M.S.; Juliano, L.; Lapa, A.J.; Souccar, C.; Moura-Da-Silva, A.M. Kininogenase activity of Thalassophryne nattereri fish venom. Biochem. Pharm. 2004, 68, 2151–2157. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Ferreira, M.; Sosa-Rosales, I.; Bruni, F.M.; Ramos, A.D.; Vieira Portaro, F.C.; Conceição, K.; Lima, C. Analysis of the intersexual variation in Thalassophryne maculosa fish venoms. Toxicon 2016, 115, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Rosales, J.; D’suze, G.; Salazar, V.; Fox, J.; Sevcik, C. Purification of a myotoxin from the toadfish Thalassophryne maculosa (günter) venom. Toxicon 2005, 45, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Pareja-Santos, A.; Oliveira Souza, V.M.; Bruni, F.M.; Sosa-Rosales, J.I.; Lopes-Ferreira, M.; Lima, C. Delayed polymorphonuclear leukocyte infiltration is an important component of Thalassophryne maculosa venom pathogenesis. Toxicon 2008, 52, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Rosales, J.I.; Piran-Soares, A.A.; Farsky, S.H.; Takehara, H.A.; Lima, C.; Lopes-Ferreira, M. Important biological activities induced by Thalassophryne maculosa fish venom. Toxicon 2005, 45, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, N.; Liu, S. Determination of peptide and protein disulfide linkages by maldi mass spectrometry. Top. Curr. Chem. 2013, 331, 79–116. [Google Scholar]
- Zelensky, A.N.; Gready, J.E. The c-type lectin-like domain superfamily. Febs J. 2005, 272, 6179–6217. [Google Scholar] [CrossRef] [PubMed]
- Cummings, R.D. The repertoire of glycan determinants in the human glycome. Mol. Biosyst. 2009, 5, 1087–1104. [Google Scholar] [CrossRef]
- Goldstein, I.J.; Hayes, C.E. The lectins: Carbohydrate-binding proteins of plants and animals. Adv. Carbo. Chem. Biochem. 1978, 35, 127–340. [Google Scholar]
- Chou, R.C.; Kim, N.D.; Sadik, C.D.; Seung, E.; Lan, Y.; Byrne, M.H.; Haribabu, B.; Iwakura, Y.; Luster, A.D. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity 2010, 33, 266–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezu-Ndubuisi, O.J.; Maheshwari, A. The role of integrins in inflammation and angiogenesis. Pediatr. Res. 2021, 89, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Ferreira, M.; Grund, L.Z.; Lima, C. Thalassophryne nattereri fish venom: From the envenoming to the understanding of the immune system. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grund, L.Z.; Souza, V.M.; Faquim-Mauro, E.L.; Lima, C.; Lopes-Ferreira, M. Experimental immunization with Thalassophryne nattereri fish venom: Striking IL-5 production and impaired of B220+ cells. Toxicon 2006, 48, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Bruni, F.M.; Coutinho, E.; Andrade-Barros, A.I.; Grund, L.Z.; Lopes-Ferreira, M.; Lima, C. Anaphylaxis induced by Thalassophryne nattereri venom in mice is an IgE/IgG1-mediated, IL-4-dependent phenomenon. Sci. Rep. 2020, 10, 584. [Google Scholar] [CrossRef]
- Magalhaes, G.S.; Lopes-Ferreira, M.; Junqueira-De-Azevedo, I.L.; Spencer, P.J.; Araujo, M.S.; Portaro, F.C.; Ma, L.; Valente, R.H.; Juliano, L.; Fox, J.W.; et al. Natterins, a new class of proteins with kininogenase activity characterized from Thalassophryne nattereri fish venom. Biochimie 2005, 87, 687–699. [Google Scholar] [CrossRef]
- Magalhaes, G.S.; Junqueira-De-Azevedo, I.L.; Lopes-Ferreira, M.; Lorenzini, D.M.; Ho, P.L.; Moura-Da-Silva, A.M. Transcriptome analysis of expressed sequence tags from the venom glands of the fish Thalassophryne nattereri. Biochimie 2006, 88, 693–699. [Google Scholar] [CrossRef]
- Komegae, E.N.; Grund, L.Z.; Lopes-Ferreira, M.; Lima, C. The longevity of Th2 humoral response induced by proteases natterins requires the participation of long-lasting innate-like B cells and plasma cells in spleen. PLoS ONE 2013, 8, e67135. [Google Scholar] [CrossRef] [Green Version]
- Komegae, E.N.; Grund, L.Z.; Lopes-Ferreira, M.; Lima, C. TLR2, TLR4 and the MyD88 signaling are crucial for the in vivo generation and the longevity of long-lived antibody-secreting cells. PLoS ONE 2013, 8, e71185. [Google Scholar] [CrossRef]
- Ferreira, M.J.; Lima, C.; Lopes-Ferreira, M. Anti-inflammatory effect of Natterins, the major toxins from the Thalassophryne nattereri fish venom is dependent on TLR4/MyD88/PI3K signaling pathway. Toxicon 2014, 87, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.; Disner, G.R.; Falcão, M.A.P.; Seni-Silva, A.C.; Maleski, A.L.A.; SouzA, M.M.; Reis Tonello, M.C.; Lopes-Ferreira, M. The natterin proteins diversity: A review on phylogeny, structure, and immune function. Toxins 2021, 13, 538. [Google Scholar] [CrossRef]
- Komegae, E.N.; Ramos, A.D.; Oliveira, A.K.; Serrano, S.M.; Lopes-Ferreira, M.; Lima, C. Insights into the local pathogenesis induced by fish toxins: Role of natterins and nattectin in the disruption of cell-cell and cell-extracellular matrix interactions and modulation of cell migration. Toxicon 2011, 58, 509–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishizuka, E.K.; Ferreira, M.J.; Grund, L.Z.; Coutinho, E.M.; Komegae, E.N.; Cassado, A.A.; Bortoluci, K.R.; Lopes-Ferreira, M.; Lima, C. Role of interplay between IL-4 and IFN-γ in the in regulating M1 macrophage polarization induced by Nattectin. Int. Immunopharmacol. 2012, 14, 513–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraiva, T.C.; Grund, L.Z.; Komegae, E.N.; Ramos, A.D.; Conceição, K.; Orii, N.M.; Lopes-Ferreira, M.; Lima, C. Nattectin a fish C-type lectin drives Th1 responses in vivo: Licenses macrophages to differentiate into cells exhibiting typical DC function. Int. immunopharmacol. 2011, 11, 1546–1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drickamer, K.; Fadden, A.J. Genomic analysis of c-type lectins. Biochem. Soc. Symp. 2002, 69, 59–72. [Google Scholar] [CrossRef]
- Moremen, K.W.; Tiemeyer, M.; Nairn, A.V. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012, 13, 448–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drickamer, K. C-type lectin-like domains. Curr. Opin. Struct. Biol. 1999, 9, 585–590. [Google Scholar] [CrossRef]
- Yang, R.; Rabinovich, G.; Liu, F. Galectins: Structure, function and therapeutic potential. Expert Rev. Mol. Med. 2008, 10, E17. [Google Scholar] [CrossRef]
- Vasta, G.R. Roles of galectins in infection. Nat. Rev. Microbiol. 2009, 7, 424–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thulasitha, W.S.; Umasuthan, N.; Whang, I.; Nam, B.H.; Lee, J. Antimicrobial response of galectin-1 from rock bream Ople-gnathus fasciatus: Molecular, transcriptional, and biological characterization. Fish Shellfish. Immunol. 2016, 50, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Nita-Lazar, M.; Mancini, J.; Feng, C.; González-Montalbán, N.; Ravindran, C.; Jackson, S.; de Las Heras-Sánchez, A.; Giomarelli, B.; Ahmed, H.; Haslam, S.M.; et al. The zebrafish galectins Drgal1-L2 and Drgal3-L1 bind in vitro to the infectious hematopoietic necrosis virus (IHNV) glycoprotein and reduce viral adhesion to fish epithelial cells. Dev. Comp. Immunol. 2016, 55, 241–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takayama, S.; Saitoh, E.; Kimizuka, R.; Yamada, S.; Kato, T. Effect of eel galectin ajl-1 on periodontopathic bacterial biofilm formation and their lipopolysaccharide-mediated inflammatory cytokine induction. Int. J. Antimicrob. Agents 2009, 34, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.G.; Li, L.; Chen, S.N.; Mao, M.G.; Nie, P. Expression and antibacterial analysis of galectin-8 and -9 genes in mandarin fish, Siniperca Chuatsi. Fish Shellfish. Immunol. 2020, 107, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Vasta, G.R.; Ahmed, H.; Shao, J.D.; Henrikson, D. Galectins in teleost fish: Zebrafish (Danio rerio) as a model species to address their biological roles in development and innate immunity. Glycoconj. J. 2004, 21, 503–521. [Google Scholar] [CrossRef]
- Nishi, N.; Shoji, H.; Seki, M.; Itoh, A.; Miyanaka, H.; Yuube, K.; Hirashima, M.; Nakamura, T. Galectin-8 modulates neutrophil function via interaction with integrin Alpha, M. Glycobiology 2003, 13, 755–763. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, N.; Nishi, N.; Seki, M.; Matsumoto, R.; Kuwabara, I.; Liu, F.T.; Hata, Y.; Nakamura, T.; Hirashima, M. Requirement of divalent galactoside-binding activity of ecalectin/galectin-9 for eosinophil chemoattraction. J. Biol. Chem. 2000, 275, 8355–8360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, B.; Pittman, K.; Menezes, G.B.; Hirota, S.A.; Slaba, I.; Waterhouse, C.C.M.; Beck., P.L.; Muruve, D.A.; Kubes, P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 2010, 330, 362–366. [Google Scholar] [CrossRef]
- Mcdonald, J.A.; Quade, B.J.; Broekelmann, T.J.; Lachance, R.; Forsman, K.; Hasegawa, E.; Akiyama, S. Fibronectin’s cell-adhesive domain and an amino-terminal matrix assembly domain participate in its assembly into fibroblast pericellular matrix. J. Biol. Chem. 1987, 262, 2957–2967. [Google Scholar] [CrossRef]
- Carroll, J.M.; Romero, M.R.; Watt, F.M. Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell 1995, 83, 957–968. [Google Scholar] [CrossRef] [Green Version]
- Lishko, V.K.; Yakubenko, V.P.; Ugarova, T.P. The interplay between integrins alphambeta2 and Alpha5beta1 during cell migration to fibronectin. Exp. Cell Res. 2003, 283, 116–126. [Google Scholar] [CrossRef]
- Rao, S.P.; Ge, X.N.; Sriramarao, P. Regulation of eosinophil recruitment and activation by galectins in allergic asthma. Front. Med. 2017, 4, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laemmli, U.K. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Marshall, A.G. A universal algorithm for fast and automated charge state deconvolution of electrospray mass-to-charge ratio spectra. J. Am. Soc. Mass Spectrom. 1998, 3, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Haab, B.B. Antibody-lectin sandwich arrays for biomarker and glycobiology studies. Expert Rev. Proteom. 2010, 7, 9–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, D.C.; Muñoz, J.E.; Carvalho, D.D.; Belmonte, R.; Faintuch, B.; Borelli, P.; Miranda, A.; Taborda, C.P.; Daffre, S. Therapeutic use of a cationic antimicrobial peptide from the spider Acanthoscurria gomesiana in the control of experimental candidiasis. BMC Microbiol. 2012, 12, 28. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes-Ferreira, M.; Sosa-Rosales, I.; Silva Junior, P.I.; Conceicao, K.; Maleski, A.L.A.; Balan-Lima, L.; Disner, G.R.; Lima, C. Molecular Characterization and Functional Analysis of the Nattectin-like Toxin from the Venomous Fish Thalassophryne maculosa. Toxins 2022, 14, 2. https://doi.org/10.3390/toxins14010002
Lopes-Ferreira M, Sosa-Rosales I, Silva Junior PI, Conceicao K, Maleski ALA, Balan-Lima L, Disner GR, Lima C. Molecular Characterization and Functional Analysis of the Nattectin-like Toxin from the Venomous Fish Thalassophryne maculosa. Toxins. 2022; 14(1):2. https://doi.org/10.3390/toxins14010002
Chicago/Turabian StyleLopes-Ferreira, Monica, Ines Sosa-Rosales, Pedro Ismael Silva Junior, Katia Conceicao, Adolfo Luis Almeida Maleski, Leticia Balan-Lima, Geonildo Rodrigo Disner, and Carla Lima. 2022. "Molecular Characterization and Functional Analysis of the Nattectin-like Toxin from the Venomous Fish Thalassophryne maculosa" Toxins 14, no. 1: 2. https://doi.org/10.3390/toxins14010002





