Establishing Cell Models to Understand Cellular Toxicity: Lessons Learned from an Unconventional Cell Type
Abstract
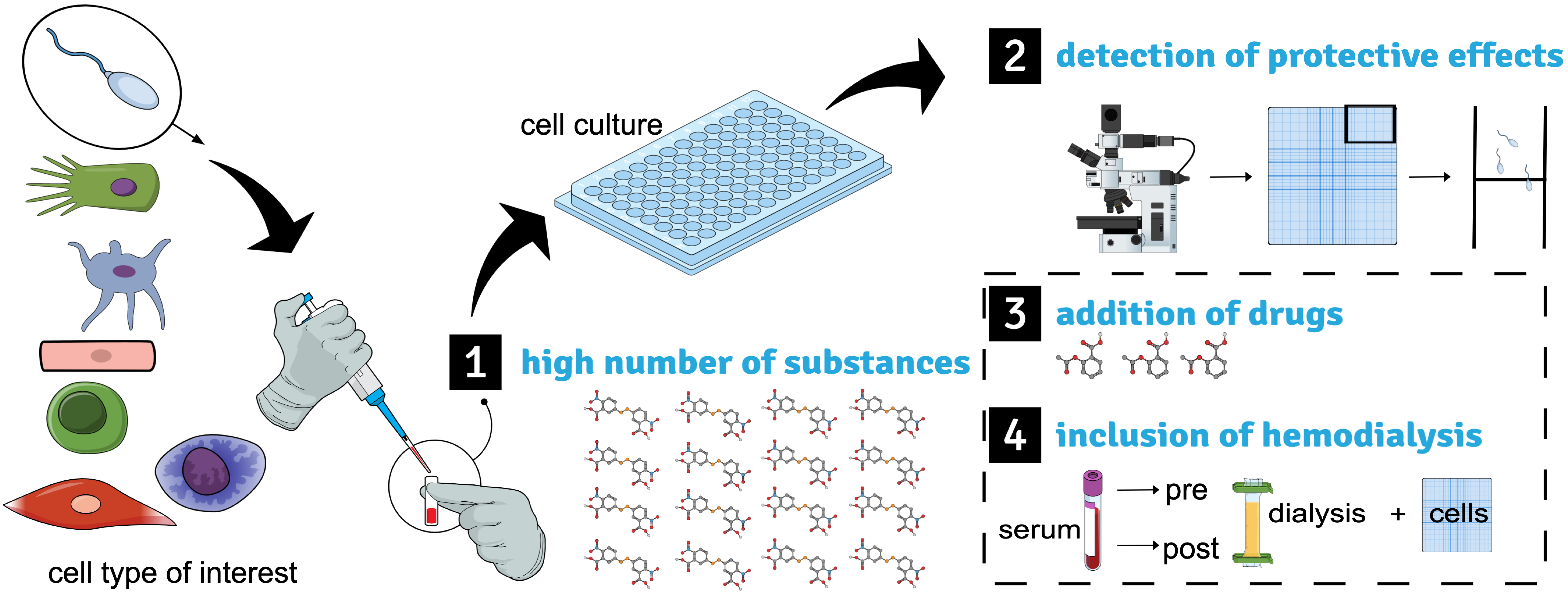
:1. Introduction
2. Serial Testing of Uremic Substances
3. Detection of Protective Effects by Cellular Responses
4. Addition of Drugs to the In-Vitro Setting
5. Translation for Clinical Application
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A.; on behalf of the European Uremic Toxin Work Group. Normal and Pathologic Concentrations of Uremic Toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, G.; Glorieux, G.; Thornalley, P.; Schepers, E.; Meert, N.; Jankowski, J.; Jankowski, V.; Argiles, A.; Anderstam, B.; Brunet, P.; et al. Review on uraemic toxins III: Recommendations for handling uraemic retention solutes in vitro towards a standardized approach for research on uraemia. Nephrol. Dial. Transplant. 2007, 22, 3381–3390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanholder, R.; Pletinck, A.; Schepers, E.; Glorieux, G.L. Biochemical and Clinical Impact of Organic Uremic Retention Solutes: A Comprehensive Update. Toxins 2018, 10, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, W.J.; Hagge, W.W.; Wagoner, R.D.; DiNapoli, R.P.; Rosevear, J.W. Effects of urea loading in patients with far-advanced renal failure. Mayo Clin. Proc. 1972, 47, 21–29. [Google Scholar] [PubMed]
- Vollmer, T.; Ljungberg, B.; Jankowski, V.; Jankowski, J.; Glorieux, G.; Stegmayr, B.G. An in-vitro assay using human spermatozoa to detect toxicity of biologically active substances. Sci. Rep. 2019, 9, 14525. [Google Scholar] [CrossRef]
- Jodar, M.; Sendler, E.; Krawetz, S.A. The protein and transcript profiles of human semen. Z. Für Zellforsch. Und Mikrosk. Anat. 2016, 363, 85–96. [Google Scholar] [CrossRef] [PubMed]
- A Baker, M.; Aitken, R.J. Proteomic insights into spermatozoa: Critiques, comments and concerns. Expert Rev. Proteom. 2009, 6, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.; Castillo, J.; Estanyol, J.M.; Ballescà, J.L.; Ramalho-Santos, J.; Oliva, R. Human Sperm Tail Proteome Suggests New Endogenous Metabolic Pathways. Mol. Cell. Proteom. 2013, 12, 330–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2011, 35, 109–124. [Google Scholar] [CrossRef] [Green Version]
- Moscatelli, N.; Spagnolo, B.; Pisanello, M.; Lemma, E.D.; De Vittorio, M.; Zara, V.; Pisanello, F.; Ferramosca, A. Single-cell-based evaluation of sperm progressive motility via fluorescent assessment of mitochondria membrane potential. Sci. Rep. 2017, 7, 17931. [Google Scholar] [CrossRef]
- Pereira, C.; Moreira, A.C.; Pereira, S.; Machado, N.; Carvalho, F.; Sardão, V.; Oliveira, P. Investigating Drug-induced Mitochondrial Toxicity: A Biosensor to Increase Drug Safety? Curr. Drug Saf. 2009, 4, 34–54. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.N.; Leung, M.C.K.; Rooney, J.P.; Sendoel, A.; Hengartner, M.O.; Kisby, G.E.; Bess, A.S. Mitochondria as a Target of Environmental Toxicants. Toxicol. Sci. 2013, 134, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, J.N.; Hartman, J.H.; Mello, D.F. Mitochondrial Toxicity. Toxicol. Sci. 2018, 162, 15–23. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Lin, K.-H.; Huang, C.-J.; Wei, A.-C. MitoTox: A comprehensive mitochondrial toxicity database. BMC Bioinform. 2021, 22, 369. [Google Scholar] [CrossRef]
- Wallace, K.B. Multiple Targets for Drug-Induced Mitochondrial Toxicity. Curr. Med. Chem. 2015, 22, 2488–2492. [Google Scholar] [CrossRef]
- Dykens, J.A.; Will, Y. The significance of mitochondrial toxicity testing in drug development. Drug Discov. Today 2007, 12, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, A.V.; Edebert, I.; Garside, H.; Cotgreave, I.; Rigler, R.; Loitto, V.; Magnusson, K.-E.; Rodríguez-Martínez, H. Boar spermatozoa successfully predict mitochondrial modes of toxicity: Implications for drug toxicity testing and the 3R principles. Toxicol. Vitr. 2015, 29, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Carrillo, A. The Usefulness of Sperm Kinematics in Drug-Induced Toxicity Assessment. Basic Clin. Pharmacol. Toxicol. 2018, 123, 3–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castagnoli, E.; Salo, J.; Toivonen, M.S.; Marik, T.; Mikkola, R.; Kredics, L.; Vicente-Carrillo, A.; Nagy, S.; Andersson, M.T.; Andersson, M.A.; et al. An Evaluation of Boar Spermatozoa as a Biosensor for the Detection of Sublethal and Lethal Toxicity. Toxins 2018, 10, 463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stegmayr, B.; Ronquist, G. Stimulation of Sperm Progressive Motility by Organelles in Human Seminal Plasma. Scand. J. Urol. Nephrol. 1982, 16, 85–90. [Google Scholar] [CrossRef]
- Rosselli, M.; Dubey, R.K.; Imthurn, B.; Macas, E.; Keller, P.J. Andrology: Effects of nitric oxide on human spermatozoa: Evidence that nitric oxide decreases sperm motility and induces sperm toxicity. Hum. Reprod. 1995, 10, 1786–1790. [Google Scholar] [CrossRef] [PubMed]
- Pons-Rejraji, H.; Brugnon, F.; Sion, B.; Maqdasy, S.; Gouby, G.; Pereira, B.; Marceau, G.; Grémeau, A.-S.; Drevet, J.; Grizard, G.; et al. Evaluation of atorvastatin efficacy and toxicity on spermatozoa, accessory glands and gonadal hormones of healthy men: A pilot prospective clinical trial. Reprod. Biol. Endocrinol. 2014, 12, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harchegani, A.B.; Niha, M.M.; Sohrabiyan, M.; Ghatrehsamani, M.; Tahmasbpour, E.; Shahriary, A. Cellular and molecular mechanisms of sulfur mustard toxicity on spermatozoa and male fertility. Toxicol. Res. 2018, 7, 1029–1035. [Google Scholar] [CrossRef] [Green Version]
- Uremic Solutes Database. Available online: https://database.uremic-toxins.org/soluteList.php (accessed on 16 December 2021).
- Vanholder, R.; Gryp, T.; Glorieux, G. Urea and chronic kidney disease: The comeback of the century? (in uraemia research). Nephrol. Dial. Transplant. 2017, 33, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Cohen, G.; Rudnicki, M.; Hörl, W.H. Uremic toxins modulate the spontaneous apoptotic cell death and essential functions of neutrophils. Kidney Int. 2001, 59, S48–S52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, R.J.; Small, D.M.; Ng, K.L.; Vesey, D.A.; Vitetta, L.; Francis, R.S.; Gobe, G.C.; Morais, C. Indoxyl Sulfate Induces Apoptosis and Hypertrophy in Human Kidney Proximal Tubular Cells. Toxicol. Pathol. 2018, 46, 449–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, X.; Cao, X.; Zhang, P.; Xiang, F.; Teng, J.; Zou, J.; Ding, X. Endoplasmic reticulum stress associated apoptosis as a novel mechanism in indoxyl sulfate-induced cardiomyocyte toxicity. Mol. Med. Rep. 2018, 18, 5117–5122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollmer, T.; Schlickeiser, S.; Amini, L.; Schulenberg, S.; Wendering, D.J.; Banday, V.; Jurisch, A.; Noster, R.; Kunkel, D.; Brindle, N.R.; et al. The intratumoral CXCR3 chemokine system is predictive of chemotherapy response in human bladder cancer. Sci. Transl. Med. 2021, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, E.; Gadêlha, H.; Smith, D.; Blake, J.; Kirkman-Brown, J. Mammalian Sperm Motility: Observation and Theory. Annu. Rev. Fluid Mech. 2011, 43, 501–528. [Google Scholar] [CrossRef] [Green Version]
- WHO Laboratory Manual for the Examination and Processing of Human Semen. Sixth Edition. 2021. Available online: https://www.who.int/publications/i/item/9789240030787 (accessed on 1 December 2021).
- Kamp, G.; Büsselmann, G.; Lauterwein, J. Spermatozoa: Models for studying regulatory aspects of energy metabolism. Cell. Mol. Life Sci. 1996, 52, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Donadio, C.; Kanaki, A.; Sami, N.; Tognotti, D. High-Flux Dialysis: Clinical, Biochemical, and Proteomic Comparison with Low-Flux Dialysis and On-Line Hemodiafiltration. Blood Purif. 2017, 44, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Eloot, S.; Van Biesen, W.; Dhondt, A.; Van de Wynkele, H.; Glorieux, G.; Verdonck, P.; Vanholder, R. Impact of hemodialysis duration on the removal of uremic retention solutes. Kidney Int. 2008, 73, 765–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vollmer, T.; Stegmayr, B. Establishing Cell Models to Understand Cellular Toxicity: Lessons Learned from an Unconventional Cell Type. Toxins 2022, 14, 54. https://doi.org/10.3390/toxins14010054
Vollmer T, Stegmayr B. Establishing Cell Models to Understand Cellular Toxicity: Lessons Learned from an Unconventional Cell Type. Toxins. 2022; 14(1):54. https://doi.org/10.3390/toxins14010054
Chicago/Turabian StyleVollmer, Tino, and Bernd Stegmayr. 2022. "Establishing Cell Models to Understand Cellular Toxicity: Lessons Learned from an Unconventional Cell Type" Toxins 14, no. 1: 54. https://doi.org/10.3390/toxins14010054




