Effect of an Eco-Friendly Cuminaldehyde Guanylhydrazone Disinfectant on Shiga Toxin Production and Global Transcription of Escherichia coli
Abstract
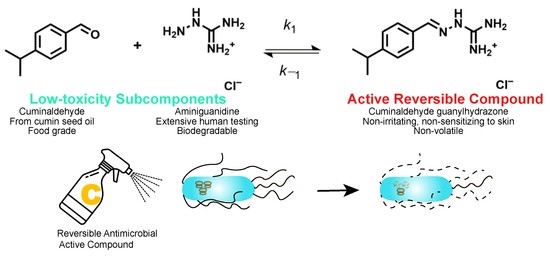
:1. Introduction
2. Results and Discussion
2.1. Susceptibility of E. coli Strains to the Guanylhydrazone and the Sublethal Dose for Treatment
2.2. Effect of the Guanylhydrazone on the Production of Stx1
2.3. Global Gene Expression Profiles of Two Strains Exposed to the Guanylhydrazone
2.4. Shared Gene Expression Patterns in Two E. coli Strains
2.5. Differences in Gene Expression between Two E. coli Strains
2.6. Expression of Resistance Genes and Virulence Genes in E. coli Challenged with the Guanylhydrazone
3. Conclusions
4. Materials and Methods
4.1. Cuminaldehyde Guanylhydrazone
4.2. Bacterial Strains and Growth Conditions
4.3. ELISA for Stx Production
4.4. Statistical Analysis
4.5. RNA Isolation and RNA Sequencing
4.6. Transcriptomic Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, C.S.; Jelacic, S.; Habeeb, R.L.; Watkins, S.L.; Tarr, P.I. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli o157:H7 infections. N. Engl. J. Med. 2000, 342, 1930–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mir, R.A.; Kudva, I.T. Antibiotic-resistant Shiga toxin-producing Escherichia coli: An overview of prevalence and intervention strategies. Zoonoses Public Health 2019, 66, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merchel Piovesan Pereira, B.; Tagkopoulos, I. Benzalkonium chlorides: Uses, regulatory status, and microbial resistance. Appl. Environ. Microbiol. 2019, 85, e00377-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.H.; Lai, Y.S.; Zheng, C.; Ilhan, Z.E.; Ontiveros-Valencia, A.; Long, X.; Krajmalnik-Brown, R.; Rittmann, B.E. Increased expression of antibiotic-resistance genes in biofilm communities upon exposure to cetyltrimethylammonium bromide (ctab) and other stress conditions. Sci. Total Environ. 2021, 765, 144264. [Google Scholar] [CrossRef]
- Hart-Cooper, W.M.; Orts, W.J.; Johnson, K.; Lynn, L.E.; Franqui-Villanueva, D.M. Self-Assembled Active Agents. U.S. Patent 11,166,464 B2 9 November 2021. [Google Scholar]
- Dirksen, A.; Dawson, P.E. Rapid oxime and hydrazone ligations with aromatic aldehydes for biomolecular labeling. Bioconjug. Chem. 2008, 19, 2543–2548. [Google Scholar] [CrossRef] [Green Version]
- Kolmel, D.K.; Kool, E.T. Oximes and hydrazones in bioconjugation: Mechanism and catalysis. Chem. Rev. 2017, 117, 10358–10376. [Google Scholar] [CrossRef]
- Dirksen, A.; Dirksen, S.; Hackeng, T.M.; Dawson, P.E. Nucleophilic catalysis of hydrazone formation and transimination: Implications for dynamic covalent chemistry. J. Am. Chem. Soc. 2006, 128, 15602–15603. [Google Scholar] [CrossRef]
- Kakoullis, L.; Papachristodoulou, E.; Chra, P.; Panos, G. Shiga toxin-induced haemolytic uraemic syndrome and the role of antibiotics: A global overview. J. Infect. 2019, 79, 75–94. [Google Scholar] [CrossRef]
- Alekshun, M.N.; Levy, S.B. Molecular mechanisms of antibacterial multidrug resistance. Cell 2007, 128, 1037–1050. [Google Scholar] [CrossRef] [Green Version]
- Lazic, J.; Ajdacic, V.; Vojnovic, S.; Zlatovic, M.; Pekmezovic, M.; Mogavero, S.; Opsenica, I.; Nikodinovic-Runic, J. Bis-guanylhydrazones as efficient anti-candida compounds through DNA interaction. Appl. Microbiol. Biotechnol 2018, 102, 1889–1901. [Google Scholar] [CrossRef]
- Brandi, L.; Fabbretti, A.; La Teana, A.; Abbondi, M.; Losi, D.; Donadio, S.; Gualerzi, C.O. Specific, efficient, and selective inhibition of prokaryotic translation initiation by a novel peptide antibiotic. Proc. Natl. Acad. Sci. USA 2006, 103, 39–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenner, L.; Starosta, A.L.; Terry, D.S.; Mikolajka, A.; Filonava, L.; Yusupov, M.; Blanchard, S.C.; Wilson, D.N.; Yusupova, G. Structural basis for potent inhibitory activity of the antibiotic tigecycline during protein synthesis. Proc. Natl. Acad. Sci. USA 2013, 110, 3812–3816. [Google Scholar] [CrossRef] [Green Version]
- Marks, J.; Kannan, K.; Roncase, E.J.; Klepacki, D.; Kefi, A.; Orelle, C.; Vazquez-Laslop, N.; Mankin, A.S. Context-specific inhibition of translation by ribosomal antibiotics targeting the peptidyl transferase center. Proc. Natl. Acad. Sci. USA 2016, 113, 12150–12155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, T. Antibiotics that affect the ribosome. Rev. Sci. Tech. 2012, 31, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pruss, B.M.; Besemann, C.; Denton, A.; Wolfe, A.J. A complex transcription network controls the early stages of biofilm development by Escherichia coli. J. Bacteriol. 2006, 188, 3731–3739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, Q.; Zhou, M.; Zhu, L.; Zhu, G. Flagella and bacterial pathogenicity. J. Basic Microbiol. 2013, 53, 1–8. [Google Scholar] [CrossRef]
- Haiko, J.; Westerlund-Wikstrom, B. The role of the bacterial flagellum in adhesion and virulence. Biology 2013, 2, 1242–1267. [Google Scholar] [CrossRef] [Green Version]
- Giacomucci, S.; Cros, C.D.; Perron, X.; Mathieu-Denoncourt, A.; Duperthuy, M. Flagella-dependent inhibition of biofilm formation by sub-inhibitory concentration of polymyxin b in Vibrio cholerae. PLoS ONE 2019, 14, e0221431. [Google Scholar] [CrossRef] [Green Version]
- Pittman, M.S.; Goodwin, M.; Kelly, D.J. Chemotaxis in the human gastric pathogen Helicobacter pylori: Different roles for chew and the three chev paralogues, and evidence for chev2 phosphorylation. Microbiology (Reading) 2001, 147, 2493–2504. [Google Scholar] [CrossRef] [Green Version]
- O’Toole, R.; Lundberg, S.; Fredriksson, S.A.; Jansson, A.; Nilsson, B.; Wolf-Watz, H. The chemotactic response of Vibrio anguillarum to fish intestinal mucus is mediated by a combination of multiple mucus components. J. Bacteriol. 1999, 181, 4308–4317. [Google Scholar] [CrossRef]
- Heinzl, G.A.; Huang, W.; Yu, W.; Giardina, B.J.; Zhou, Y.; MacKerell, A.D., Jr.; Wilks, A.; Xue, F. Iminoguanidines as allosteric inhibitors of the iron-regulated heme oxygenase (hemo) of Pseudomonas aeruginosa. J. Med. Chem. 2016, 59, 6929–6942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Kong, Q.; Patfield, S.; Skinner, C.; Rasooly, R. A new immunoassay for detecting all subtypes of Shiga toxins produced by Shiga toxin-producing E. coli in ground beef. PLoS ONE 2016, 11, e0148092. [Google Scholar] [CrossRef] [PubMed]
- Skinner, C.; Patfield, S.; Stanker, L.H.; Fratamico, P.; He, X. New high-affinity monoclonal antibodies against Shiga toxin 1 facilitate the detection of hybrid Stx1/Stx2 in vivo. PLoS ONE 2014, 9, e99854. [Google Scholar] [CrossRef] [PubMed]



| Strain | Serotype | stx1 | Origin |
|---|---|---|---|
| RM2367 | O157:H7 | + | Human |
| RM6649 | O157:H7 | + | Human |
| RM7370 | O111 | + | Water |
| RM7375 | O26 | + | Human |
| RM7543 | O157:H7 | + | Human |
| RM7927 | O26 | + | Water |
| RM7958 | O113 | + | Cow feces |
| RM8082 | O121 | + | Cow feces |
| RM8385 | O103 | + | Cow feces |
| RM8426 | O26 | + | Water |
| RM8876 | O145 | + | Water |
| RM9306 | O145 | + | Cow feces |
| RM9322 | O111 | + | Water |
| RM9413 | O45 | + | Cow feces |
| RM9882 | O103 | + | Cow feces |
| RM9907 | O111 | + | Feral pig |
| RM9917 | O145 | + | Feral pig |
| RM9975 | O111 | + | Crow |
| RM10061 | O103 | + | Feral pig |
| RM10408 | O103 | + | Crow |
| RM10817 | O26 | + | Cow feces |
| RM12788 | O111 | + | Human |
| RM13506 | O45 | + | Human |
| RM13508 | O103 | + | Human |
| RM13752 | O45 | + | Cow feces |
| ATCC25922 | O6 | - | ATCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Hart-Cooper, W.M.; Rasooly, R.; Carter, M.Q.; Orts, W.J.; Gu, Y.; He, X. Effect of an Eco-Friendly Cuminaldehyde Guanylhydrazone Disinfectant on Shiga Toxin Production and Global Transcription of Escherichia coli. Toxins 2022, 14, 752. https://doi.org/10.3390/toxins14110752
Wang Y, Hart-Cooper WM, Rasooly R, Carter MQ, Orts WJ, Gu Y, He X. Effect of an Eco-Friendly Cuminaldehyde Guanylhydrazone Disinfectant on Shiga Toxin Production and Global Transcription of Escherichia coli. Toxins. 2022; 14(11):752. https://doi.org/10.3390/toxins14110752
Chicago/Turabian StyleWang, Yan, William M. Hart-Cooper, Reuven Rasooly, Michelle Qiu Carter, William J. Orts, Yongqiang Gu, and Xiaohua He. 2022. "Effect of an Eco-Friendly Cuminaldehyde Guanylhydrazone Disinfectant on Shiga Toxin Production and Global Transcription of Escherichia coli" Toxins 14, no. 11: 752. https://doi.org/10.3390/toxins14110752
APA StyleWang, Y., Hart-Cooper, W. M., Rasooly, R., Carter, M. Q., Orts, W. J., Gu, Y., & He, X. (2022). Effect of an Eco-Friendly Cuminaldehyde Guanylhydrazone Disinfectant on Shiga Toxin Production and Global Transcription of Escherichia coli. Toxins, 14(11), 752. https://doi.org/10.3390/toxins14110752