A Review of the Proteomic Profiling of African Viperidae and Elapidae Snake Venoms and Their Antivenom Neutralisation
Abstract
1. Introduction
2. Characteristics of Snake Venom Proteins
2.1. Snake Venom Phospholipase A2s
2.2. Snake Venom Metalloproteinases
2.3. Snake Venom Serine Proteases
2.4. Cysteine-Rich Secretory Proteins
2.5. Three-Finger Toxins
2.6. Other Toxins
3. Bottom-Up Snake Venom Proteomics
4. Proteome Components of Venoms of African Viperidae and Elapidae
4.1. Dominant Viperidae Toxins
| Dominant | Secondary | Minor | Others | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Country/Region | SVMP | PLA2 | SVSP | CTL | DIS | LAAO | KUN | CRISP | VEGF | CYS | Others | Ref. |
| B. arietans | Ghana | 38.5 | 4.3 | 19.5 | 13.2 | 17.8 | – | 4.1 | – | – | 1.7 | 0.9 | [85] |
| B. arietans | Nigeria | 21.1 | 10.1 | 22.3 | 10.7 | 3.4 | 8.7 | 1.1 | 2.1 | 8.1 | – | 8.6 | [84] |
| B. nasicornis | West Africa | 40.9 | 20.1 | 21.9 | 4.2 | 3.5 | 3.2 | – | 1.3 | – | 4.2 | 0.8 | [82] |
| B. caudalis | West Africa | 11.5 | 59.8 | 15.1 | 4.9 | 2.3 | 1.7 | 3.2 | 1.2 | – | – | 0.3 | [82] |
| B. g. gabonica | East Africa | 22.9 | 11.4 | 26.4 | 14.3 | 3.4 | 1.3 | 3 | 2 | 1 | 9.8 | 4.5 | [83] |
| B. g. rhinoceros | West Africa | 30.8 | 4.8 | 23.9 | 14.1 | 8.5 | 2.2 | 7.5 | 1.2 | – | 5.3 | 1.7 | [82] |
| E. ocellatus | Nigeria | 66.5 | 12.6 | 2 | 7 | 6.8 | 1.4 | – | 1.5 | – | – | 2.2 | [81] |
| E. ocellatus | Nigeria | 34.8 | 21.2 | 15.5 | 3.9 | 1.9 | 2.2 | 1.5 | 2.9 | 2.7 | 12.8 | [84] | |
| C. cerastes | Egypt | 28.18 | 16.01 | 24.87 | 9.1 | 9.69 | – | – | – | – | – | 12.15 | [86] |
| C. cerastes | Tunisia | 37 | 20 | 9 | 24 | 8 | 12 | – | – | – | – | – | [87] |
| C. cerastes | Tunisia | 55.9 | 16.6 | 13.2 | 3.2 | 4.9 | 6.2 | – | – | – | – | – | [88] |
| C. cerastes | Morocco | 63.1 | 19.1 | 6.9 | 1.7 | 8.5 | – | – | 0.7 | – | – | – | [88] |
| C. vipera | Tunisia | 48 | 21 | 20 | 1 | <1 | 9 | – | – | – | – | – | [87] |
| M. mauritanica | Morocco | 45.4 | 5.5 | 8.3 | 8.1 | 13.8 | – | 2.5 | – | 4.9 | – | 5.6 | [88] |
| M. lebetina | Tunisia | 63.1 | 5 | 5.5 | 3.2 | 15.1 | – | 3.1 | – | 3.3 | – | 1.7 | [88] |
| Dominant | Secondary | Minor | Others | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Country/Region | 3FTx | PLA2 | SVMP | CRISP | KUN | LAAO | CVF | PDE | Others | Ref. |
| D. polylepis | Kenya | 31 | <0.1 | 3.2 | – | 61.1 | – | – | 0.1 | 4.5 | [77] |
| D. angusticeps | Tanzania | 69.2 | – | 6.7 | 2 | 16.3 | – | – | – | 6 | [89] |
| N. nigricollis | Nigeria | 73.3 | 21.9 | 2.4 | 0.2 | – | – | – | – | 2.2 | [90] |
| N. nigricollis | Nigeria | 41.25 | 36.5 | 7.91 | 3.4 | – | 4.02 | 1 | – | 3.24 | [91] |
| N. katiensis | Burkina Faso | 67.1 | 29 | 3.3 | 0.2 | – | – | – | – | 0.5 | [90] |
| N. pallida | Kenya | 67.7 | 30.1 | 1.6 | – | – | – | – | – | 0.7 | [90] |
| N. nubiae | North Africa | 70.9 | 26.4 | 2.6 | – | – | – | – | – | 0.1 | [90] |
| N. mossambica | Tanzania | 69.3 | 27.1 | 2.6 | – | – | – | – | – | 0.4 | [90] |
| N. melanoleuca | Uganda | 57.1 | 12.9 | 9.7 | 7.6 | – | – | – | – | 7.7 | [59] |
| N. annulifera | Mozambique | 78 | – | 11.18 | 0.61 | – | 5.01 | 1.08 | – | 2.53 | [76] |
| N. annulifera | Mozambique | 79.2 | 2.7 | 12.3 | 3.2 | 0.5 | 0.5 | – | 0.45 | 1.7 | [92] |
| N. ashei | Kenya | 69 | 27 | 2.1 | 0.7 | – | – | 0.12 | – | 1.014 | [93] |
| N. senegalensis | West Africa | 75.9 | – | 6.78 | 9.23 | 3.15 | – | 0.79 | 3.61 | 0.53 | [94] |
| N. haje | Nigeria | 52.14 | 24.02 | 7.2 | 4.85 | – | 3.63 | 3.93 | – | 3.85 | [91] |
| N. katiensis | Nigeria | 52.2 | 26 | 4.72 | 7 | – | 4.36 | 2.83 | – | 2.2 | [91] |
| H. haemachatus | South Africa | 63.3 | 22.8 | 7.1 | 4.1 | 1.5 | – | – | – | 0.6 | [95] |
| A. s. intermedius | Southern Africa | 82.7 | 6.1 | 2.9 | 4.9 | 1 | – | – | 0.4 | 1.88 | [96] |
| A. l. cowlesi | Southern Africa | 76.1 | 4.9 | 5.1 | 3.5 | 8.6 | 1 | – | 0.5 | 0.1 | [96] |
| A. l. lubricus | Southern Africa | 77.8 | 5.7 | 4 | 5.2 | 5.5 | 1 | – | 1.1 | 0.17 | [96] |
4.2. Secondary Viperidae Toxins
4.3. Minor Viperidae Toxins
4.4. Other Viperidae Toxins
4.5. Dominant Elapidae Toxins
4.6. Secondary Elapidae Toxins
4.7. Minor Elapidae Toxins
4.8. Other Elapidae Toxins
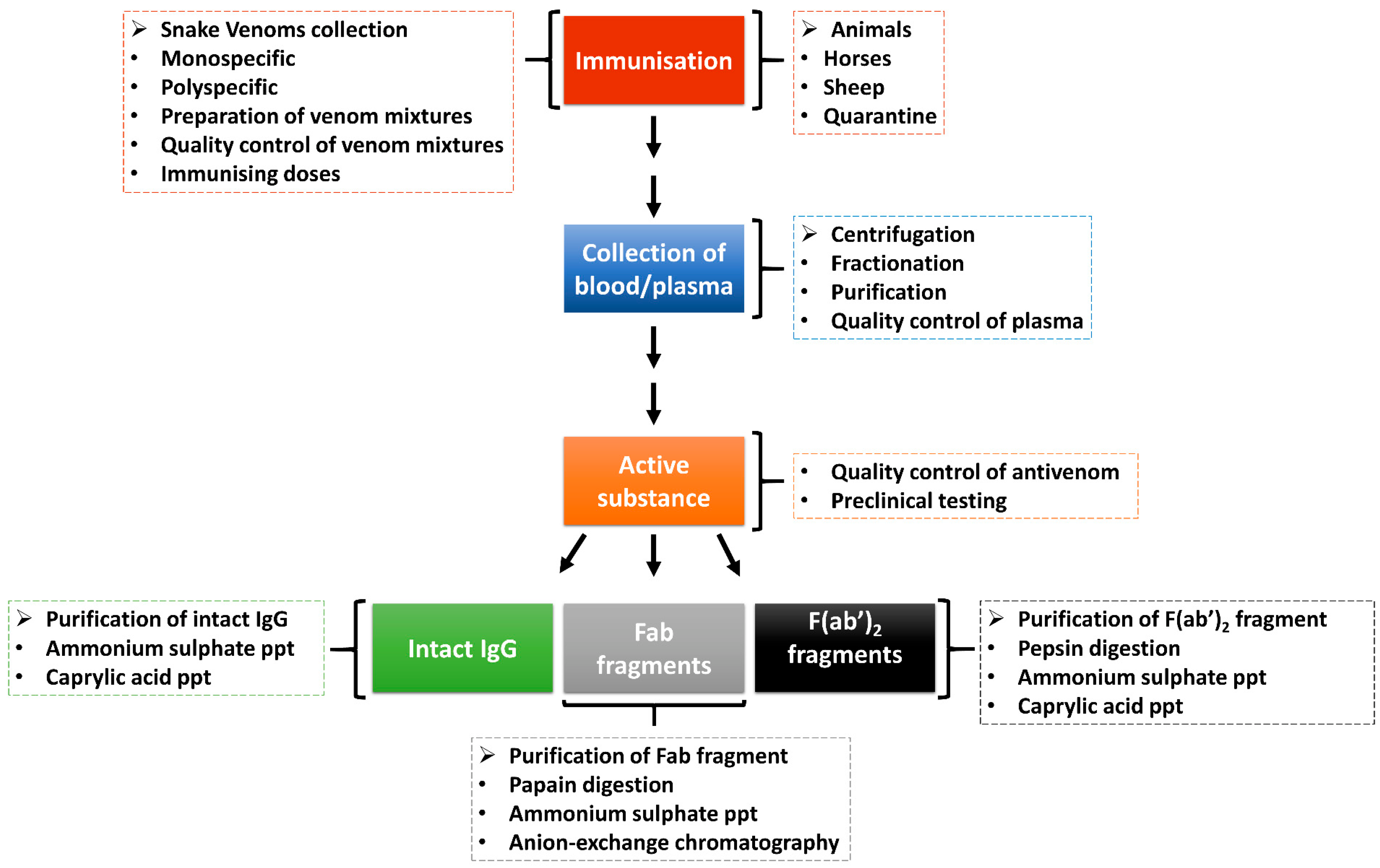
5. Antivenom for African Snakebite Envenomation
| Antivenom | Active Substance | Antivenom Producing Company | Venom Used for Immunisation | Venom Cross-Neutralisation | Ref. | ||
|---|---|---|---|---|---|---|---|
| Viperidae | Elapidae | Viperidae | Elapidae | ||||
| EchiTAb-Plus-ICP | IgG | Instituto Clodomiro Picado, Costa Rica | E. ocellatus, B. arietans | N. nigricollis | E. ocellatus, E. leucogaster, E. pyramidium leakeyi, B. arietans, B. gabonica, B. rhinoceros, B. nasicornis | D. poylepis, N. nigricollis, N. mosaambica, N. annulifera, N. nubiae, N. katiensis, N. pallida, H. haemachatus | [90,92,109,110,111,112,118] |
| EchiTAb-Plus + ICP (expanded) | IgG | Instituto Clodomiro Picado, Costa Rica | E. ocellatus, B. arietans | D. poylepis, N. nigricollis, N. mosaambica, N. annulifera, H. haemachatus | E. ocellatus, B. arietans | D. polylepis, N. mossambica, N. annulifera, N. nigricollis, H. haemachatus | [112] |
| FAV Afrique | F (ab’)2 | Sanofi-Pasteur, France | E. ocellatus, E. leucogaster, B. arietans, B. gabonica | D. polylepis, D. jamesoni, D. viridis, N. nigricollis, N. haje | B. arietans, E. ocellatus | D. polylepis, N. mossambica, N. annulifera, H. haemachatus | [112,115] |
| ASNA antivenom C (ASNA-C) | F (ab’)2 | Bherat Serum and Vaccines, India | E. carinatus, B. arietans, B. gabonica, B. nasiconrnis | D. polylepis, D. jamesoni, D. angusticeps, N. gigricollis, N. annulifera, N. nivea | B. arietans | D. polylepis, N. mossambica, N. annulifera, H. haemachatus | [112] |
| South African Institute for Medical research (SAIMR) Polyvalent | F (ab’)2 | SAVP, South Africa | B. arietans, B. gabonica | D. polylepis, D. jamesoni, D. angusticeps, N. melanoleuca, N. nivea, N. annulifera, N. mossambica, H. haemachatus | B. arietans | D. angusticeps, D. polylepis, N. mossambica, N. annulifera, H. haemachatus, A. s. intermedius, A. l. cowlesi, A. l. lubricus | [59,89,96,97,108,112] |
| Snake venom antiserum (Central African) antivenom | F (ab’)2 | VINS Bioproducts, India | B. g. rhinoceros, Vipera russelli, E. carinatus | D. polylepis | D. angusticeps, D. polylepis | [77,89,97] | |
| Premium serum Pan African polyvalent antivenom (PANAF) | F (ab’)2 | Premium Serum and Vaccines, India | B. arietans, B. gabonica, B. nasicornis, B. rhinoceros, E. leucogaster, E. ocellatus, E. carinatus | D. jamesoni, D. polylepis D. viridis, D. angusticeps, N. haje, N. melanoleuca, N. nigrocollis | N. annulifera | [76] | |
| VINS African polyvalent antivenom (VAPAV) | F (ab’)2 | VINS Bioproducts, India | B. arietans, B. gabonica, E. leucogaster, E. ocellatus | D. jamesoni, D. polylepis D. viridis, N. haje, N. melanoleuca, N. nigrocollis | B. arietans, B. gabonica, E. leucogaster, E. ocellatus | D. polylepis, D. angusticeps, D. jamesoni, D. viridis, N. annulifera, N. senegalensis, N. haje, N. mossambica, N. nigricollis, N. melanoleuca, H. haemachatus | [76,77,89,94,97,112,113] |
| EchiTAb G | IgG | MicroPharm, UK | E. ocellatus | E. ocellatus | [115] | ||
| Inoserp-Panafricain (Inoserp-P) | F (ab’)2 | INOSAN Biopharma, Spain | E. ocellatus, E. leucogaster, E. pyramidium, B. arietans, B. gabonica | D. polylepis, D. jamesoni, N. nigricollis, N. melanoleuca, N. haje, N. pallida | E. ocellatus | [115] | |
| anti-Hemachatus-ICP | Instituto Clodomiro Picado, Costa Rica | H. haemachatus | H. haemachatus, N. mossambica, N. nigricollis, N. annulifera | [92] | |||
| Anti-Moroccan C. cerastes antivenom (CcMo_AV) | F (ab’)2 | Instituto Butantan, Brazil | C. cerastes | C. cerastes | [88] | ||
| Gamma-VIP | F (ab’)2 | Institut Pasteur de Tunis, Tunisia | C. cerastes, M. lebetina | C. cerastes, M. mauritanica, M. lebetina | [88,114] | ||
| Anti-Moroccan M. mauritanica antivenom (MmMo_AV) | F (ab’)2 | Instituto Butantan, Brazil | M. mauritanica | M. mauritanica, M. lebetina | [114] | ||
6. Alternative Antivenom Therapy
7. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Prim. 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Hargreaves, A.; Wagstaff, S.C.; Faragher, B.; Lalloo, D.G. Snake envenoming: A disease of poverty. PLoS Negl. Trop. Dis. 2009, 3, e569. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.P. Snake-bites: Appraisal of the global situation. Bull. World Health Organ. 1998, 76, 515–524. [Google Scholar]
- Kasturiratne, A.; Wickremasinghe, A.R.; De Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; De Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, 1591–1604. [Google Scholar] [CrossRef] [PubMed]
- WHO. Snakebite Envenoming: Strategy for Prevention and Control; World Health Organization: Geneva, Switzerland, 2019; Available online: https://apps.who.int/iris/handle/10665/312195 (accessed on 29 August 2022).
- Chippaux, J.P. Estimate of the burden of snakebites in sub-Saharan Africa: A meta-analytic approach. Toxicon 2011, 57, 586–599. [Google Scholar] [CrossRef]
- Halilu, S.; Iliyasu, G.; Hamza, M.; Chippaux, J.P.; Kuznik, A.; Habib, A.G. Snakebite burden in sub-Saharan Africa: Estimates from 41 countries. Toxicon 2019, 159, 1–4. [Google Scholar] [CrossRef]
- Chippaux, J.P. Snakebite envenomation turns again into a neglected tropical disease! J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 38. [Google Scholar] [CrossRef]
- Casewell, N.R.; Jackson, T.N.W.; Laustsen, A.H.; Sunagar, K. Causes and consequences of snake venom variation. Trends Pharmacol. Sci. 2020, 41, 570–581. [Google Scholar] [CrossRef]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Casewell, N.R.; Wagstaff, S.C.; Wus̈ter, W.; Cook, D.A.N.; Bolton, F.M.S.; King, S.I.; Pla, D.; Sanz, L.; Calvete, J.J.; Harrison, R.A. Medically important differences in snake venom composition are dictated by distinct postgenomic mechanisms. Proc. Natl. Acad. Sci. USA 2014, 111, 9205–9210. [Google Scholar] [CrossRef]
- Currier, R.B.; Harrison, R.A.; Rowley, P.D.; Laing, G.D.; Wagstaff, S.C. Intra-specific variation in venom of the African Puff Adder (Bitis arietans): Differential expression and activity of snake venom metalloproteinases (SVMPs). Toxicon 2010, 55, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Damm, M.; Hempel, B.F.; Süssmuth, R.D. Old world vipers-a review about snake venom proteomics of viperinae and their variations. Toxins 2021, 13, 427. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.; Viegas, M.F.; da Silva, S.L.; Soares, A.M.; Ramos, M.J.; Fernandes, P.A. The chemistry of snake venom and its medicinal potential. Nat. Rev. Chem. 2022, 6, 452–469. [Google Scholar] [CrossRef]
- Lu, Q.; Clemetson, J.M.; Clemetson, K.J. Snake venoms and hemostasis. J. Thromb. Haemost. 2005, 3, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.J.; Faiz, M.A.; Abela-Ridder, B.; Ainsworth, S.; Bulfone, T.C.; Nickerson, A.D.; Habib, A.G.; Junghanss, T.; Fan, H.W.; Turner, M.; et al. Strategy for a globally coordinated response to a priority neglected tropical disease: Snakebite envenoming. PLoS Negl. Trop. Dis. 2019, 13, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.M.; Abo, B.N.; Brandehoff, N. Review article: Snake envenomation in Africa. Curr. Trop. Med. Rep. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- Xiao, H.; Pan, H.; Liao, K.; Yang, M.; Huang, C. Snake Venom PLA2, a promising target for broad-spectrum antivenom drug development. Biomed Res. Int. 2017, 2017, 6592820. [Google Scholar] [CrossRef] [PubMed]
- Tasoulis, T.; Isbister, G.K. A review and database of snake venom proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef]
- Slagboom, J.; Mladić, M.; Xie, C.; Kazandjian, T.D.; Vonk, F.; Somsen, G.W.; Casewell, N.R.; Kool, J. High throughput screening and identification of coagulopathic snake venom proteins and peptides using nanofractionation and proteomics approaches. PLoS Negl. Trop. Dis. 2020, 14, e0007802. [Google Scholar] [CrossRef]
- Koh, C.Y.; Kini, R.M. From snake venom toxins to therapeutics—Cardiovascular examples. Toxicon 2012, 59, 497–506. [Google Scholar] [CrossRef]
- McCleary, R.J.R.; Kini, R.M. Non-enzymatic proteins from snake venoms: A gold mine of pharmacological tools and drug leads. Toxicon 2013, 62, 56–74. [Google Scholar] [CrossRef]
- Six, D.A.; Dennis, E.A. The expanding superfamily of phospholipase A2 enzymes: Classification and characterization. Biochim. Biophys. Acta 2000, 1488, 1–19. [Google Scholar] [PubMed]
- Kang, T.S.; Georgieva, D.; Genov, N.; Murakami, M.T.; Sinha, M.; Kumar, R.P.; Kaur, P.; Kumar, S.; Dey, S.; Sharma, S.; et al. Enzymatic toxins from snake venom: Structural characterization and mechanism of catalysis. FEBS J. 2011, 278, 4544–4576. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, C.R.; Arrahman, A.; Xie, C.; Casewell, N.R.; Lewis, R.J.; Kool, J.; Cardoso, F.C. Multifunctional toxins in snake venoms and therapeutic implications: From pain to hemorrhage and necrosis. Front. Ecol. Evol. 2019, 7, 1–19. [Google Scholar] [CrossRef]
- Escalante, T.; Shannon, J.; Moura-da-Silva, A.M.; María Gutiérrez, J.; Fox, J.W. Novel insights into capillary vessel basement membrane damage by snake venom hemorrhagic metalloproteinases: A biochemical and immunohistochemical study. Arch. Biochem. Biophys. 2006, 455, 144–153. [Google Scholar] [CrossRef]
- Kini, R.M. Serine proteases affecting blood coagulation and fibrinolysis from snake venoms. Pathophysiol. Haemost. Thromb. 2006, 34, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Morita, T. Structure and function of snake venom cysteine-rich secretory proteins. Toxicon 2004, 44, 227–231. [Google Scholar] [CrossRef]
- Frangieh, J.; Rima, M.; Fajloun, Z.; Henrion, D.; Sabatier, J.M.; Legros, C.; Mattei, C. Snake venom components: Tools and cures to target cardiovascular diseases. Molecules 2021, 26, 2223. [Google Scholar] [CrossRef]
- Rivel, M.; Solano, D.; Herrera, M.; Vargas, M.; Villalta, M.; Segura, Á.; Arias, A.S.; León, G.; Gutiérrez, J.M. Pathogenesis of dermonecrosis induced by venom of the spitting cobra, Naja nigricollis: An experimental study in mice. Toxicon 2016, 119, 171–179. [Google Scholar] [CrossRef]
- Kini, R.M.; Doley, R. Structure, function and evolution of three-finger toxins: Mini proteins with multiple targets. Toxicon 2010, 56, 855–867. [Google Scholar] [CrossRef]
- Ogawa, T.; Chijiwa, T.; Oda-Ueda, N.; Ohno, M. Molecular diversity and accelerated evolution of C-type lectin-like proteins from snake venom. Toxicon 2005, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kamiguti, A.S.; Zuzel, M.; Theakston, R.D.G. Snake venom metalloproteinases and disintegrins: Interactions with cells. Braz. J. Med. Biol. Res. 1998, 31, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J. The continuing saga of snake venom disintegrins. Toxicon 2013, 62, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Schweitz, H.; Heurteaux, C.; Bois, P.; Moinier, D.; Romey, G.; Lazdunski, M. Calcicludine, a venom peptide of the Kunitz-type protease inhibitor family, is a potent blocker of high-threshold Ca2+ channels with a high affinity for L-type channels in cerebellar granule neurons. Proc. Natl. Acad. Sci. USA 1994, 91, 878–882. [Google Scholar] [CrossRef]
- Tan, K.K.; Bay, B.H.; Gopalakrishnakone, P. L-amino acid oxidase from snake venom and its anticancer potential. Toxicon 2018, 144, 7–13. [Google Scholar] [CrossRef]
- Mashiko, H.; Takahashi, H. Cysteine proteinase inhibitors in elapid and hydrophiid snake venoms. Toxicon 2002, 40, 1275–1281. [Google Scholar] [CrossRef]
- Inagaki, H. Snake venom proteases inhibitors: Enhanced identification, expanding biological function, and promising future. In Snake Venoms, Toxinology; Springer: Dordrecht, The Netherland, 2017; pp. 161–186. [Google Scholar]
- Hiu, J.J.; Yap, M.K.K. Cytotoxicity of snake venom enzymatic toxins: Phospholipase A2 and L-amino acid oxidase. Biochem. Soc. Trans. 2020, 48, 719–731. [Google Scholar] [CrossRef]
- Cendron, L.; Mičetić, I.; Polverino De Laureto, P.; Paoli, M. Structural analysis of trimeric phospholipase A2 neurotoxin from the Australian taipan snake venom. FEBS J. 2012, 279, 3121–3135. [Google Scholar] [CrossRef]
- Wang, C.R.; Bubner, E.R.; Jovcevski, B.; Mittal, P.; Pukala, T.L. Interrogating the higher order structures of snake venom proteins using an integrated mass spectrometric approach. J. Proteom. 2020, 216, 103680. [Google Scholar] [CrossRef]
- Mora-Obando, D.; Fernández, J.; Montecucco, C.; Gutiérrez, J.M.; Lomonte, B. Synergism between basic Asp49 and Lys49 phospholipase A2 myotoxins of viperid snake venom in vitro and in vivo. PLoS ONE 2014, 9, e109846. [Google Scholar] [CrossRef]
- Liu, C.C.; Wu, C.J.; Hsiao, Y.C.; Yang, Y.H.; Liu, K.L.; Huang, G.J.; Hsieh, C.H.; Chen, C.K.; Liaw, G.W. Snake venom proteome of Protobothrops mucrosquamatus in Taiwan: Delaying venom-induced lethality in a rodent model by inhibition of phospholipase A2 activity with varespladib. J. Proteom. 2021, 234, 104084. [Google Scholar] [CrossRef] [PubMed]
- Seo, T.; Sakon, T.; Nakazawa, S.; Nishioka, A.; Watanabe, K.; Matsumoto, K.; Akasaka, M.; Shioi, N.; Sawada, H.; Araki, S. Haemorrhagic snake venom metalloproteases and human ADAMs cleave LRP5/6, which disrupts cell–cell adhesions in vitro and induces haemorrhage in vivo. FEBS J. 2017, 284, 1657–1671. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Escalante, T.; Rucavado, A.; Herrera, C.; Fox, J.W. A comprehensive view of the structural and functional alterations of extracellular matrix by snake venom metalloproteinases (SVMPs): Novel perspectives on the pathophysiology of envenoming. Toxins 2016, 8, 304. [Google Scholar] [CrossRef]
- Arias, A.S.; Rucavado, A.; Gutiérrez, J.M. Peptidomimetic hydroxamate metalloproteinase inhibitors abrogate local and systemic toxicity induced by Echis ocellatus (saw-scaled) snake venom. Toxicon 2017, 132, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.M.T.; Maroun, R.C. Snake venom serine proteinases: Sequence homology vs. substrate specificity, a paradox to be solved. Toxicon 2005, 45, 1115–1132. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.J.; Rawlings, N.D. Families and clans of serine peptidases. Arch. Biochem. Biophys. 1995, 318, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Serrano, S.M.T. The long road of research on snake venom serine proteinases. Toxicon 2013, 62, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Masood, R.; Ali, I.; Ullah, K.; Ali, H.; Akbar, H.; Betzel, C. Thrombin-like enzymes from snake venom: Structural characterization and mechanism of action. Int. J. Biol. Macromol. 2018, 114, 788–811. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.V.; Grillo, G.; Fonseca, H.; Stanisic, D.; Tasic, L. Hesperetin as an inhibitor of the snake venom serine protease from Bothrops jararaca. Toxicon 2021, 198, 64–72. [Google Scholar] [CrossRef]
- Da Silva, G.M.; de Souza, D.H.B.; Waitman, K.B.; Ebram, M.C.; Fessel, M.R.; Zainescu, I.C.; Portaro, F.C.; Heras, M.; de Andrade, S.A. Design, synthesis, and evaluation of Bothrops venom serine protease peptidic inhibitors. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- Silva, G.M.; Berto, D.H.; Lima, C.A.; Waitman, K.B.; Lima, C.F.G.; Prezoto, B.C.; Vieira, M.L.; Rocha, M.M.T.; Gonçalves, L.R.C.; Andrade, S.A. Synergistic effect of serine protease inhibitors and a bothropic antivenom in reducing local hemorrhage and coagulopathy caused by Bothrops jararaca venom. Toxicon 2021, 199, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Hyodo, F.; Morita, T. Wide distribution of cysteine-rich secretory proteins in snake venoms: Isolation and cloning of novel snake venom cysteine-rich secretory proteins. Arch. Biochem. Biophys. 2003, 412, 133–141. [Google Scholar] [CrossRef]
- Kjeldsen, L.; Cowland, J.B.; Johnsen, A.H.; Borregaard, N. SGP28, a novel matrix glycoprotein in specific granules of human neutrophils with similarity to a human testis-specific gene product and to a rodent sperm-coating glycoprotein. FEBS Lett. 1996, 380, 246–250. [Google Scholar] [CrossRef]
- Koppers, A.J.; Reddy, T.; O’Bryan, M.K. The role of cysteine-rich secretory proteins in male fertility. Asian J. Androl. 2011, 13, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, T.; Modahl, C.; Maenaka, K.; Aoki-Shioi, N. Cysteine-rich secretory proteins (CRISPs) from venomous snakes: An overview of the functional diversity in a large and underappreciated superfamily. Toxins 2020, 12, 175. [Google Scholar] [CrossRef]
- Osipov, A.V.; Levashov, M.Y.; Tsetlin, V.I.; Utkin, Y.N. Cobra venom contains a pool of cysteine-rich secretory proteins. Biochem. Biophys. Res. Commun. 2005, 328, 177–182. [Google Scholar] [CrossRef]
- Lauridsen, L.P.; Laustsen, A.H.; Lomonte, B.; Gutiérrez, J.M. Exploring the venom of the forest cobra snake: Toxicovenomics and antivenom profiling of Naja melanoleuca. J. Proteom. 2017, 150, 98–108. [Google Scholar] [CrossRef]
- Nirthanan, S.; Gwee, M.C.E. Three-finger α-neurotoxins and the nicotinic acetylcholine receptor, forty years on. J. Pharmacol. Sci. 2004, 94, 1–17. [Google Scholar] [CrossRef]
- Wang, C.I.A.; Reeks, T.; Vetter, I.; Vergara, I.; Kovtun, O.; Lewis, R.J.; Alewood, P.F.; Durek, T. Isolation and structural and pharmacological characterization of α-elapitoxin-Dpp2d, an amidated three finger toxin from black mamba venom. Biochemistry 2014, 53, 3758–3766. [Google Scholar] [CrossRef]
- Pessatti, M.L.; Fontana, J.D.; Furtado, M.F.D.; Guimãraes, M.F.; Zanette, L.R.S.; Costa, W.T.; Baron, M. Screening of Bothrops snake venoms for L-amino acid oxidase activity. Appl. Biochem. Biotechnol. 1995, 55, 276. [Google Scholar] [CrossRef]
- Arlinghaus, F.T.; Eble, J.A. C-type lectin-like proteins from snake venoms. Toxicon 2012, 60, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M.; Evans, H.J. Structural domains in venom proteins: Evidence that metalloproteinases and nonenzymatic platelet aggregation inhibitors (disintegrins) from snake venoms are derived by proteolysis from a common precursor. Toxicon 1992, 30, 265–293. [Google Scholar] [CrossRef]
- Selistre-de-Araujo, H.S.; Pontes, C.L.S.; Montenegro, C.F.; Martin, A.C.B.M. Snake venom disintegrins and cell migration. Toxins 2010, 2, 2606–2621. [Google Scholar] [CrossRef] [PubMed]
- Župunski, V.; Kordiš, D.; Gubenšek, F. Adaptive evolution in the snake venom Kunitz/BPTI protein family. FEBS Lett. 2003, 547, 131–136. [Google Scholar] [CrossRef]
- Cardle, L.; Dufton, M.J. Foci of amino acid residue conservation in the 3D structures of the Kunitz BPTI proteinase inhibitors: How do variants from snake venom differ? Protein Eng. 1997, 10, 131–136. [Google Scholar] [CrossRef]
- Harvey, A.L. Twenty years of dendrotoxins. Toxicon 2001, 39, 15–26. [Google Scholar] [CrossRef]
- Ritonja, A.; Evans, H.J.; Machleidt, W.; Barrett, A.J. Amino acid sequence of a cystatin from venom of the African puff adder (Bitis arietans). Biochem. J. 1987, 246, 799–802. [Google Scholar] [CrossRef]
- Brillard-Bourdet, M.; Nguyên, V.; Ferrer-Di Martino, M.; Gauthier, F.; Moreau, T. Purification and characterization of a new cystatin inhibitor from Taiwan cobra (Naja naja atra) venom. Biochem. J. 1998, 331, 239–244. [Google Scholar] [CrossRef]
- Xie, Q.; Tang, N.; Wan, R.; Qi, Y.; Lin, X.; Lin, J. Recombinant snake venom cystatin inhibits the growth, invasion and metastasis of B16F10 cells and MHCC97H cells in vitro and in vivo. Toxicon 2011, 57, 704–711. [Google Scholar] [CrossRef]
- Tan, C.H. Snake Venomics: Fundamentals, Recent Updates, and a Look to the Next Decade. Toxins 2022, 14, 247. [Google Scholar] [CrossRef]
- Slagboom, J.; Kaal, C.; Arrahman, A.; Vonk, F.J.; Somsen, G.W.; Calvete, J.J.; Wüster, W.; Kool, J. Analytical strategies in venomics. Microchem. J. 2022, 175, 107187. [Google Scholar] [CrossRef]
- Li, L.; Huang, J.; Lin, Y. Snake venoms in cancer therapy: Past, present and future. Toxins 2018, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Tasoulis, T.; Pukala, T.L.; Isbister, G.K. Investigating toxin diversity and abundance in snake venom proteomes. Front. Pharmacol. 2022, 12, 768015. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.Y.; Wong, K.Y.; Tan, N.H.; Tan, C.H. Quantitative proteomics of Naja annulifera (sub-Saharan snouted cobra) venom and neutralization activities of two antivenoms in Africa. Int. J. Biol. Macromol. 2020, 158, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H.; Lomonte, B.; Lohse, B.; Fernández, J.; Gutiérrez, J.M. Unveiling the nature of black mamba (Dendroaspis polylepis) venom through venomics and antivenom immunoprofiling: Identification of key toxin targets for antivenom development. J. Proteom. 2015, 119, 126–142. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Calvete, J.J. Strategies in “snake venomics” aiming at an integrative view of compositional, functional, and immunological characteristics of venoms. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mouchbahani-Constance, S.; Sharif-Naeini, R. Proteomic and transcriptomic techniques to decipher the molecular evolution of venoms. Toxins 2021, 13, 154. [Google Scholar] [CrossRef]
- Tan, C.H.; Tan, K.Y.; Tan, N.H. A proein decomplexation strategy in snake venom proteomics. Funct. Proteom. Methods Protoc. 2019, 1871, 83–92. [Google Scholar]
- Wagstaff, S.C.; Sanz, L.; Juárez, P.; Harrison, R.A.; Calvete, J.J. Combined snake venomics and venom gland transcriptomic analysis of the ocellated carpet viper, Echis ocellatus. J. Proteom. 2009, 71, 609–623. [Google Scholar] [CrossRef]
- Calvete, J.J.; Escolano, J.; Sanz, L. Snake venomics of Bitis species reveals large intragenus venom toxin composition variation: Application to taxonomy of congeneric taxa. J. Proteome Res. 2007, 6, 2732–2745. [Google Scholar] [CrossRef]
- Calvete, J.J.; Marcinkiewicz, C.; Sanz, L. Snake venomics of Bitis gabonica gabonica. Protein family composition, subunit organization of venom toxins, and characterization of dimeric disintegrins bitisgabonin-1 and bitisgabonin-2. J. Proteome Res. 2007, 6, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Dingwoke, E.J.; Adamude, F.A.; Mohamed, G.; Klein, A.; Salihu, A.; Abubakar, M.S.; Sallau, A.B. Venom proteomic analysis of medically important Nigerian viper Echis ocellatus and Bitis arietans snake species. Biochem. Biophys. Rep. 2021, 28, 101164. [Google Scholar] [CrossRef]
- Juárez, P.; Wagstaff, S.C.; Oliver, J.; Sanz, L.; Harrison, R.A.; Calvete, J.J. Molecular cloning of disintegrin-like transcript BA-5A from a Bitis arietans venom gland cDNA library: A putative intermediate in the evolution of the long-chain disintegrin bitistatin. J. Mol. Evol. 2006, 63, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Ozverel, C.S.; Damm, M.; Hempel, B.F.; Göçmen, B.; Sroka, R.; Süssmuth, R.D.; Nalbantsoy, A. Investigating the cytotoxic effects of the venom proteome of two species of the Viperidae family (Cerastes cerastes and Cryptelytrops purpureomaculatus) from various habitats. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 220, 20–30. [Google Scholar] [CrossRef]
- Bazaa, A.; Marrakchi, N.; El Ayeb, M.; Sanz, L.; Calvete, J.J. Snake venomics: Comparative analysis of the venom proteomes of the Tunisian snakes Cerastes cerastes, Cerastes vipera and Macrovipera lebetina. Proteomics 2005, 5, 4223–4235. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, L.; Makran, B.; Pla, D.; Sanz, L.; Oukkache, N.; Lkhider, M.; Harrison, R.A.; Ghalim, N.; Calvete, J.J. Venomics and antivenomics profiles of North African Cerastes cerastes and C. vipera populations reveals a potentially important therapeutic weakness. J. Proteom. 2012, 75, 2442–2453. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, L.P.; Laustsen, A.H.; Lomonte, B.; Gutiérrez, J.M. Toxicovenomics and antivenom profiling of the Eastern green mamba snake (Dendroaspis angusticeps). J. Proteom. 2016, 136, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Petras, D.; Sanz, L.; Segura, Á.; Herrera, M.; Villalta, M.; Solano, D.; Vargas, M.; León, G.; Warrell, D.A.; Theakston, R.D.G.; et al. Snake venomics of African spitting cobras: Toxin composition and assessment of congeneric cross-reactivity of the Pan-African EchiTAb-Plus-ICP antivenom by antivenomics and neutralization approaches. J. Proteome Res. 2011, 10, 1266–1280. [Google Scholar] [CrossRef]
- Adamude, F.A.; Dingwoke, E.J.; Abubakar, M.S.; Ibrahim, S.; Mohamed, G.; Klein, A.; Sallau, A.B. Proteomic analysis of three medically important Nigerian Naja (Naja haje, Naja katiensis and Naja nigricollis) snake venoms. Toxicon 2021, 197, 24–32. [Google Scholar] [CrossRef]
- Sánchez, A.; Segura, Á.; Pla, D.; Munuera, J.; Villalta, M.; Quesada-Bernat, S.; Chavarría, D.; Herrera, M.; Gutiérrez, J.M.; León, G.; et al. Comparative venomics and preclinical efficacy evaluation of a monospecific Hemachatus antivenom towards sub-Saharan Africa cobra venoms. J. Proteom. 2021, 240, 104196. [Google Scholar] [CrossRef]
- Hus, K.K.; Buczkowicz, J.; Petrilla, V.; Petrillová, M.; Łyskowski, A.; Legáth, J.; Bocian, A. First look at the venom of Naja ashei. Molecules 2018, 23, 609. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.Y.; Tan, K.Y.; Tan, N.H.; Tan, C.H. A neurotoxic snake venom without phospholipase A2: Proteomics and cross-neutralization of the venom from Senegalese cobra, Naja senegalensis (Subgenus: Uraeus). Toxins 2021, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Herrera, M.; Villalta, M.; Solano, D.; Segura, Á.; Lomonte, B.; Gutiérrez, J.M.; León, G.; Vargas, M. Proteomic and toxinological characterization of the venom of the South African Ringhals cobra Hemachatus haemachatus. J. Proteom. 2018, 181, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, G.; Casewell, N.R.; Pla, D.; Quesada-Bernat, S.; Logan, R.A.E.; Bolton, F.M.S.; Wagstaff, S.C.; Gutiérrez, J.M.; Calvete, J.J.; Harrison, R.A. Defining the pathogenic threat of envenoming by South African shield-nosed and coral snakes (genus Aspidelaps), and revealing the likely efficacy of available antivenom. J. Proteom. 2019, 198, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, S.; Petras, D.; Engmark, M.; Süssmuth, R.D.; Whiteley, G.; Albulescu, L.O.; Kazandjian, T.D.; Wagstaff, S.C.; Rowley, P.; Wüster, W.; et al. The medical threat of mamba envenoming in sub-Saharan Africa revealed by genus-wide analysis of venom composition, toxicity and antivenomics profiling of available antivenoms. J. Proteom. 2018, 172, 173–189. [Google Scholar] [CrossRef]
- Williams, D.J.; Gutiérrez, J.M.; Calvete, J.J.; Wüster, W.; Ratanabanangkoon, K.; Paiva, O.; Brown, N.I.; Casewell, N.R.; Harrison, R.A.; Rowley, P.D.; et al. Ending the drought: New strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J. Proteom. 2011, 74, 1735–1767. [Google Scholar] [CrossRef]
- Habib, A.G. Public health aspects of snakebite care in West Africa: Perspectives from Nigeria. J. Venom. Anim. Toxins Incl. Trop. Dis. 2013, 19, 27. [Google Scholar] [CrossRef]
- WHO. WHO Expert Committee on Biological Standardization, Sixty-Seventh Report; World Health Organization: Geneva, Switzerland, 2017; WHO technical report series; no. 1004. [Google Scholar]
- Gutiérrez, J.M.; Vargas, M.; Segura, Á.; Herrera, M.; Villalta, M.; Solano, G.; Sánchez, A.; Herrera, C.; León, G. In vitro tests for assessing the neutralizing ability of snake antivenoms: Toward the 3Rs principles. Front. Immunol. 2021, 11, 617429. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; León, G.; Burnouf, T. Antivenoms for the treatment of snakebite envenomings: The road ahead. Biologicals 2011, 39, 129–142. [Google Scholar] [CrossRef]
- Guidolin, F.R.; Caricati, C.P.; Marcelino, J.R.; da Silva, W.D. Development of equine IgG antivenoms against major snake groups in Mozambique. PLoS Negl. Trop. Dis. 2016, 10, e0004325. [Google Scholar] [CrossRef]
- Eursakun, S.; Simsiriwong, P.; Ratanabanangkoon, K. Studies on the fractionation of equine antivenom IgG by combinations of ammonium sulfate and caprylic acid. Toxicon 2012, 60, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Simsiriwong, P.; Eursakun, S.; Ratanabanangkoon, K. A study on the use of caprylic acid and ammonium sulfate in combination for the fractionation of equine antivenom F(ab’)2. Biologicals 2012, 40, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Rojas, G.; Jiménez, J.; Gutiérrez, J. Caprylic acid fractionation of hyperimmune horse plasma: Description of a simple procedure for antivenom production. Toxicon 1994, 32, 351–363. [Google Scholar] [CrossRef]
- Moran, N.F.; Newman, W.J.; Theakston, R.D.G.; Warrell, D.A.; Wilkinson, D. High incidence of early anaphylactoid reaction to SAIMR polyvalent snake antivenom. Trans. R. Soc. Trop. Med. Hyg. 1998, 92, 69–70. [Google Scholar] [CrossRef]
- Fernandez, S.; Hodgson, W.; Chaisakul, J.; Kornhauser, R.; Konstantakopoulos, N.; Smith, A.I.; Kuruppu, S. In vitro toxic effects of puff adder (Bitis arietans) venom, and their neutralization by antivenom. Toxins 2014, 6, 1586–1597. [Google Scholar] [CrossRef]
- Segura, Á.; Villalta, M.; Herrera, M.; León, G.; Harrison, R.; Durfa, N.; Nasidi, A.; Calvete, J.J.; Theakston, R.D.G.; Warrell, D.A.; et al. Preclinical assessment of the efficacy of a new antivenom (EchiTAb-Plus-ICP®) for the treatment of viper envenoming in sub-Saharan Africa. Toxicon 2010, 55, 369–374. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Rojas, E.; Quesada, L.; León, G.; Núñez, J.; Laing, G.D.; Sasa, M.; Renjifo, J.M.; Nasidi, A.; Warrell, D.A.; et al. Pan-African polyspecific antivenom produced by caprylic acid purification of horse IgG: An alternative to the antivenom crisis in Africa. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 468–475. [Google Scholar] [CrossRef]
- Calvete, J.J.; Cid, P.; Sanz, L.; Segura, Á.; Villalta, M.; Herrera, M.; León, G.; Harrison, R.; Durfa, N.; Nasidi, A.; et al. Antivenomic assessment of the immunological reactivity of EchiTAb-Plus-ICP, an antivenom for the treatment of snakebite envenoming in sub-Saharan Africa. Am. J. Trop. Med. Hyg. 2010, 82, 1194–1201. [Google Scholar] [CrossRef]
- Sánchez, A.; Segura, Á.; Vargas, M.; Herrera, M.; Villalta, M.; Estrada, R.; Wu, F.; Litschka-Koen, T.; Perry, M.A.; Alape-Girón, A.; et al. Expanding the neutralization scope of the EchiTAb-plus-ICP antivenom to include venoms of elapids from Southern Africa. Toxicon 2017, 125, 59–64. [Google Scholar] [CrossRef]
- Djameh, G.I.; Nyarko, S.; Tetteh-Tsifoanya, M.; Marfo, F.M.; Adjei, S.; Blay, E.A.; Anang, A.K.; Ayi, I. Preclinical immuno-recognition and neutralization of lethality assessment of a new polyvalent antivenom, VINS snake venom antiserum—African IHS®, against envenomation of ten African viperid and elapid snakes. J. Sci. Res. Rep. 2021, 27, 25–43. [Google Scholar]
- Makran, B.; Fahmi, L.; Pla, D.; Sanz, L.; Oukkache, N.; Lkhider, M.; Ghalim, N.; Calvete, J.J. Snake venomics of Macrovipera mauritanica from Morocco, and assessment of the para-specific immunoreactivity of an experimental monospecific and a commercial antivenoms. J. Proteom. 2012, 75, 2431–2441. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, L.V.; Pla, D.; Herrera, M.; Chippaux, J.P.; Calvete, J.J.; Gutiérrez, J.M. Evaluation of the preclinical efficacy of four antivenoms, distributed in sub-Saharan Africa, to neutralize the venom of the carpet viper, Echis ocellatus, from Mali, Cameroon, and Nigeria. Toxicon 2015, 106, 97–107. [Google Scholar] [CrossRef]
- Potet, J.; Smith, J.; McIver, L. Reviewing evidence of the clinical effectiveness of commercially available antivenoms in sub-saharan Africa identifies the need for a multi-centre, multi-antivenom clinical trial. PLoS Negl. Trop. Dis. 2019, 13, e0007551. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Oluoch, G.O.; Ainsworth, S.; Alsolaiss, J.; Bolton, F.; Arias, A.S.; Gutiérrez, J.M.; Rowley, P.; Kalya, S.; Ozwara, H.; et al. Preclinical antivenom-efficacy testing reveals potentially disturbing deficiencies of snakebite treatment capability in East Africa. PLoS Negl. Trop. Dis. 2017, 11, e0005969. [Google Scholar] [CrossRef] [PubMed]
- Méndez, I.; Gutiérrez, J.M.; Angulo, Y.; Calvete, J.J.; Lomonte, B. Comparative study of the cytolytic activity of snake venoms from African spitting cobras (Naja spp., Elapidae) and its neutralization by a polyspecific antivenom. Toxicon 2011, 58, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Alangode, A.; Rajan, K.; Nair, B.G. Snake antivenom: Challenges and alternate approaches. Biochem. Pharmacol. 2020, 181, 114135. [Google Scholar] [CrossRef]
- Harrison, R.A. Development of venom toxin-specific antibodies by DNA immunisation: Rationale and strategies to improve therapy of viper envenoming. Vaccine 2004, 22, 1648–1655. [Google Scholar] [CrossRef]
- Wagstaff, S.C.; Laing, G.D.; Theakston, R.D.G.; Papaspyridis, C.; Harrison, R.A. Bioinformatics and multiepitope DNA immunization to design rational snake antivenom. PLoS Med. 2006, 3, 0832–0844. [Google Scholar] [CrossRef] [PubMed]
- Laustsen, A.H.; María Gutiérrez, J.; Knudsen, C.; Johansen, K.H.; Bermúdez-Méndez, E.; Cerni, F.A.; Jürgensen, J.A.; Ledsgaard, L.; Martos-Esteban, A.; Øhlenschlæger, M.; et al. Pros and cons of different therapeutic antibody formats for recombinant antivenom development. Toxicon 2018, 146, 151–175. [Google Scholar] [CrossRef]
- Lipps, B.V. Anti-lethal factor from opossum serum is a potent antidote for animal, plant and bacterial Toxins. J. Venom. Anim. Toxins 1999, 5, 56–66. [Google Scholar] [CrossRef]
- Komives, C.F.; Sanchez, E.E.; Rathore, A.S.; White, B.; Balderrama, M.; Suntravat, M.; Cifelli, A.; Joshi, V. Opossum peptide that can neutralize rattlesnake venom is expressed in Escherichia coli. Biotechnol. Prog. 2017, 33, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Albulescu, L.O.; Xie, C.; Ainsworth, S.; Alsolaiss, J.; Crittenden, E.; Dawson, C.A.; Softley, R.; Bartlett, K.E.; Harrison, R.A.; Kool, J.; et al. A therapeutic combination of two small molecule toxin inhibitors provides broad preclinical efficacy against viper snakebite. Nat. Commun. 2020, 11, 6094. [Google Scholar] [CrossRef] [PubMed]
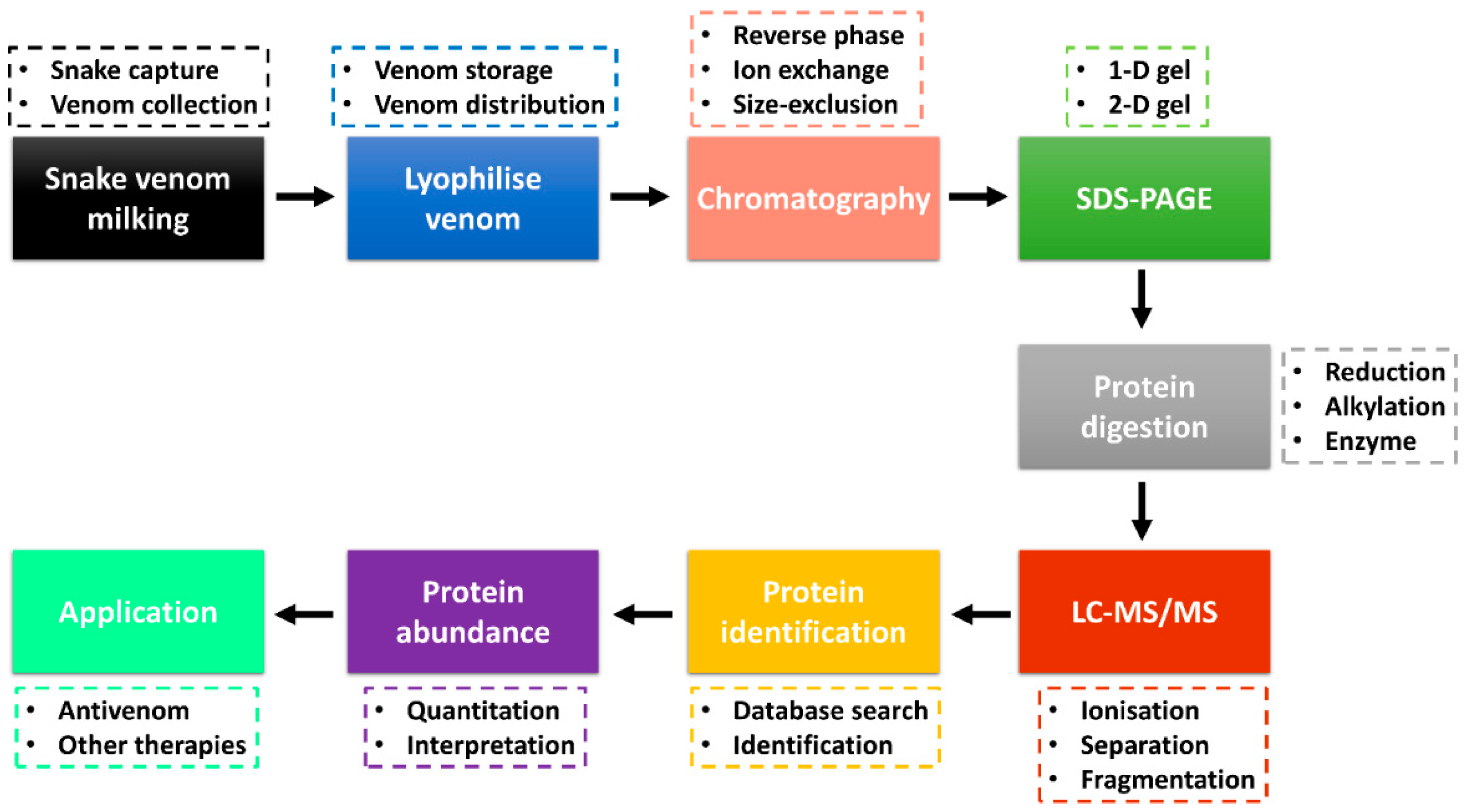

| Protein Family | MW (kDa) | Description | Mode of Action | Elapidae | Viperidae | Ref. |
|---|---|---|---|---|---|---|
| PLA2s | 13–15 | Lipolytic enzymes hydrolyse ester bonds at the sn-2 position of the glycerophospholipids to release lysophospholipids and fatty acids. | Cause neurotoxicity, cytotoxicity, myotoxicity, cardiotoxicity, oedema and anticoagulant effects | V | V | [18,23] |
| SVMPs | 20–100 | Zinc-dependent proteinases | Hydrolyse vital constituents of the capillary vessel of the basement membrane leading to the weakening of the microvessels and local tissue damage | V | V | [14,24,25,26] |
| SVSPs | 25–67 | Monomeric or multimeric glycoproteins | Haemotoxic enzymes that affect coagulation factors, fibrinolysis, plasminogen or platelets | ● | V | [14,27] |
| CRISP | 20–30 | Secreted proteins containing conserved cysteine mainly at the C-terminal | Has both smooth muscle contraction and cyclic nucleotide-gated ion channel-blocking activities | V | V | [28] |
| 3FTxs | 6–9 | Non-enzymatic secreted protein superfamily of small toxins | Inhibit neuromuscular transmission by targeting nicotinic acid and muscarinic acetylcholine receptors, ion channels and membranes | V | ● | [29,30,31] |
| CTL | 13–15 | Non-enzymatic protein with carbohydrate recognition domain | Prevents clotting and affects platelet activity | ● | V | [19,32] |
| DIS | 5–10 | Inhibitors of integrin | Alters activity of platelet and promotes haemorrhage | ● | V | [33,34] |
| KUN | 6–7 | Members of serine protease inhibitors | Causes neurotoxicity and disrupts haemostasis | V | V | [35] |
| LAAO | 50–70 | Converts L-amino acid into alpha-keto acid, release hydrogen peroxide and ammonia | Causes cell damage | V | V | [36] |
| CYS | 12–13 | Protease inhibitor family alters prey homeostasis | Affects blood coagulation and blood pressure | V | V | [37,38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Offor, B.C.; Muller, B.; Piater, L.A. A Review of the Proteomic Profiling of African Viperidae and Elapidae Snake Venoms and Their Antivenom Neutralisation. Toxins 2022, 14, 723. https://doi.org/10.3390/toxins14110723
Offor BC, Muller B, Piater LA. A Review of the Proteomic Profiling of African Viperidae and Elapidae Snake Venoms and Their Antivenom Neutralisation. Toxins. 2022; 14(11):723. https://doi.org/10.3390/toxins14110723
Chicago/Turabian StyleOffor, Benedict C., Beric Muller, and Lizelle A. Piater. 2022. "A Review of the Proteomic Profiling of African Viperidae and Elapidae Snake Venoms and Their Antivenom Neutralisation" Toxins 14, no. 11: 723. https://doi.org/10.3390/toxins14110723
APA StyleOffor, B. C., Muller, B., & Piater, L. A. (2022). A Review of the Proteomic Profiling of African Viperidae and Elapidae Snake Venoms and Their Antivenom Neutralisation. Toxins, 14(11), 723. https://doi.org/10.3390/toxins14110723





