1. Introduction
Staphylococcus aureus is the most common species of coagulase-positive staphylococci (CPS). This opportunistic pathogen is widely distributed in nature and has a major impact on human health, making it one of the world’s most common pathogens [
1]. Long known as a commensal microorganism residing on the skin and in mucosa, it has recently been recognized as a facultative intracellular pathogen. As such, it plays a critical role in recurrent infection and chronic illness [
2].
The pathogenicity of
S. aureus is attributable to its ability to secrete more than 20 types of toxins [
3,
4] and invasive enzymes, leading to moderate or potentially fatal illness [
5]. The risk of infection is relatively high in humans: an estimated 50–60% are intermittently or permanently colonized with
S. aureus [
6]. While sensitive, rapid, and specific testing can aid in diagnosis and treatment [
7], many strains have developed antibiotic resistance. For example, methicillin-resistant strains of
S. aureus (MRSA) are common in clinical settings, where antibiotic-resistant forms continue to pose a serious problem for hospital-, livestock-, and community-associated infection. The World Health Organization (WHO) has classified them as high-priority health issues [
8].
Foodborne disease (FBD) of infectious or toxic nature results from the consumption of contaminated food or water. Many different pathogens are implicated in foodborne infection, one of which is
S. aureus [
9].
S. aureus is a common cause of FBD and staphylococcal food poisoning (SFP). SFP occurs after the accidental ingestion of superantigens (e.g.,
S. aureus enterotoxins, SEs) pre-formed in food by
S. aureus enterotoxigenic strains. Symptoms have a rapid onset (2–8 h): nausea, abdominal cramping, violent vomiting, and diarrhea [
10,
11] typically resolve within 24 to 48 h after onset.
Staphylococcal contamination of food products occurs through respiratory secretions or via manual contact by individuals carrying the pathogen in the nose or on the hands. Dust, air, and food contact surfaces can also be vehicles of contamination of food items [
12]. Due to its biological characteristics,
S. aureus contamination results mainly from incorrect handling of processed or cooked foods or from storage conditions that allow staphylococcal growth and enterotoxin production. Various food types are subject to
S. aureus contamination, with products of animal origin frequently involved in cases of SFP. The potential presence of staphylococcal toxins has been reported in meat, ham, eggs, cheese, milk, and dairy items, as well as salads, bakery products, and sandwiches [
13,
14,
15]. In 2009 the European Food Safety Authority (EFSA) stated that the two main vehicles in documented food poisoning outbreaks caused by
S. aureus toxins were cheese and buffet/mixed meals [
16].
Food microbiological safety in Europe is regulated by Regulation (EC) 2073/2005 [
17] based on microbiological criteria for foodstuffs. The Regulation sets two types of microbiological criteria with which food businesses must comply, as listed in Annex I: food safety criteria and process hygiene criteria. If test results for either type of criteria are unsatisfactory, food businesses must take specific action in compliance with the Regulation.
The aims of this study were: to determine seasonal changes in CPS concentration and the occurrence of S. aureus enterotoxins (SEs) in food product samples from supermarkets, factories, and producers in Italy. The samples were collected and analyzed over a five-year period (2016–2020). The study was designed to evaluate whether the presence of enterotoxins in food products is influenced by food type or seasonality.
3. Discussion
Coagulase-positive staphylococci (CPS), and
S. aureus, in particular, are pathogenic microorganisms of great interest to the food industry.
S. aureus food poisoning can occur when a minimum of 18 µg SE is present in 100 g of ingested food [
1]. The presence of heat-stable enterotoxins in food products can have serious consequences for public health. In
S. aureus ecology, both seasonality and food type can influence bacterial count and the presence of enterotoxins [
18,
19,
20].
Among the 52 food samples positive for the presence of staphylococcal enterotoxins, 44 were dairy products: 22 were declared to be made of raw milk and no declaration was available for the other 22 samples. Raw milk cheese can be generally considered riskier than pasteurized products because of the potential presence of a higher concentration of bacterial population carrying toxins genes and with an active metabolism to synthesize and secrete toxic proteins. This kind of food should be generally consumed very carefully by young or elderly people.
We found considerable differences in bacterial count and enterotoxin prevalence. Dairy products were most often contaminated with
S. aureus or staphylococcal enterotoxins: 88.57% of the samples (1550/1750) had a bacterial count score of ≥1 (>10 CFU/g to ≤100 CFU/g). Dairy products, milk, and related foods are at high risk of bacterial contamination, particularly for CPS [
21,
22].
Analysis of bacterial count of
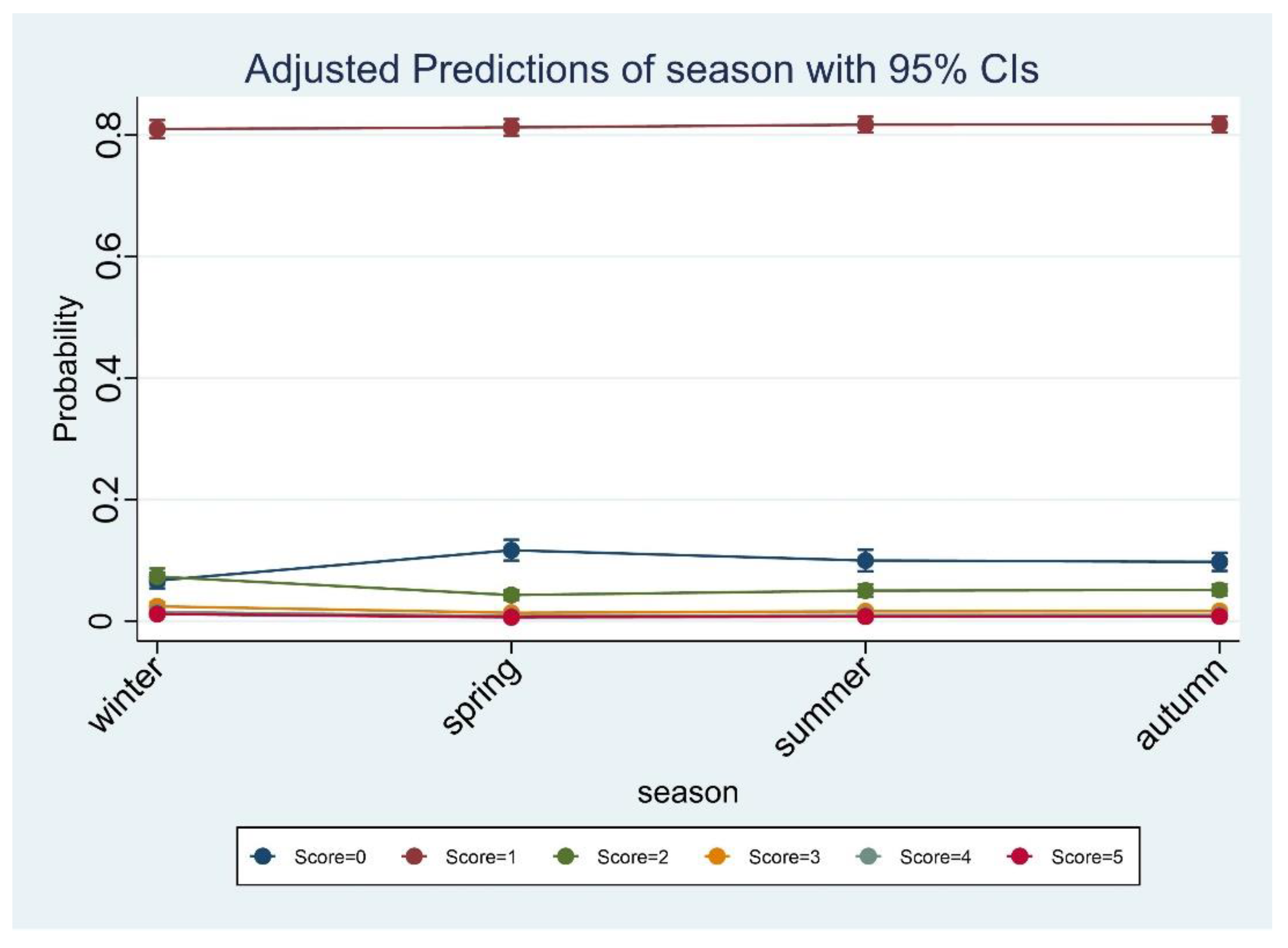
S. aureus showed seasonal differences. Compared to the other months, April was most likely to have a score of 0. Although there was no obvious match between the other seasons, the risk of contamination was greater during winter than in summer. Our findings are shared by those of Sentitula and colleagues [
23], who based their hypothesis on PCR findings that showed that pathogens (i.e., staphylococci) remain dormant in the udder and then proliferate under suitable climatic conditions. Unlike other studies [
18,
24] that analyzed the seasonality of
S. aureus contamination in foods, our dataset was composed of a variety of food types of different origins. CPS thrives at an optimum of 37 °C (range, 7–48 °C); however, the scientific evidence comes largely from dairy products and products of animal origin. The diversity of the food matrices we examined may explain why the incidence of contamination was higher for the colder (winter) compared to the warmer season (spring or summer).
In our dataset, the percentage of samples that tested positive for toxins compared to the total was 6.71% (52 vs. 774), and is consistent with similar studies [
25]. We found a marked difference between seasons for the presence of toxins, with autumn being the season most at risk of contamination. A plausible explanation is that there is a greater release of toxins by
S. aureus in autumn after the warmth of summer, ensuring a higher incidence of toxins in food. A previous study [
26] on UHT milk documented a higher production of staphylococcal enterotoxins when the milk was exposed to temperatures between 37 and 42 °C or to fluctuating temperatures.
The European Food Safety approach to protect consumers’ health and guarantee a high safety level in foods, define actions to be undertaken when hygiene or safety criteria are not satisfied. In the frame of Regulation (EC) 2073/2005 [
17] coagulase-positive staphylococci hygiene criteria vary from 10 to 10
5 ufc/g or ufc/mL according to the different food matrices; staphylococcal enterotoxins presence can be investigated on the choice of a competent authority in high-risk foodstuff or must be investigated if CPS concentration is higher than 10
5 ufc/g or ufc/mL in foods. If any of the hygiene criteria results are uncompliant, the competent authority provides additional investigation on hygiene and good manufacturing practices, own-check analysis results, and cleaning and disinfection protocols. Furthermore, if safety criteria are not satisfied the activation of withdrawal or recall of food products ensures that unsafe products are no longer available on the market.
5. Materials and Methods
5.1. Food Samples
Overall, 3604 food samples were grouped by food category (
Table 1). The survey included samples collected according to the official national plan for food safety by Public Health Services between January 2016 and December 2020 at different points in the food chain, from primary production to retail or catering, in northern Italy. Analysis was performed according to five sampling plans: (1) official food control activities (national plans); (2) criteria verification in mass catering; (3) consultancy activities; (4) official control in the food milk sector, and (5) monitoring and audits by control agencies.
5.2. Enumeration of CPS
A total of 3604 analyses for CPS enumeration were performed according to ISO 6888-2:1999 using rabbit plasma fibrinogen agar medium. Test portion, initial suspension, and dilution were performed under aseptic conditions according to ISO 6887-1. Prepared Petri dishes were incubated at 37 °C for 18 h to 24 h. The staphylococci formed black, grey, or small white colonies surrounded by a halo of precipitation, indicating coagulase activity. At the beginning of incubation, proteus colonies may appear like CPS colonies. After incubation for 24 h or 48 h, they appear to spread and are more or less brownish in color, which distinguishes them from staphylococci. Results are expressed as N of CPS/milliliter or per gram of product as weighted.
Some tests were performed using TEMPO
®: this automated system combines a card with an adapted medium to ensure rapid enumeration of quality indicators. The TEMPO
® STA test consists of a vial of culture medium and a card, which are specific to the test. The culture medium is inoculated with the sample to be tested and homogeneously transferred by the TEMPO
® Filler into the card containing 48 wells of three different volumes. The TEMPO
® Filler seals the card to prevent contamination during subsequent handling. The culture medium contains a fluorescent pH indicator which, when pH is neutral, emits a signal detected by the TEMPO
® Reader. During incubation, the CPS present in the card assimilates the nutrients in the culture medium, thus lowering the pH and the fluorescent signal extinguishes. Depending on the number and size of positive wells, the TEMPO
® system deduces the number of CPS present in the original sample calculated by the Most Probable Number (MPN) method. Card reading, interpretation, and reporting are managed by the TEMPO
® system after incubation for 24 h at 35 °C. The TEMPO
® STA method enumerates CPS. Both methods are fully validated and accredited according to ISO 17025: 2017 [
27] at the Food Safety Laboratory, Istituto Zooprofilattico Sperimentale del Piemonte Liguria e Valle d’Aosta.
5.3. Qualitative Detection of Staphylococcal Enterotoxins
In total, 826 analyses were performed for the qualitative detection of staphylococcal enterotoxins. The protocol proceeds by the following steps according to ISO 19020:2017: extraction, the concentration of the extract, and recovery of the concentrated extract [
28]. For the detection of staphylococcal enterotoxins (SEA to SEE) commercially available immunoenzyme detection kits were used: VIDAS® STAPH ENTEROTOXIN II (SET2) (BioMérieux, France) and RIDASCREEN® SET Total (R-Biopharm, Germany). The tests were performed according to the manufacturer’s instructions. All diagnostic protocols are fully validated and accredited at the Food Safety Laboratory, Istituto Zooprofilattico Sperimentale del Piemonte Liguria e Valle d’Aosta.
5.4. Statistical Analysis
Data in the Laboratory Information Management System (LIMS) of our Institute and covering a five-year period (2016–2020) were extracted and analyzed. Only data for S. aureus count were considered. The bacterial count was scored as follows:
Score 0 CPS < 10 CFU/g or mL
Score 1 >10 and ≤100 CFU/g or mL
Score 2 >100 and ≤1000 CFU/g or mL
Score 3 >1000 and ≤10,000 CFU/g or mL
Score 4 >10,000 and ≤100,000 CFU/g or mL
Score 5 >1,000,000 CFU/g or Ml
The food matrixes were grouped into 26 categories; after data cleaning (all categories with less than 15 records were excluded from analysis) 11 categories remained for analysis (
Table 1). The variable season was created by grouping the months January to March (winter), April to June (spring), July to September (summer), and October to December (autumn).