Corynebacterium sp. 2-TD Mediated Toxicity of 2-Tridecanone to Helicoverpa armigera
Abstract
:1. Introduction
2. Results
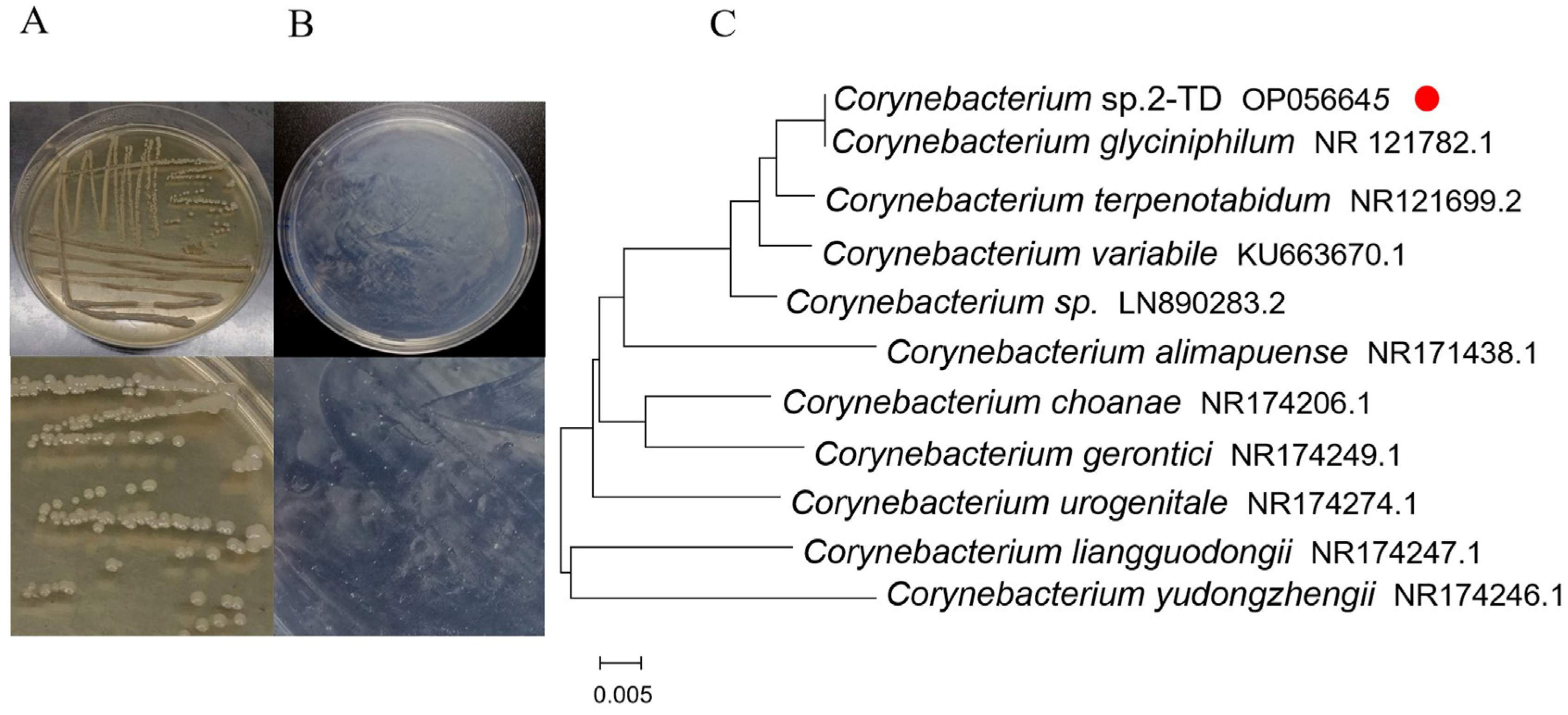
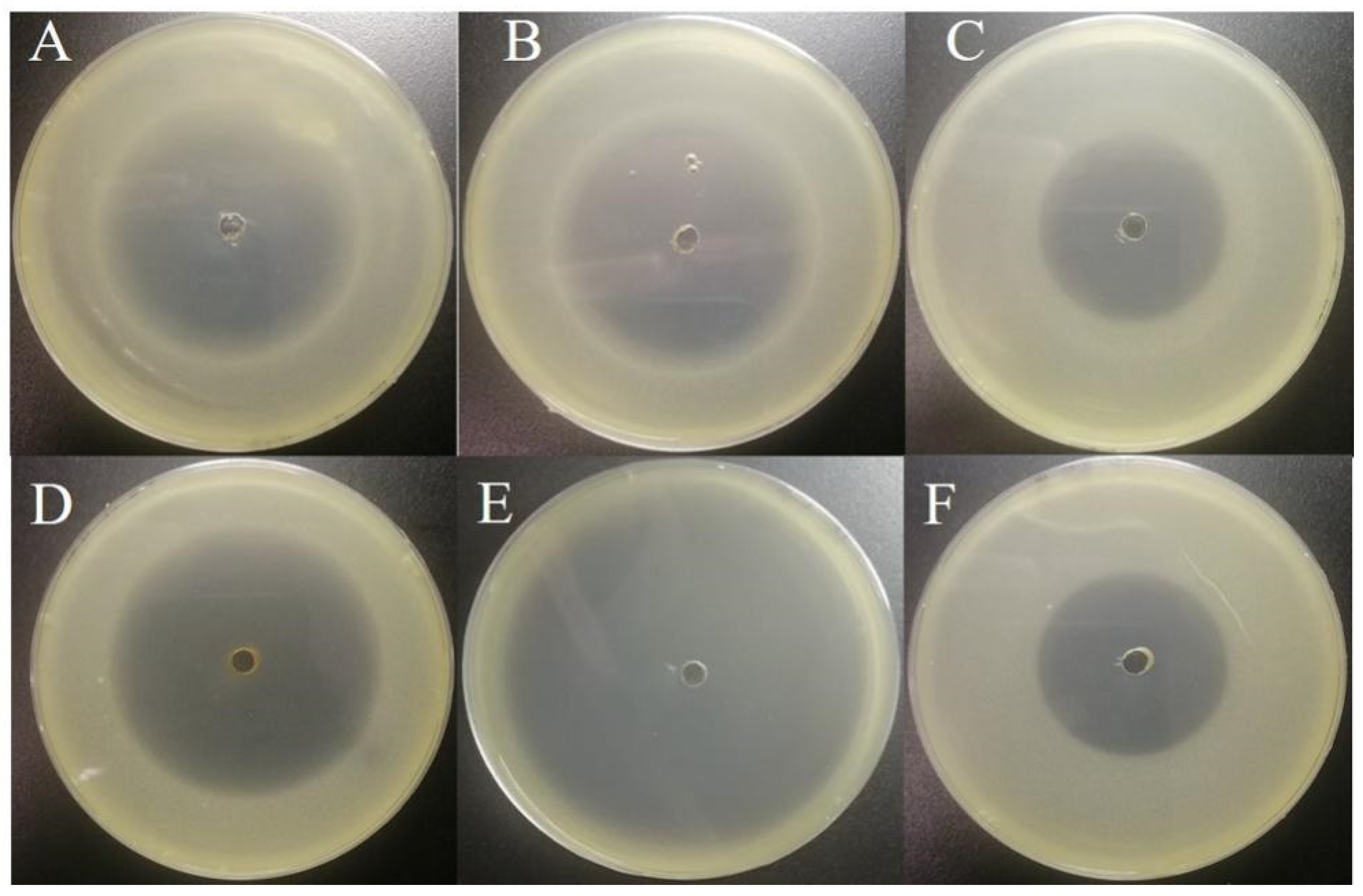
2.1. Isolation and Identification of 2-Tridecanone Degrading Bacteria
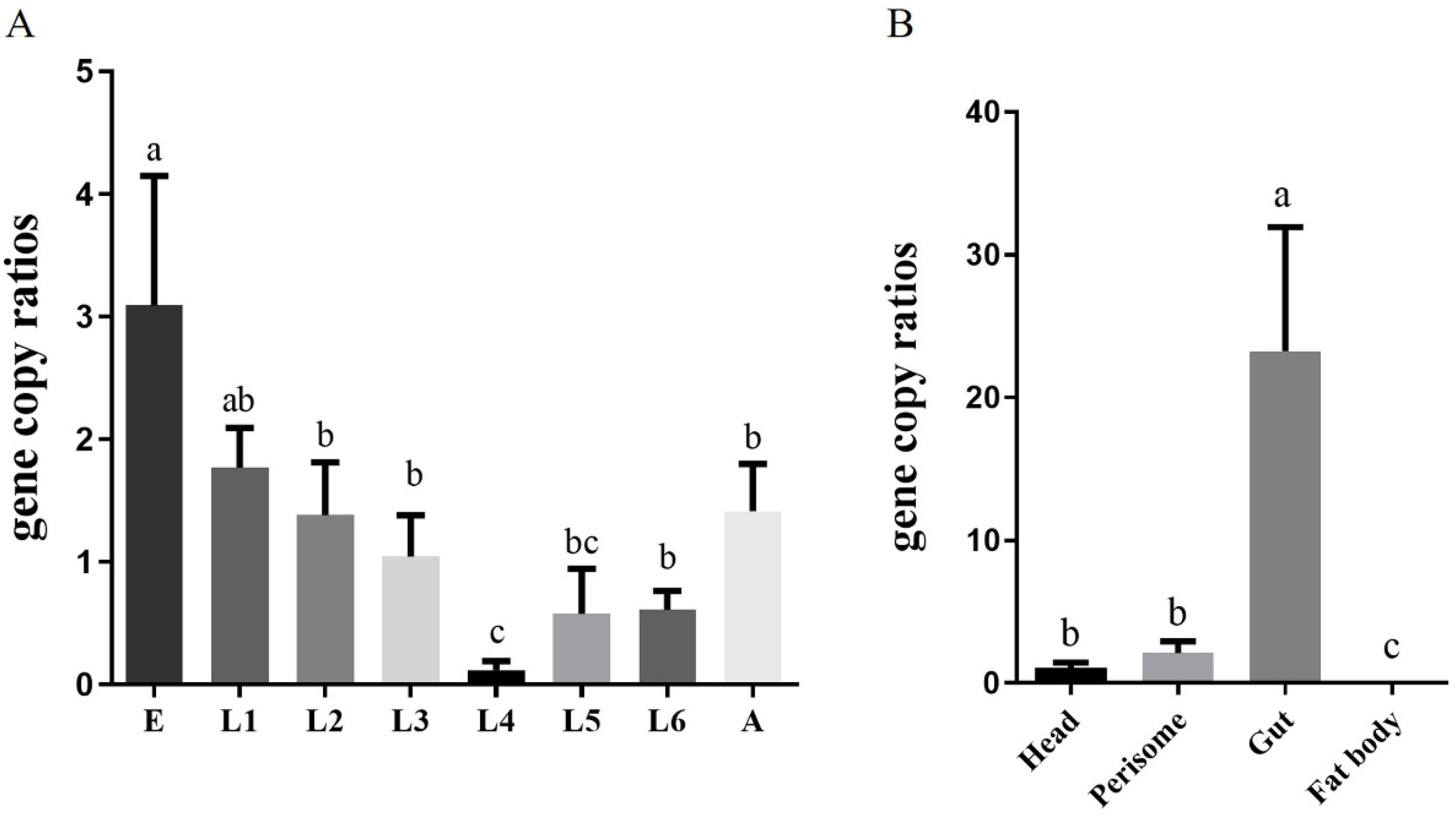
2.2. Localization and Density of Corynebacterium sp. 2-TD in H. armigera
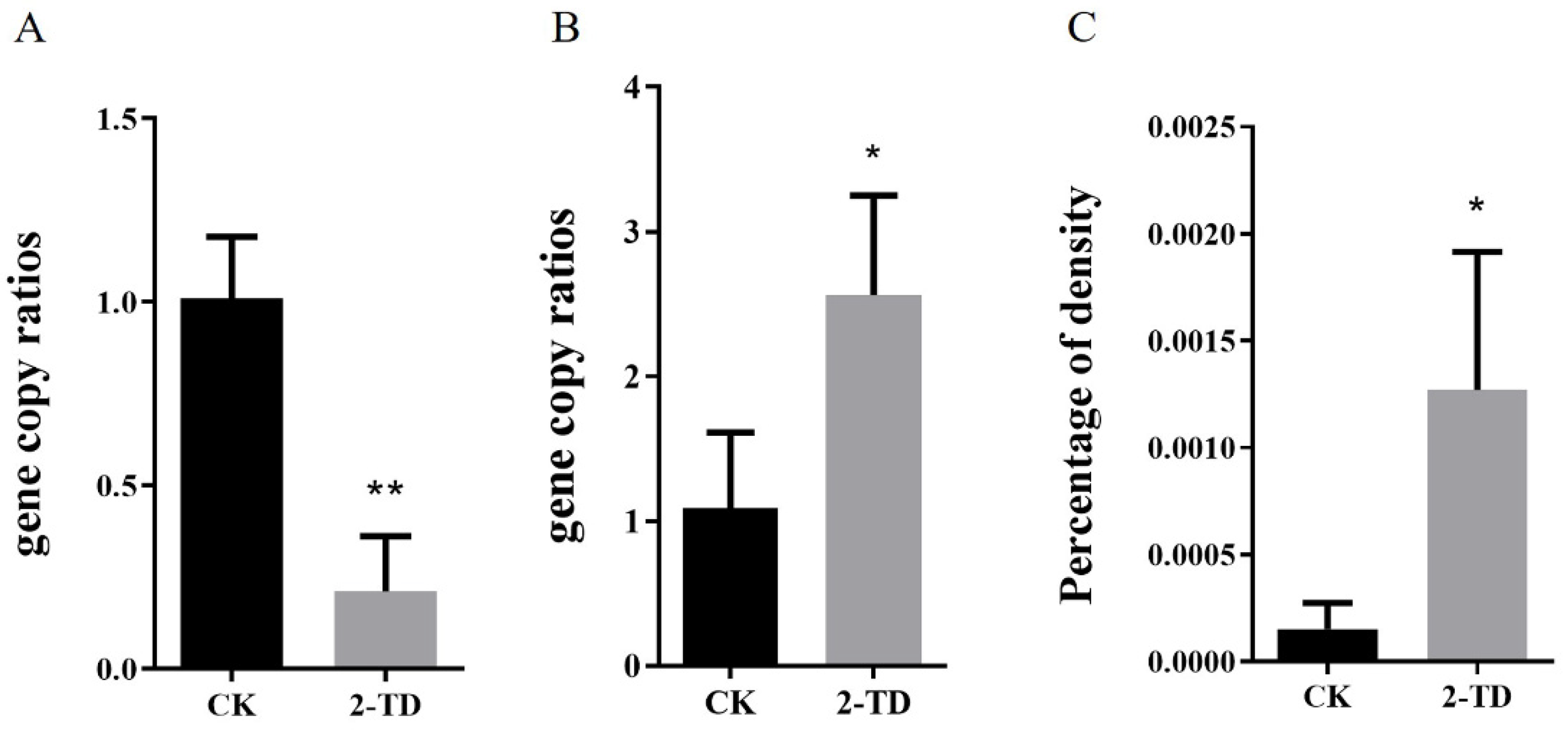
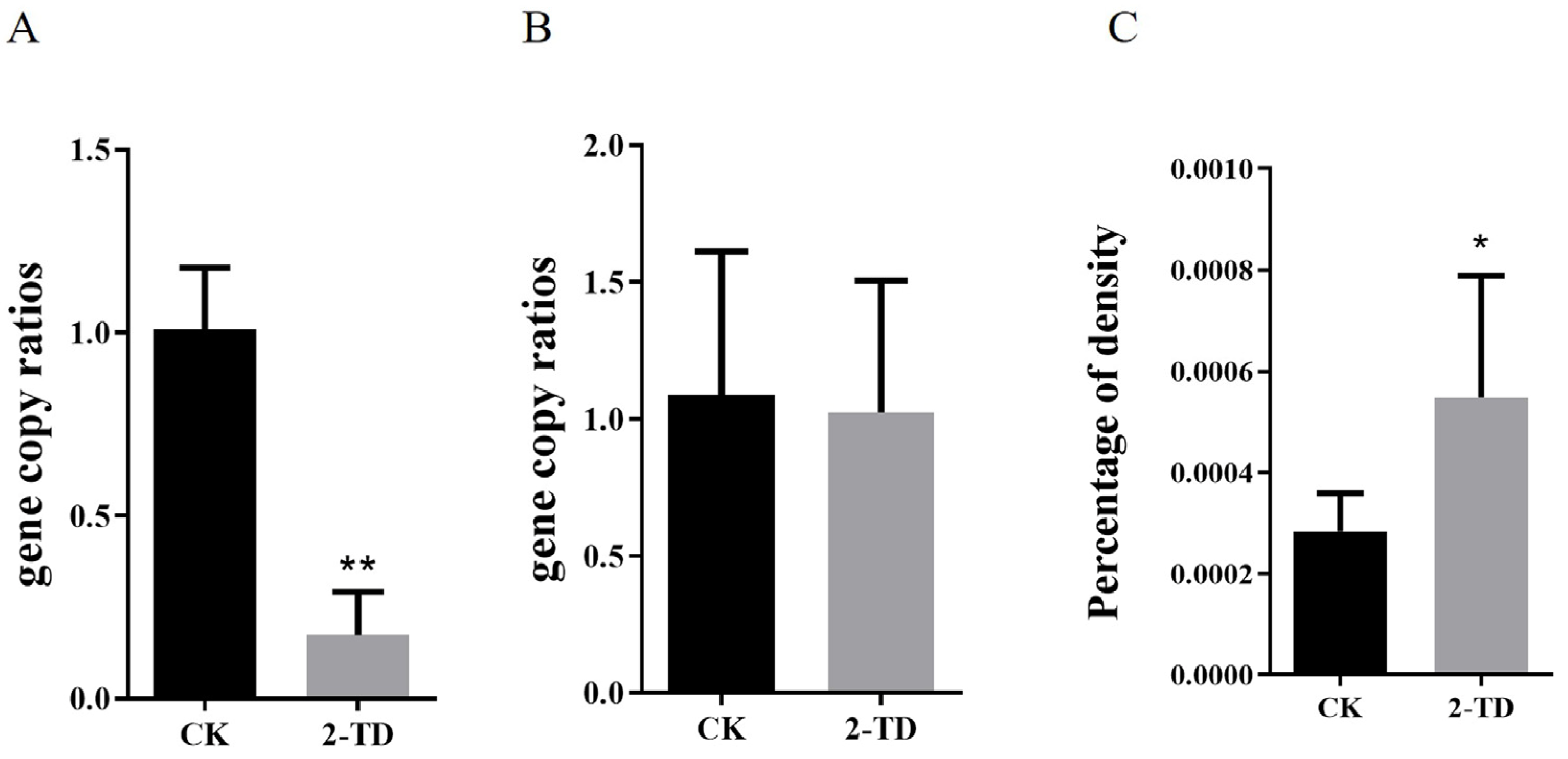
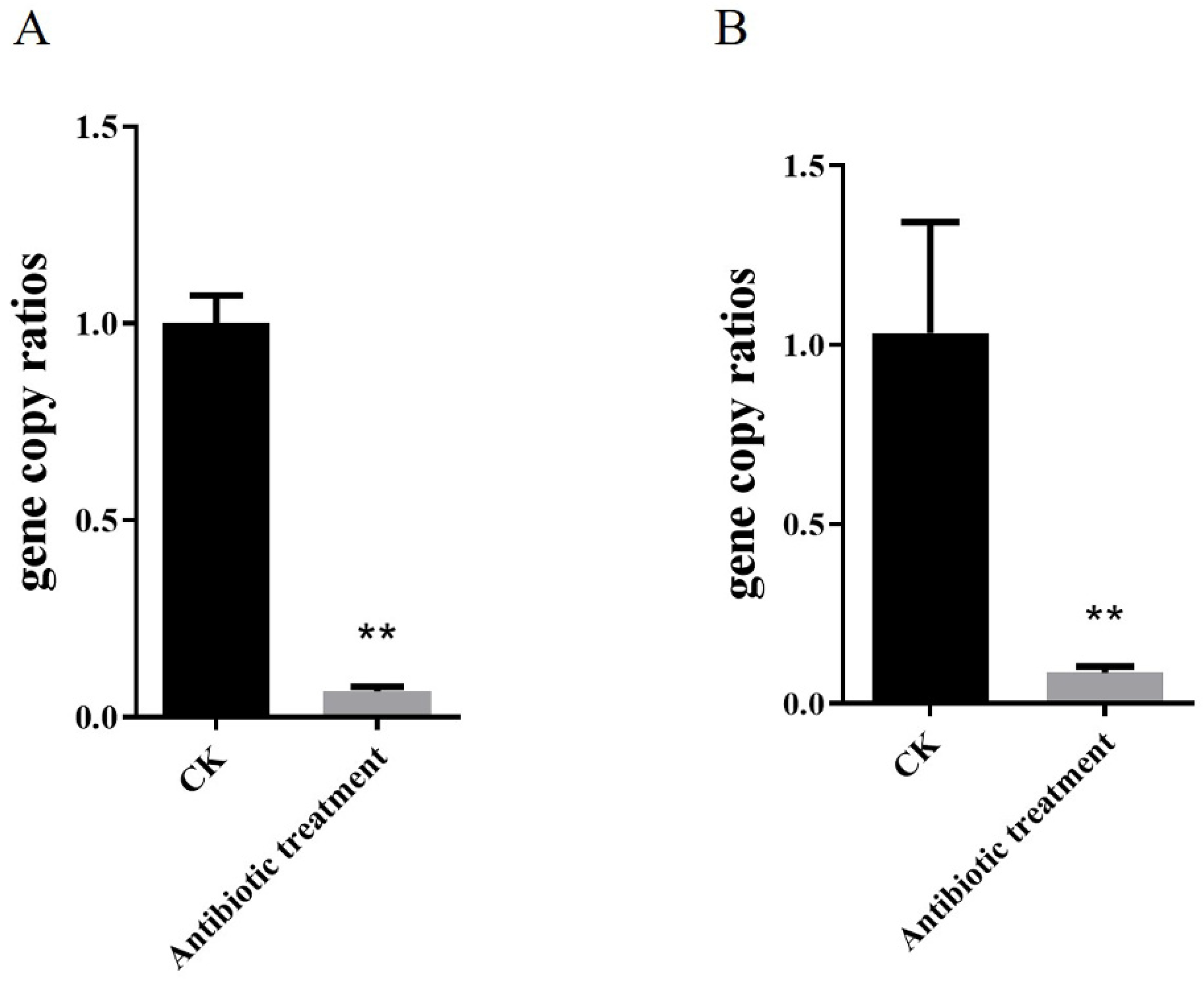
2.3. Density of Corynebacterium in H. armigera after 2-Tridecanone Treatment
2.4. Corynebacterium sp. 2-TD Improve the Survival Rate of H. armigera to 2-Tridecanone
2.5. Degradation of 2-Tridecanone by Corynebacterium sp. 2-TD
3. Discussion
4. Materials and Methods
4.1. Insect Strain and Rearing
4.2. Isolation of H. armigera Bacteria
4.3. Screening and Identifying 2-Tridecanone Degrading Bacteria
4.4. Detecting Density of Corynebacterium sp. 2-TD in H. armigera
4.5. Detecting Distribution of Corynebacterium sp. 2-TD in H. armigera
4.6. Antibiotic Treatment and Reinoculation of Corynebacterium sp. 2-TD in H. armigera
4.7. Testing of the 2-Tridecanone Degradation Characteristics of Corynebacterium sp. 2-TD
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fitt, G.P. The ecology of heliothis species in relation to agroecosystems. Annu. Rev. Entomol. 1989, 34, 17–52. [Google Scholar] [CrossRef]
- Gujar, N.; Nikte, V.S.; Joshi, R.S.; Joshi, M. Molecular Characterization characterization of the β2-like Octopamine octopamine Receptor receptor of Helicoverpa armigera. J. Membr. Biol. 2021, 254, 311–319. [Google Scholar] [CrossRef]
- Xia, J.X.; Guo, Z.J.; Yang, Z.Z.; Han, H.L.; Wang, S.L.; Xu, H.F.; Yang, X.; Yang, F.S.; Wu, Q.J.; Xie, W.; et al. Whitefly hijacks a plant detoxification gene that neutralizes plant toxins. Cell 2021, 184, 1693. [Google Scholar] [CrossRef]
- Abe, H.; Tateishi, K.; Seo, S.; Kugimiya, S.; Hirai, M.Y.; Sawada, Y.; Murata, Y.; Yara, K.; Shimoda, T.; Kobayashi, M. Disarming the Jasmonate-Dependent Plant Defense Makes Nonhost Arabidopsis Plants Accessible to the American Serpentine leafminer. Plant. Physiol. 2013, 163, 1242–1253. [Google Scholar] [CrossRef] [Green Version]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant. Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [Green Version]
- Dimock, M.B.; Kennedy, G.G.; Williams, W.G. Toxicity studies of analogs of 2-tridecanone, a naturally-occurring toxicant from a wild tomato. J. Chem. Ecol. 1982, 8, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Williams, W.G.; Kennedy, G.G.; Yamamoto, R.T.; Thacker, J.D.; Bordner, J. 2-tridecanone—Naturally-occurring insecticide from the wild tomato Lycopersicon hirsutum f. glabratum. Science 1980, 207, 888–889. [Google Scholar] [CrossRef]
- Kimps, N.W.; Bissinger, B.W.; Apperson, C.S.; Sonenshine, D.E.; Roe, R.M. First report of the repellency of 2-tridecanone against ticks. Med. Vet. Entomol. 2011, 25, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Fridman, E.; Wang, J.H.; Iijima, Y.; Froehlich, J.E.; Gang, D.R.; Ohlrogge, J.; Pichersky, E. Metabolic, genomic, and biochemical analyses of glandular trichomes from the wild tomato species Lycopersicon hirsutum identify a key enzyme in the biosynthesis of methylketones. Plant. Cell 2005, 17, 1252–1267. [Google Scholar] [CrossRef] [Green Version]
- Sabree, Z.L.; Kambhampati, S.; Moran, N.A. Nitrogen recycling and nutritional provisioning by Blattabacterium, the cockroach endosymbiont. Proc. Natl. Acad. Sci. USA 2009, 106, 19521–19526. [Google Scholar] [CrossRef]
- Frago, E.; Dicke, M.; Godfray, H.C.J. Insect symbionts as hidden players in insect-plant interactions. Trends Ecol. Evol. 2012, 27, 705–711. [Google Scholar] [CrossRef]
- Leftwich, P.T.; Clarke, N.V.E.; Hutchings, M.I.; Chapman, T. Gut microbiomes and reproductive isolation in Drosophila (vol 114, pg 12767, 2017). Proc. Natl. Acad. Sci. USA 2018, 115, E2487. [Google Scholar] [CrossRef] [Green Version]
- Blanton, A.G.; Peterson, B.F. Symbiont-Mediated mediated Insecticide insecticide detoxification as an emerging problem in insect pests. Front. Microbiol. 2020, 11, 547108. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Gurr, G.M.; Vasseur, L.; Zheng, D.; Zhong, H.; Qin, B.; Lin, J.; Wang, Y.; Song, F.; Li, Y.; et al. Metagenomic sequencing of diamondback moth gut microbiome unveils key holobiont adaptations for herbivory. Front. Microbiol. 2017, 8, 663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welte, C.U.; de Graaf, R.M.; van den Bosch, T.J.; Op den Camp, H.J.; van Dam, N.M.; Jetten, M.S. Plasmids from the gut microbiome of cabbage root fly larvae encode SaxA that catalyses the conversion of the plant toxin 2-phenylethyl isothiocyanate. Environ. Microbiol. 2016, 18, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- Sureshan, S.C.; Mohideen, H.S.; Nair, T.S. Gut metagenomic profiling of gossypol induced Oxycarenus laetus (Hemiptera: Lygaeidae) reveals gossypol tolerating bacterial species. Indian J. Microbiol. 2022, 62, 54–60. [Google Scholar] [CrossRef]
- Ben-Yosef, M.; Pasternak, Z.; Jurkevitch, E.; Yuval, B. Symbiotic bacteria enable olive fly larvae to overcome host defences. R. Soc. Open Sci. 2015, 2, 150170. [Google Scholar] [CrossRef] [Green Version]
- Adams, A.S.; Aylward, F.O.; Adams, S.M.; Erbilgin, N.; Aukema, B.H.; Currie, C.R.; Suen, G.; Raffa, K.F. Mountain pine beetles colonizing historical and naive host trees are associated with a bacterial community highly enriched in genes contributing to terpene metabolism. Appl. Environ. Microbiol. 2013, 79, 3468–3475. [Google Scholar] [CrossRef] [Green Version]
- Hosokawa, T.; Kikuchi, Y.; Shimada, M.; Fukatsu, T. Obligate symbiont involved in pest status of host insect. Proc. Biol. Sci. 2007, 274, 1979–1984. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Xiao, G.; Du, G.; Chang, L.; Yang, Y.; Ye, J.; Chen, B. Glutamicibacter halophytocola-mediated host fitness of potato tuber moth on Solanaceae crops. Pest. Manag. Sci. 2022, 78, 3920–3930. [Google Scholar] [CrossRef]
- Hacker, E.; Antunes, C.A.; Mattos-Guaraldi, A.L.; Burkovski, A.; Tauch, A. Corynebacterium ulcerans, an emerging human pathogen. Future Microbiol. 2016, 11, 1191–1208. [Google Scholar] [CrossRef] [PubMed]
- Poojary, I.; Kurian, A.; Jayalekshmi, V.A.; Devapriya, J.D.; Thirunarayan, M.A. Corynebacterium species causing breast abscesses among patients attending a tertiary care hospital in Chennai, South India. Infect. Dis. 2017, 49, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Sakane, T.; Nihira, T.; Yamada, Y.; Imai, K. Corynebacterium terpenotabidum sp. nov., a bacterium capable of degrading squalene. Int. J. Syst. Bacteriol. 1999, 49 Pt 1, 223–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rückert, C.; Albersmeier, A.; Al-Dilaimi, A.; Bednarz, H.; Niehaus, K.; Szczepanowski, R.; Kalinowski, J. Genome sequence of the squalene-degrading bacterium Corynebacterium terpenotabidum type strain Y-11(T) (= DSM 44721(T)). Stand. Genom. Sci. 2014, 9, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Kubota, K.; Yamada, K.; Okumura, S.; Maeyashiki, I.; Kageyama, K. Fermentative production of l-serine production of l-serine from glycine by Corynebacterium-glycinophilum nov sp. J. Gen. Appl. Microbiol. 1972, 18, 365. [Google Scholar] [CrossRef]
- Al-Dilaimi, A.; Bednarz, H.; Lömker, A.; Niehaus, K.; Kalinowski, J.; Rückert, C. Revisiting Corynebacterium glyciniphilum (ex Kubota et al., 1972) sp. nov., nom. rev., isolated from putrefied banana. Int. J. Syst. Evol. Microbiol. 2015, 65, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herren, J.K.; Paredes, J.C.; Schüpfer, F.; Lemaitre, B. Vertical transmission of a Drosophila endosymbiont via cooption of the yolk transport and internalization machinery. mBio 2013, 4, e00532-12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, K.; Yukuhiro, F.; Matsuura, Y.; Fukatsu, T.; Noda, H. Intrasperm vertical symbiont transmission. Proc. Natl. Acad. Sci. USA 2014, 111, 7433–7437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brumin, M.; Levy, M.; Ghanim, M. Transovarial transmission of Rickettsia spp. and organ-specific infection of the whitefly Bemisia tabaci. Appl. Environ. Microbiol. 2012, 78, 5565–5574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowd, P.F. Insitu production of hydrolytic detoxifying enzymes by symbiotic yeasts in the cigarette beetle (Coleoptera, Anobiidae). J. Econ. Entomol. 1989, 82, 396–400. [Google Scholar] [CrossRef]
- Cheng, D.; Guo, Z.; Riegler, M.; Xi, Z.; Liang, G.; Xu, Y. Gut symbiont enhances insecticide resistance in a significant pest, the oriental fruit fly Bactrocera dorsalis (Hendel). Microbiome 2017, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceja-Navarro, J.A.; Vega, F.E.; Karaoz, U.; Hao, Z.; Jenkins, S.; Lim, H.C.; Kosina, P.; Infante, F.; Northen, T.R.; Brodie, E.L. Gut microbiota mediate caffeine detoxification in the primary insect pest of coffee. Nat. Commun. 2015, 6, 7618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forney, F.W.; Markovetz, A.J.; Kallio, R.E. Bacterial oxidation of 2-tridecanone to 1-undecanol. J. Bacteriol. 1967, 93, 649–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forney, F.W.; Markovetz, A.J. Oxidative degradation of methyl ketones. II. Chemical pathway for degradation of 2-tridecanone by Pseudomonas multivorans and Pseudomonas aeruginosa. J. Bacteriol. 1968, 96, 1055–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shum, A.C.; Markovetz, A.J. Specificity and induction of undecyl acetate esterase from Pseudomonas cepacia grown on 2-tridecanone. J. Bacteriol. 1974, 118, 890–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Britton, L.N.; Markovetz, A.J. A novel ketone monooxygenase from Pseudomonas cepacia. Purification and properties. J. Biol. Chem. 1977, 252, 8561–8566. [Google Scholar] [CrossRef]
- Hammer, T.J.; Bowers, M.D. Gut microbes may facilitate insect herbivory of chemically defended plants. Oecologia 2015, 179, 1–14. [Google Scholar] [CrossRef]
- Sinno, M.; Bézier, A.; Vinale, F.; Giron, D.; Laudonia, S.; Garonna, A.P.; Pennacchio, F. Symbiosis disruption in the olive fruit fly, Bactrocera oleae (Rossi), as a potential tool for sustainable control. Pest. Manag. Sci. 2020, 76, 3199–3207, Epub 26 May 2020. [Google Scholar] [CrossRef]
- Login, F.H.; Balmand, S.; Vallier, A.; Vincent-Monégat, C.; Vigneron, A.; Weiss-Gayet, M.; Rochat, D.; Heddi, A. Antimicrobial peptides keep insect endosymbionts under control. Science 2011, 334, 362–365. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, M.; Xue, Z.; Lv, S.; Cai, Y.; Zhang, L.; Gao, X. Corynebacterium sp. 2-TD Mediated Toxicity of 2-Tridecanone to Helicoverpa armigera. Toxins 2022, 14, 698. https://doi.org/10.3390/toxins14100698
Gu M, Xue Z, Lv S, Cai Y, Zhang L, Gao X. Corynebacterium sp. 2-TD Mediated Toxicity of 2-Tridecanone to Helicoverpa armigera. Toxins. 2022; 14(10):698. https://doi.org/10.3390/toxins14100698
Chicago/Turabian StyleGu, Meng, Zhaoxiang Xue, Shenglan Lv, Yuhao Cai, Lei Zhang, and Xiwu Gao. 2022. "Corynebacterium sp. 2-TD Mediated Toxicity of 2-Tridecanone to Helicoverpa armigera" Toxins 14, no. 10: 698. https://doi.org/10.3390/toxins14100698





