Mitigative Potential of Novel Lactobacillus plantarum TISTR 2076 against the Aflatoxins-Associated Oxidative Stress and Histopathological Alterations in Liver and Kidney of Broiler Chicks during the Entire Growth Period
Abstract
1. Introduction
2. Results
2.1. Feed Intake
2.2. Body Weight Gain and Organ Weight
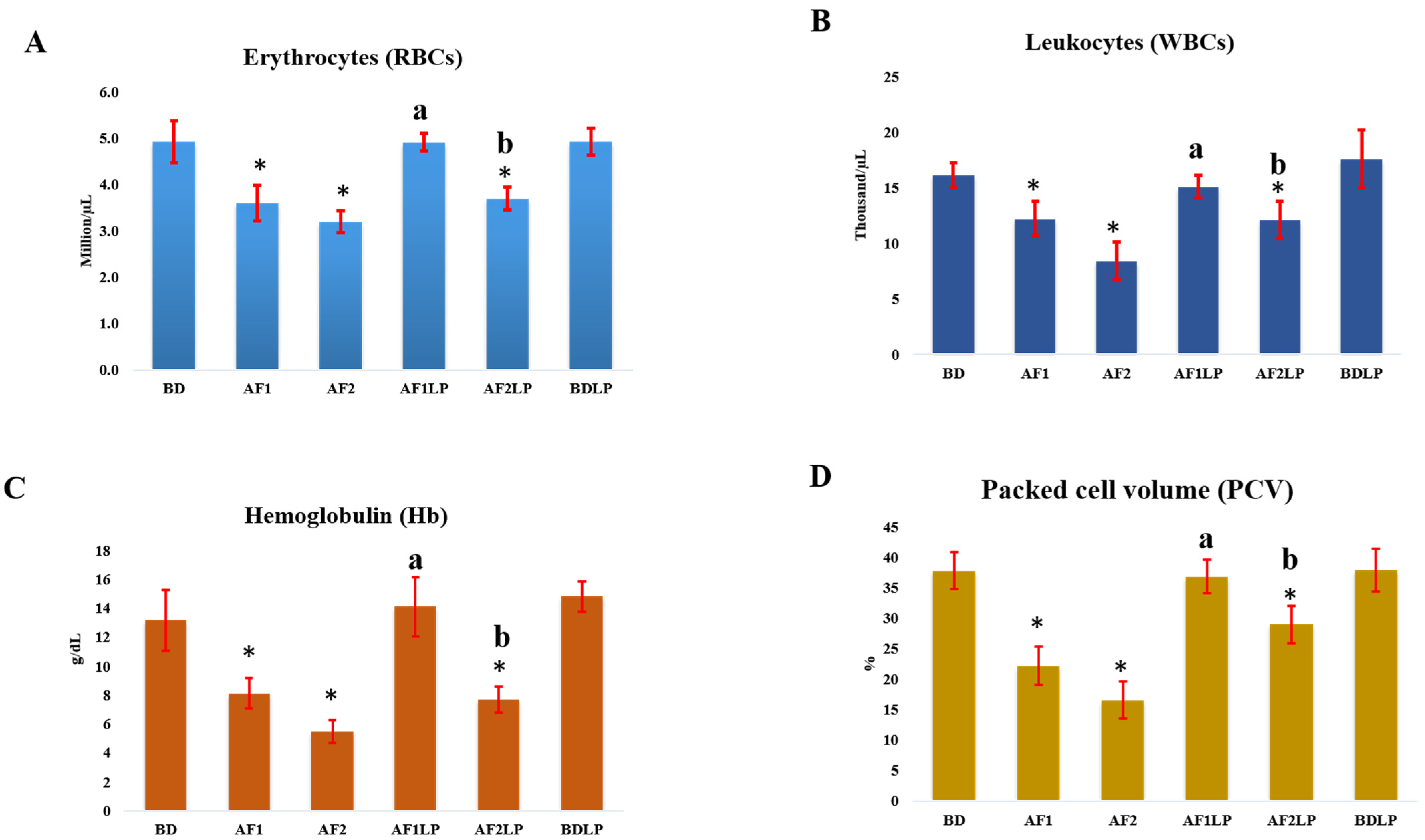
2.3. Hematological Examination
2.4. Serum Chemistry
2.4.1. Total Proteins, Albumin and Globulin
2.4.2. Hepatic Biomarkers
2.4.3. Renal Biomarkers

2.4.4. Serum TAC (Total Antioxidant Capacity) and TOS (Total Oxidant Status)
2.4.5. Serum Interleukins
2.5. Gross Pathology
2.6. Histopathological Examinations
2.7. Residues of Aflatoxins in Liver and Kidney
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Ethical Approval
5.2. Aflatoxins Production and Propagation of Lactobacillus plantarum
5.3. Plan of Study
5.4. Parameters Studied
5.4.1. Performance Parameters
5.4.2. Gross and Microscopic Pathology
5.4.3. Hematological Examination
5.4.4. Serum Biochemistry
5.4.5. Serum Interleukins
5.4.6. Serum Total Oxidative Stress (TOS; μmol/L)
5.4.7. Serum Total Antioxidant Capacity (TAC; mmol/L)
5.4.8. Residues of Aflatoxins in Liver and Kidney
5.4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Aflatoxins |
| AF1 | Aflatoxins 1st level |
| AF2 | Aflatoxins 2nd level |
| LP | Lactobacillus plantarum |
| RBCs | Red blood cells |
| WBCs | White blood cells |
| Hb | Hemoglobin concentration |
| PCV | Packed cell volume |
| ALT | Alanine aminotransferase |
| AST | aspartate aminotransferase |
| GGT | Gamma glutamyle transferase |
| LDH | Lactate dehydrogenase |
| ALP | Alkaline phosphatase |
References
- Eaton, D.L.; Groopman, J.D. The Toxicology of Aflatoxins: Human Health, Veterinary, and Agricultural Significance; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Diaz, D.E.; Hagler, W.M.; Hopkins, B.A.; Whitlow, L.W. Aflatoxin binders I: In vitro binding assay for aflatoxin B1 by several potential sequestering agents. Mycopathologia 2003, 156, 223–226. [Google Scholar] [CrossRef]
- Binder, E.; Tan, L.; Chin, L.; Handl, J.; Richard, J. Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim. Feed Sci. Technol. 2007, 137, 265–282. [Google Scholar] [CrossRef]
- Rawal, S.; Kim, J.E.; Coulombe, R., Jr. Aflatoxin B1 in poultry: Toxicology, metabolism and prevention. Res. Vet. Sci. 2010, 89, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, R. An overview of aflatoxicosis of poultry: Its characteristics, prevention and reduction. Vet. Res. Commun. 1986, 10, 429–443. [Google Scholar] [CrossRef]
- Sridhar, M.; Suganthi, R.U.; Thammiaha, V. Effect of dietary resveratrol in ameliorating aflatoxin B1-induced changes in broiler birds. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1094–1104. [Google Scholar] [CrossRef]
- Tessari, E.; Oliveira, C.; Cardoso, A.; Ledoux, D.; Rottinghaus, G. Effects of aflatoxin B1 and fumonisin B1 on body weight, antibody titres and histology of broiler chicks. Brit. Poult. Sci. 2006, 47, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Verma, J.; Johri, T.; Swain, B.; Ameena, S. Effect of graded levels of aflatoxin, ochratoxin and their combinations on the performance and immune response of broilers. Brit. Poult. Sci. 2004, 45, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Siloto, E.; Oliveira, E.; Sartori, J.R.; Fascina, V.; Martins, B.; Ledoux, D.; Rottinghaus, G.; Sartori, D. Lipid metabolism of commercial layers fed diets containing aflatoxin, fumonisin, and a binder. Poult. Sci. 2013, 92, 2077–2083. [Google Scholar] [CrossRef] [PubMed]
- Kolosova, A.; Stroka, J. Evaluation of the effect of mycotoxin binders in animal feed on the analytical performance of standardised methods for the determination of mycotoxins in feed. Food Addit. Contam. A 2012, 29, 1959–1971. [Google Scholar] [CrossRef] [PubMed]
- Namazi, M.; Allameh, A.; Aminshahidi, M.; Nohee, A.; Malekzadeh, F. Inhibitory effects of ammonia solution on growth and aflatoxins production by Aspergillus parasiticus NRRL-2999. Acta Pol. Toxicol. 2002, 10, 65–72. [Google Scholar]
- Isolauri, E.; Sütas, Y.; Kankaanpää, P.; Arvilommi, H.; Salminen, S. Probiotics: Effects on immunity. Am. J. Clin. Nutr. 2001, 73, 444s–450s. [Google Scholar] [CrossRef] [PubMed]
- Barati, M.; Chamani, M.; Mousavi, S.N.; Hoseini, S.A.; Taj Abadi Ebrahimi, M. Effects of biological and mineral compounds in aflatoxin-contaminated diets on blood parameters and immune response of broiler chickens. J. Appl. Anim. Res. 2018, 46, 707–713. [Google Scholar] [CrossRef]
- Huang, L.; Duan, C.; Zhao, Y.; Gao, L.; Niu, C.; Xu, J.; Li, S. Reduction of aflatoxin B1 toxicity by Lactobacillus plantarum C88: A potential probiotic strain isolated from Chinese traditional fermented food “tofu”. PLoS ONE 2017, 12, e0170109. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Yang, H.; Jiao, Y.; Pang, Q.; Wang, Y.; Wang, M.; Shan, A.; Feng, X. Dietary curcumin alleviated acute ileum damage of ducks (Anas platyrhynchos) induced by AFB1 through regulating Nrf2-ARE and NF-κB signaling pathways. Foods 2021, 10, 1370. [Google Scholar] [CrossRef] [PubMed]
- Rodricks, J.V.; Stoloff, L. Aflatoxin residue in edible tissues of food-producing animals resulting from feed contamination. In Proceedings of the Annual Meeting of the United States Animal Health Association, Miami, FL, USA, 7–12 November 1976; Volume 80, pp. 442–456. [Google Scholar]
- Hamilton, P.B.; Tung, H.T.; Harris, J.R.; Gainer, J.H.; Donaldson, W.E. The effect of dietary fat on aflatoxicosis in turkeys. Poult. Sci. 1972, 51, 165–170. [Google Scholar] [CrossRef]
- Smith, E.E.; Kubena, L.F.; Braithwaite, R.B.; Harvey, R.B.; Phillips, T.D.; Reine, A.H. Toxicological evaluation of aflatoxin and cyclopiazonic acid in broiler chickens. Poult. Sci. 1992, 71, 1136–1144. [Google Scholar] [CrossRef]
- Quezada, T.; Cuéllar, H.; Jaramillo-Juárez, F.; Valdivia, A.G.; Reyes, J.L. Effects of aflatoxin B1 on the liver and kidney of broiler chickens during development. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2000, 125, 265–272. [Google Scholar] [CrossRef]
- Wade, M.; Sapcota, D. Effect of Dietary Esterified Glucomannan on the Performance of Broiler Chickens During Experimental Aflatoxicosis. Anim. Nutr. Feed Technol. 2017, 16, 107–116. [Google Scholar] [CrossRef]
- Kumar, C.B.; Reddy, B.; Gloridoss, R.G.; Prabhu, T.; Suresh, B.; Kumar, S.N. Amelioration of Aflatoxicosis through a Bio-Technologically Derived Aflatoxin Degrading Commercial Product in Broilers. Pak. Vet. J. 2015, 35, 217–221. [Google Scholar]
- Nemati, Z.; Karimi, A.; Besharati, M. Impact of Aflatoxin Contaminated Feed and Yeast Cell Wall Supplementation on Immune System in Broiler Chickens. In Proceedings of the International Conference on Innovations in Chemical & Agricultural Engineering, Kuala Lumpur, Malaysia, 8–9 February 2015. [Google Scholar]
- Peng, Q.; Zeng, X.; Zhu, J.; Wang, S.; Liu, X.; Hou, C.; Thacker, P.; Qiao, S. Effects of dietary Lactobacillus plantarum B1 on growth performance, intestinal microbiota, and short chain fatty acid profiles in broiler chickens. Poult. Sci. 2016, 95, 893–900. [Google Scholar] [CrossRef]
- Śliżewska, K.; Cukrowska, B.; Smulikowska, S.; Cielecka-Kuszyk, J. The effect of probiotic supplementation on performance and the histopathological changes in liver and kidneys in broiler chickens fed diets with aflatoxin B1. Toxins 2019, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Ortatatli, M.; Ciftci, M.; Tuzcu, M.; Kaya, A. The effects of aflatoxin on the reproductive system of roosters. Res. Vet. Sci. 2002, 72, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Aravind, K.; Patil, V.; Devegowda, G.; Umakantha, B.; Ganpule, S. Efficacy of esterified glucomannan to counteract mycotoxicosis in naturally contaminated feed on performance and serum biochemical and hematological parameters in broilers. Poult. Sci. 2003, 82, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Ortatatli, M.; Oğuz, H.; Hatipoğlu, F.; Karaman, M. Evaluation of pathological changes in broilers during chronic aflatoxin (50 and 100 ppb) and clinoptilolite exposure. Res. Vet. Sci. 2005, 78, 61–68. [Google Scholar] [CrossRef]
- Magnoli, A.; Monge, M.; Miazzo, R.; Cavaglieri, L.; Magnoli, C.; Merkis, C.; Cristofolini, A.; Dalcero, A.; Chiacchiera, S. Effect of low levels of aflatoxin B1 on performance, biochemical parameters, and aflatoxin B1 in broiler liver tissues in the presence of monensin and sodium bentonite. Poult. Sci. 2011, 90, 48–58. [Google Scholar] [CrossRef]
- Oguz, H.; Kurtoglu, V.; Coskun, B. Preventive efficacy of clinoptilolite in broilers during chronic aflatoxin (50 and 100 ppb) exposure. Res. Vet. Sci. 2000, 69, 197–201. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Liu, M.; Zhou, X.; Wang, M.; Cao, K.; Jin, S.; Shan, A.; Feng, X. Curcumin mitigates aflatoxin B1-induced liver injury via regulating the NLRP3 inflammasome and Nrf2 signaling pathway. Food Chem. Toxicol. 2022, 161, 112823. [Google Scholar] [CrossRef]
- Çelýk, K.; Denlý, M.; Savas, T. Reduction of toxic effects of aflatoxin B1 by using baker yeast (Saccharomyces cerevisiae) in growing broiler chicks diets. Braz. J. Anim. Sci. 2003, 32, 615–619. [Google Scholar] [CrossRef]
- Chen, X.; Naehrer, K.; Applegate, T. Interactive effects of dietary protein concentration and aflatoxin B1 on performance, nutrient digestibility, and gut health in broiler chicks. Poult. Sci. 2016, 95, 1312–1325. [Google Scholar] [CrossRef]
- Hassan, Z.U.; Khan, M.Z.; Saleemi, M.K.; Khan, A.; Javed, I.; Noreen, M. Immunological responses of male White Leghorn chicks kept on ochratoxin A (OTA)-contaminated feed. J. Immunotoxicol. 2012, 9, 56–63. [Google Scholar] [CrossRef]
- Rathod, P.; Gangadhar, K.; Gangane, G.; Bhojane, N. Effect of aflatoxin on haematological and biochemical alteration in broilers. Int. J. Sci. Environ. Technol. 2017, 6, 824–831. [Google Scholar]
- Ali, A.; Khatoon, A.; Saleemi, M.K.; Abbas, R.Z. Aflatoxins associated oxidative stress and immunological alterations are mitigated by dietary supplementation of Pichia kudriavzevii in broiler chicks. Microb. Pathog. 2021, 161, 105279. [Google Scholar] [CrossRef]
- Lai, Y.; Sun, M.; He, Y.; Lei, J.; Han, Y.; Wu, Y.; Bai, D.; Guo, Y.; Zhang, B. Mycotoxins binder supplementation alleviates aflatoxin B1 toxic effects on the immune response and intestinal barrier function in broilers. Poult. Sci. 2022, 101, 101683. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, C.; Chen, M.; Ya, T.; Huang, W.; Gao, P.; Zhang, H. A novel Lactobacillus plantarum strain P-8 activates beneficial immune response of broiler chickens. Int. Immunopharmacol. 2015, 29, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Tejada-Castañeda, Z.I.; Ávila-Gonzalez, E.; Casaubon-Huguenin, M.T.; Cervantes-Olivares, R.A.; Vásquez-Peláez, C.; Hernández-Baumgarten, E.M.; Moreno-Martinez, E. Biodetoxification of aflatoxin-contaminated chick feed. Poult. Sci. 2008, 87, 1569–1576. [Google Scholar] [CrossRef]
- Fan, Y.; Zhao, L.; Ma, Q.; Li, X.; Shi, H.; Zhou, T.; Zhang, J.; Ji, C. Effects of Bacillus subtilis ANSB060 on growth performance, meat quality and aflatoxin residues in broilers fed moldy peanut meal naturally contaminated with aflatoxins. Food Chem. Toxicol. 2013, 59, 748–753. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Benjamin, M.M. Outline of Veterinary Clinical Pathology, 2nd ed.; Iowa State University Press: Ames, IA, USA, 1961. [Google Scholar]
- SAS Institute Inc. SAS/STAT® 15.1 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2018. [Google Scholar]




| Groups | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 |
|---|---|---|---|---|---|---|
| BD | 57.41 ± 14.12 a | 97.76 ± 12.44 a | 128.13 ± 18.76 b | 156.49 ± 6.24 b | 199.31 ± 9.56 b | 230.53 ± 11.17 b |
| AF1 | 51.14 ± 13.23 a | 84.69 ± 9.56 b | 103.42 ± 14.87 c | 145.17 ± 7.84 c | 182.74 ± 14.53 c | 198.35 ± 14.27 c |
| AF2 | 45.15 ± 11.23 a | 75.09 ± 8.57 c | 92.77 ± 16.23 d | 136.73 ± 5.53 d | 166.14 ± 8.67 d | 174.46 ± 12.54 d |
| AF1LP | 56.52 ± 14.81 a | 98.27 ± 9.32 a | 127.45 ± 17.63 b | 155.17 ± 5.45 b | 197.73 ± 8.13 b | 227.48 ± 9.21 b |
| AF2LP | 54.84 ± 13.73 a | 85.65 ± 12.74 b | 116.34 ± 28.67 c | 144.32 ± 7.51 c | 179.78 ± 7.63 c | 187.16 ± 13.23 c |
| BDLP | 60.47 ± 13.73 a | 99.11 ± 5.87 a | 148.74 ± 15.83 a | 172.63 ± 5.87 a | 216.74 ± 11.73 a | 252.84 ± 14.87 a |
| Group | Initial Weight | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 |
|---|---|---|---|---|---|---|---|
| BD | 40 ± 4.30 a | 86.23 ± 6.57 a | 237.35 ± 18.52 a | 861.83 ± 30.14 b | 1242.74 ± 44.51 b | 1728.51 ± 54.14 b | 2012.53 ± 66.49 b |
| AF1 | 45 ± 3.70 a | 83.39 ± 10.83 a | 214.49 ± 12.67 c | 799.23 ± 40.15 c | 1031.69 ± 53.23 c | 1459.23 ± 94.67 c | 1679.48 ± 64.43 c |
| AF2 | 47 ± 3.50 a | 74.74 ± 11.57 a | 165.47 ± 22.73 d | 677.09 ± 55.34 d | 880.79 ± 51.23 d | 1322.23 ± 57.57 d | 1559.21 ± 37.54 d |
| AF1LP | 39 ± 5.3 a | 72.15 ± 6.13 a | 229.63 ± 13.47 b | 859.48 ± 30.83 b | 1227.12 ± 38.19 b | 1702.27 ± 81.51 b | 1952.34 ± 51.43 b |
| AF2LP | 51 ± 3.90 a | 71.11 ± 5.38 a | 212.49 ± 10.51 c | 809.67 ± 30.52 c | 1095.72 ± 81.53 c | 1492.17 ± 79.84 c | 1632.65 ± 71.86 c |
| BDLP | 42 ± 4.31 a | 78.23 ± 6.76 a | 249.28 ± 10.64 a | 902.0 ± 33.1 a | 1368.63 ± 21.56 a | 1787.16 ± 46.23 a | 2087.29 ± 43.68 a |
| Group | Liver | Kidney | Spleen | Bursa | Thymus |
|---|---|---|---|---|---|
| BD | 3.20 ± 0.16 d | 0.66 ± 0.06 d | 0.16 ± 0.03 a | 0.19 ± 0.01 b | 0.40 ± 0.02 b |
| AF1 | 5.62 ± 0.38 b | 1.40 ± 0.06 b | 0.14 ± 0.01 a | 0.11 ± 0.01 c | 0.27 ± 0.01 c |
| AF2 | 6.50 ± 0.32 a | 1.85 ± 0.07 a | 0.12 ± 0.02 a | 0.06 ± 0.01 d | 0.21 ± 0.02 d |
| AF1LP | 3.26 ± 0.15 d | 0.69 ± 0.07 d | 0.15 ± 0.03 a | 0.18 ± 0.01 b | 0.39 ± 0.01 b |
| AF2LP | 4.80 ± 0.33 c | 0.95 ± 0.08 c | 0.15 ± 0.02 a | 0.08 ± 0.03 c | 0.23 ± 0.03 cd |
| BDLP | 3.21 ± 0.22 d | 0.59 ± 0.04 d | 0.17 ± 0.01 a | 0.24 ± 0.01 a | 0.45 ± 0.01 a |
| Group | No. of Birds | Treatment |
|---|---|---|
| BD | 30 | Control (Basal diet) |
| AF1 | 30 | Aflatoxins 1st level (300 µg/kg of b.wt) |
| AF2 | 30 | Aflatoxins 2nd level (600 µg/kg of b.wt) |
| AF1LP | 30 | Aflatoxins 1st level + Lactobacillus plantarum |
| AF2LP | 30 | Aflatoxins 2nd level + Lactobacillus plantarum |
| BDLP | 30 | Lactobacillus plantarum (1 × 108 cfu/kg) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Khatoon, A.; Almohaimeed, H.M.; Al-Sarraj, F.; Albiheyri, R.; Alotibi, I.; Abidin, Z.U. Mitigative Potential of Novel Lactobacillus plantarum TISTR 2076 against the Aflatoxins-Associated Oxidative Stress and Histopathological Alterations in Liver and Kidney of Broiler Chicks during the Entire Growth Period. Toxins 2022, 14, 689. https://doi.org/10.3390/toxins14100689
Ali A, Khatoon A, Almohaimeed HM, Al-Sarraj F, Albiheyri R, Alotibi I, Abidin ZU. Mitigative Potential of Novel Lactobacillus plantarum TISTR 2076 against the Aflatoxins-Associated Oxidative Stress and Histopathological Alterations in Liver and Kidney of Broiler Chicks during the Entire Growth Period. Toxins. 2022; 14(10):689. https://doi.org/10.3390/toxins14100689
Chicago/Turabian StyleAli, Ashiq, Aisha Khatoon, Hailah M. Almohaimeed, Faisal Al-Sarraj, Raed Albiheyri, Ibrahim Alotibi, and Zain Ul Abidin. 2022. "Mitigative Potential of Novel Lactobacillus plantarum TISTR 2076 against the Aflatoxins-Associated Oxidative Stress and Histopathological Alterations in Liver and Kidney of Broiler Chicks during the Entire Growth Period" Toxins 14, no. 10: 689. https://doi.org/10.3390/toxins14100689
APA StyleAli, A., Khatoon, A., Almohaimeed, H. M., Al-Sarraj, F., Albiheyri, R., Alotibi, I., & Abidin, Z. U. (2022). Mitigative Potential of Novel Lactobacillus plantarum TISTR 2076 against the Aflatoxins-Associated Oxidative Stress and Histopathological Alterations in Liver and Kidney of Broiler Chicks during the Entire Growth Period. Toxins, 14(10), 689. https://doi.org/10.3390/toxins14100689





