Evaluation of Zearalenones and Their Metabolites in Chicken, Pig and Lamb Liver Samples
Abstract
:1. Introduction
2. Results and Discussion
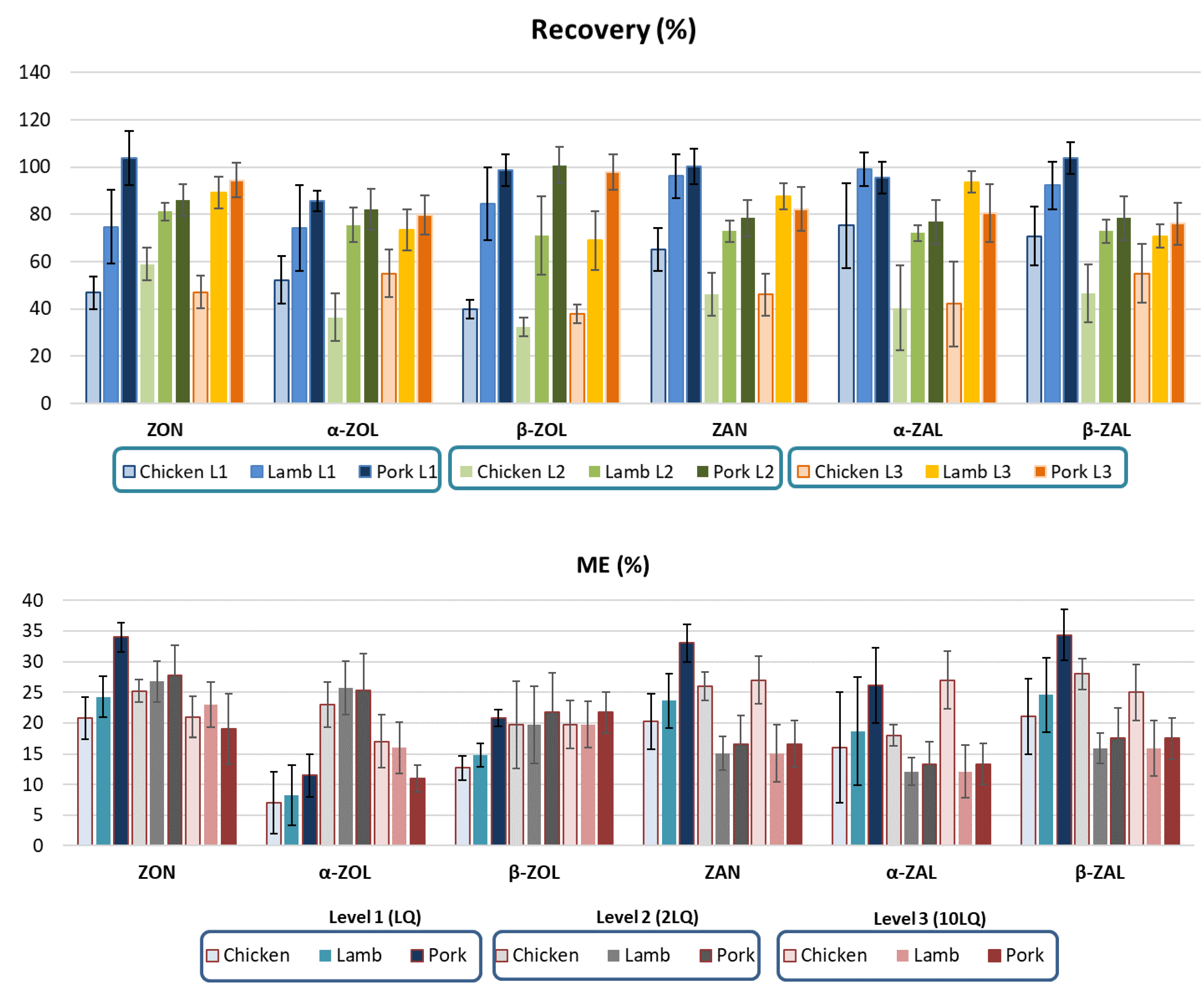
2.1. Validation of the Mycotoxin Determination in Animal Liver
2.2. Presence of Zearalenone (ZON), Zearalanone (ZAN) and Their Metabolites in Liver Samples
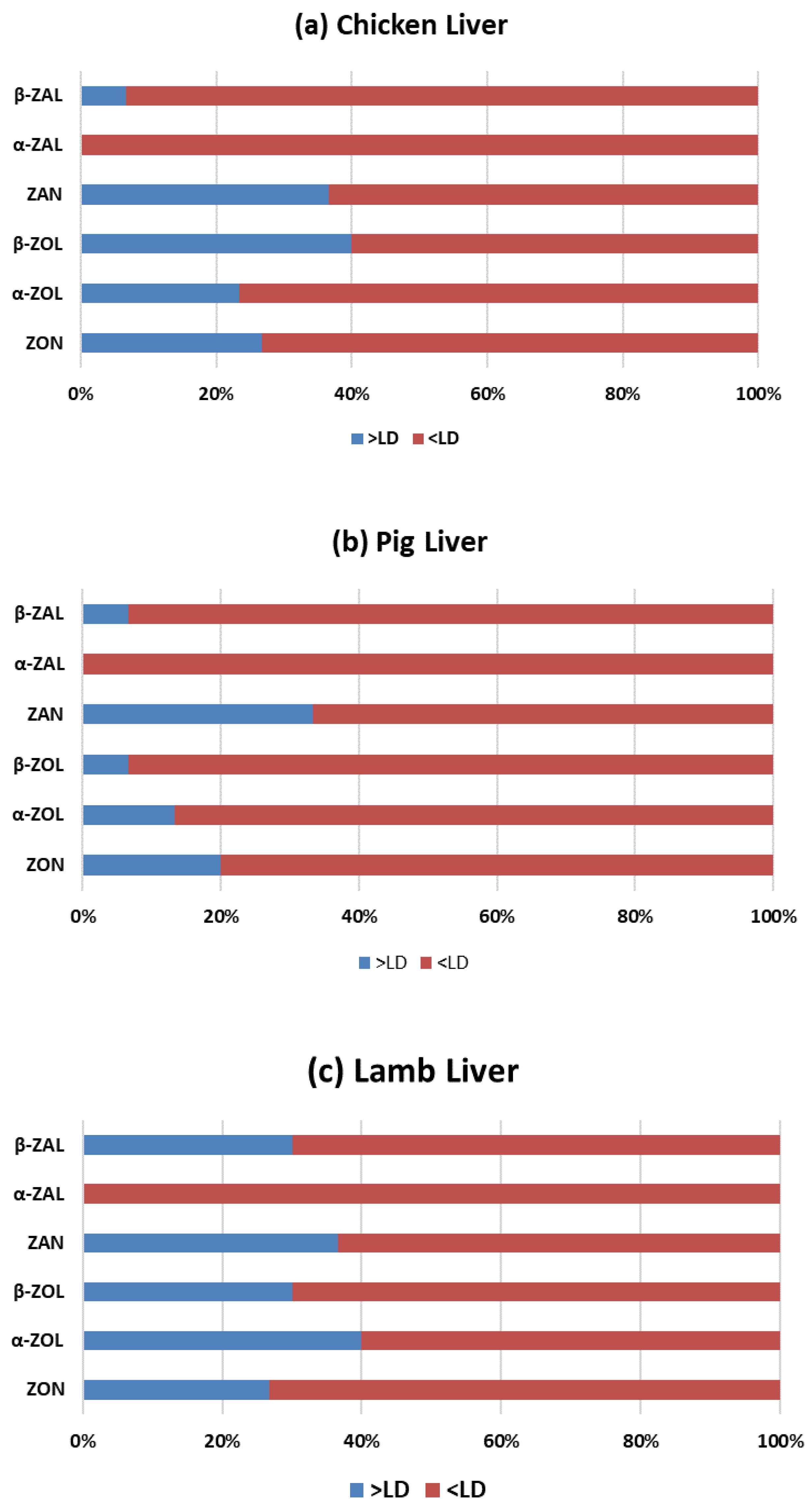
2.2.1. Zearalenone (ZON) and Its Metabolites Occurrence
2.2.2. Zearalanone (ZAN) and Its Metabolites Occurrence
2.2.3. Simultaneous Presence of Analyzed Mycotoxins and Metabolites
3. Conclusions
4. Material and Methods
4.1. Standards
4.2. Chemical, Reagents and Other Material
4.3. Apparatus
4.4. Sample Preparation
4.5. Sampling
4.6. Method Validation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ropejko, K.; Twarużek, M. Zearalenone and Its Metabolites-General Overview, Occurrence, and Toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef] [PubMed]
- Gil-Serna, J.; Vázquez, C.; González-Jaén, M.T.; Patiño, B. Mycotoxins. Toxicology. In Encyclopedia of Food Microbiology; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 887–892. [Google Scholar]
- Omar, S.S. Prevalence, level and health risk assessment of mycotoxins in the fried poultry eggs from Jordan. Environ. Res. 2021, 200, 111701. [Google Scholar] [CrossRef] [PubMed]
- Ochieng, P.E.; Scippo, M.L.; Kemboi, D.C.; Croubels, S.; Okoth, S.; Kang’ethe, E.K.; Doupovec, B.; Gathumbi, J.K.; Lindahl, J.F.; Antonissen, G. Mycotoxins in Poultry Feed and Feed Ingredients from Sub-Saharan Africa and Their Impact on the Production of Broiler and Layer Chickens: A Review. Toxins 2021, 13, 633. [Google Scholar] [CrossRef]
- Zinedine, A.; Brera, C.; Elakhdari, S.; Catano, C.; Debegnach, F.; Angelini, S.; de Santis, B.; Faid, M.; Benlemlih, M.; Minardi, V.; et al. Natural occurrence of mycotoxins in cereals and spices commercialized in Morocco. Food Control 2006, 17, 868–874. [Google Scholar] [CrossRef]
- Schollenberger, M.; Müller, H.-M.; Rüfle, M.; Suchy, S.; Plank, S.; Drochner, W. Natural Occurrence of 16 Fusarium Toxins in Grains and Feedstuffs of Plant Origin from Germany. Mycopathologia 2006, 161, 43–52. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Rabbani, T.; Asi, M.R.; Jinap, S. Assessment of aflatoxins, ochratoxin A and zearalenone in breakfast cereals. Food Chem. 2014, 157, 257–262. [Google Scholar] [CrossRef]
- European Commission. Regulation of the European Commission (EC) No. 1881/2006 of December 19, 2006, as Amended Fixing Maximum Levels for Certain Contaminants in Foodstuffs (OJ. L. 364/5 of 20.12.2006, Annex “Maximum Levels for Certain Contaminants in foodstuffs”. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- Lu, Q.; Luo, J.Y.; Ruan, H.N.; Wang, C.J.; Yang, M.H. Structure-toxicity relationships, toxicity mechanisms and health risk assessment of food-borne modified deoxynivalenol and zearalenone: A comprehensive review. Sci. Total Environ. 2022, 806, 151192. [Google Scholar] [CrossRef]
- Kinkade, C.W.; Rivera-Núñez, Z.; Gorcyzca, L.; Aleksunes, L.M.; Barrett, E.S. Impact of Fusarium-Derived Mycoestrogens on Female Reproduction: A Systematic Review. Toxins 2021, 13, 373. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, M.Y.; Zhao, A.H.; Kong, L.; Wang, J.J.; Shen, W.; Li, L. Single-cell transcriptomic profiling provides insights into the toxic effects of Zearalenone exposure on primordial follicle assembly. Theranostics 2021, 11, 5197–5213. [Google Scholar] [CrossRef] [PubMed]
- Dolenšek, T.; Švara, T.; Knific, T.; Gombač, M.; Luzar, B.; Jakovac-Strajn, B. The Influence of Fusarium Mycotoxins on the Liver of Gilts and Their Suckling Piglets. Animals 2021, 11, 2534. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives. Safety Evaluation of Certain Food Additives and Contaminants: Prepared by the Seventy-Third Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Risks for animal health related to the presence of zearalenone and its modified forms in feed. EFSA J. 2017, 15, 04851. [Google Scholar]
- Carballo, D.; Pallarés, N.; Ferrer, E.; Barba, F.J.; Berrada, H. Assessment of Human Exposure to Deoxynivalenol, Ochratoxin A, Zearalenone and Their Metabolites Biomarker in Urine Samples Using LC-ESI-qTOF. Toxins 2021, 13, 530. [Google Scholar] [CrossRef]
- Schmidt, J.; Cramer, B.; Turner, P.C.; Stoltzfus, R.J.; Humphrey, J.H.; Smith, L.E.; Humpf, H.U. Determination of Urinary Mycotoxin Biomarkers Using a Sensitive Online Solid Phase Extraction-UHPLC-MS/MS Method. Toxins 2021, 13, 418. [Google Scholar] [CrossRef]
- Foerster, C.; Ríos-Gajardo, G.; Gómez, P.; Muñoz, K.; Cortés, S.; Maldonado, C.; Ferreccio, C. Assessment of Mycotoxin Exposure in a Rural County of Chile by Urinary Biomarker Determination. Toxins 2021, 13, 439. [Google Scholar] [CrossRef]
- Pack, E.; Stewart, J.; Rhoads, M.; Knight, J.; de Vita, R.; Clark-Deener, S. Quantification of zearalenone and α-zearalenol in swine liver and reproductive tissues using GC-MS. Toxicon X 2020, 8, 100058. [Google Scholar] [CrossRef]
- Kwaśniewska, K.; Gadzała-Kopciuch, R.; Cendrowski, K. Analytical procedure for the determination of zearalenone in environmental and biological samples. Crit. Rev. Anal. Chem. 2015, 45, 119–130. [Google Scholar] [CrossRef]
- Singh, J.; Mehta, A. Rapid and sensitive detection of mycotoxins by advanced and emerging analytical methods: A review. Food Sci. Nutr. 2020, 8, 2183–2204. [Google Scholar] [CrossRef] [Green Version]
- Kinani, S.; Bouchonnet, S.; Bourcier, S.; Porcher, J.-M.; Aït-Aïssa, S. Study of the chemical derivatization of zearalenone and its metabolites for gas chromatography–mass spectrometry analysis of environmental samples. J. Chromatogr. A 2008, 1190, 307–315. [Google Scholar] [CrossRef]
- Niknejad, F.; Escrivá, L.; Adel Rad, K.B.; Khoshnia, M.; Barba, F.J.; Berrada, H. Biomonitoring of Multiple Mycotoxins in Urine by GC-MS/MS: A Pilot Study on Patients with Esophageal Cancer in Golestan Province, Northeastern Iran. Toxins 2021, 13, 243. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Ortega, N.; Caballero-Gallardo, K.; Taboada-Alquerque, M.; Franco, J.; Stashenko, E.E.; Juan, C.; Juan-García, A.; Olivero-Verbel, J. Protective Effects of the Hydroethanolic Extract of Fridericia chica on Undifferentiated Human Neuroblastoma Cells Exposed to α-Zearalenol (α-ZEL) and β-Zearalenol (β-ZEL). Toxins 2021, 13, 748. [Google Scholar] [CrossRef]
- Pleadin, J.; Mihaljević, Ž.; Barbir, T.; Vulić, A.; Kmetič, I.; Zadravec, M.; Brumen, V.; Mitak, M. Natural incidence of zearalenone in Croatian pig feed, urine and meat in 2014. Food Addit. Contam. Part B 2015, 80, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Gambacorta, L.; Olsen, M.; Solfrizzo, M. Pig Urinary Concentration of Mycotoxins and Metabolites Reflects Regional Differences, Mycotoxin Intake and Feed Contaminations. Toxins 2019, 11, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoev, S.D.; Dutton, M.F.; Njobeh, P.B.; Mosonik, J.S.; Steenkamp, P.A. Mycotoxic nephropathy in Bulgarian pigs and chickens: Complex aetiology and similarity to Balkan Endemic Nephropathy. Food Addit. Contam. Part A 2010, 27, 72–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtui, V.G.; Gareis, M.; Usleber, E.; Märtlbauer, E. Survey of Romanian slaughtered pigs for the occurrence of mycotoxins ochratoxins A and B, and zearalenone. Food Addit. Contam. 2001, 18, 730–738. [Google Scholar] [CrossRef]
- Tanaka, T.; Yoneda, A.; Inoue, S.; Sugiura, Y.; Ueno, Y. Simultaneous determination of trichothecene mycotoxins and zearalenone in cereals by gas chromatography–mass spectrometry. J. Chromatogr. A. 2000, 882, 23–28. [Google Scholar] [CrossRef]
- EC (European Commission). Commission Decision 2002/657/EC of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Communities 2002, 50, 8–36. [Google Scholar]
- Zöllner, P.; Jodlbauer, J.; Kleinova, M.; Kahlbacher, H.; Kuhn, T.; Hochsteiner, W.; Lindner, W. Concentration Levels of Zearalenone and Its Metabolites in Urine, Muscle Tissue, and Liver Samples of Pigs Fed with Mycotoxin-Contaminated Oats. J. Agric. Food Chem. 2002, 50, 2494–2501. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011, 9, 2197. [Google Scholar] [CrossRef]
- Gajęcka, M.; Sławuta, P.; Nicpoń, J.; Kołacz, R.; Kiełbowicz, Z.; Zielonka, Ł.; Dąbrowski, M.; Szweda, W.; Gajęcki, M.; Nicpoń, J. Zearalenone and its metabolites in the tissues of female wild boars exposed per os to mycotoxins. Toxicon 2016, 114, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mally, A.; Solfrizzo, M.; Degen, G.H. Biomonitoring of the mycotoxin Zearalenone: Current state-of-the art and application to human exposure assessment. Arch. Toxicol. 2016, 90, 1281–1292. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Nisar, S.; Así, M.R.; Jinap, S. Natural incidence of aflatoxins, ochratoxin A and zearalenone in chicken meat and eggs. Food Control 2014, 43, 98–103. [Google Scholar] [CrossRef]
- Meyer, K.; Usleber, E.; Märtlbauer, E.; Bauer, J. Occurrence of zearalenone, alpha- and beta-zearalenol in bile of breeding sows in relation to reproductive performance. Berl. Munch. Tierarztl. Wochenschr. 2000, 113, 374–379. [Google Scholar] [PubMed]
- Malekinejad, H.; Maas-Bakker, R.; Fink-Gremmels, J. Species differences in the hepatic biotransformation of zearalenone. Vet. J. 2006, 172, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Zinedine, A.; Soriano, J.M.; Molto, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- Mirocha, C.J.; Schauerhamer, B.; Christensen, C.M.; Niku-Paavola, M.L.; Nummi, M. Incidence of zearalenol (Fusarium mycotoxin) in animal feed. Appl. Environ. Microbiol. 1979, 38, 749–750. [Google Scholar] [CrossRef] [Green Version]
- Döll, S.; Dänicke, S.; Ueberschär, K.H.; Valenta, H.; Schnurrbusch, U.; Ganter, M.; Klobasa, F.; Flachowsky, G. Effects of graded levels of Fusarium toxin contaminated maize in diets for female weaned piglets. Arch. Tierernährung 2003, 57, 311–334. [Google Scholar]
- Biehl, M.L.; Prelusky, D.B.; Koritz, G.D.; Hartin, K.E.; Buck, W.B.; Trenholm, H.L. Biliary excretion and enterohepatic cycling of zearalenone in immature pigs. Toxicol. Appl. Pharmacol. 1993, 121, 152–159. [Google Scholar] [CrossRef]
- Jodlbauer, J.; Zöllner, P.; Lindner, W. Determination of zeranol, taleranol, zearalenone, alpha- and beta-zearalenol in urine and tissue by high-performance liquid chromatography-tandem mass spectrometry. Chromatographia 2000, 51, 681–687. [Google Scholar] [CrossRef]
- Miles, C.O.; Erasmuson, A.F.; Wilkins, A.L.; Towers, N.R.; Smith, B.L.; Garthwaite, I.; Scahill, B.G.; Hansen, R.P. Ovine metabolism of zearalenone to alpha-zearalanol (zeranol). J. Agric. Food Chem. 1996, 44, 3244–3250. [Google Scholar] [CrossRef]
- Juan, C.; Oueslati, S.; Mañes, J.; Berrada, H. Multimycotoxin Determination in Tunisian Farm Animal Feed. J. Food Sci. 2019, 84, 3885–3893. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.M.T.; Solfrizzo, M.; de Girolamo, A.; Chulze, S.N.; Torres, A.M.; Visconti, A. LC–MS/MS characterization of the urinary excretion profile of the mycotoxin deoxynivalenol in human and rat. J. Chromatogr. B 2011, 879, 707–715. [Google Scholar] [CrossRef]
- Mahmoud, A.F.; Escrivá, L.; Rodríguez-Carrasco, Y.; Moltó, J.C.; Berrada, H. Determination of trichothecenes in chicken liver using gas chromatography coupled with triple-quadrupole mass spectrometry. LWT-Food Sci. Technol. 2018, 93, 237–242. [Google Scholar] [CrossRef]

| Mycotoxin * | Rt (min) | Quantification Transition (m/z) | Confirmation Transition (m/z) | LD (ng/g) | LQ (ng/g) |
|---|---|---|---|---|---|
| ZON | 16.77 | 462 > 151 | 462 > 151 | 0.061 | 1.25 |
| α-ZOL | 16.74 | 305 > 289 | 305 > 289 | 0.031 | 0.31 |
| β-ZOL | 15.83 | 536 > 446 | 536 > 446 | 0.118 | 0.63 |
| ZAN | 15.84 | 449 > 335 | 449 > 335 | 0.017 | 0.31 |
| α-ZAL | 15.84 | 433 > 309 | 433 > 309 | 0.239 | 2.50 |
| β-ZAL | 15.89 | 307 > 292 | 307 > 292 | 0.361 | 1.25 |
| Chicken Liver (n = 31) | Pig Liver (n = 30) | Lamb Liver (n = 30) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyte * | I | M ± SD (ng/g) | Mp ± SD (ng/g) | Range (ng/g) | I | M ± SD (ng/g) | Mp ± SD (ng/g) | Range (ng/g) | I | M ± SD (ng/g) | Mp ± SD (ng/g) | Range (ng/g) |
| ZON | 9 | 1.95 ± 5.96 | 8.94 ± 13.15 | 0.09–30.79 | 6 | 0.02 ± 0.04 | 0.13 ± 0.02 | 0.09–0.13 | 8 | 0.82 ±1.51 | 4.08 ± 1.70 | 1.94–5.91 |
| α-ZOL | 8 | 1.44 ± 4.21 | 7.46 ± 9.37 | 0.11–21.50 | 4 | 0.05 ± 0.16 | 0.48 ± 0.43 | 0.11–0.83 | 12 | 6.19 ± 9.16 | 20.64 ± 10.68 | 6.62–23.81 |
| β-ZOL | 13 | 7.15 ± 27.46 | 22.73 ± 55.01 | 0.25–152.62 | 2 | 0.02 ± 0.10 | 0.49 ± 0.11 | 0.31–0.43 | 9 | 0.58 ± 1.47 | 2.60 ± 2.94 | 0.19–4.97 |
| ZAN | 12 | 2.85 ± 7.92 | 9.83 ± 15.48 | 0.78–43.33 | 10 | 0.29 ± 0.43 | 1.17 ± 0.26 | 0.75–1.36 | 11 | 1.03 ± 1.49 | 3.75 ± 1.31 | 1.50–4.94 |
| β-ZAL | 3 | 1.49 ± 6.21 | 20.54 ± 21.38 | 6.01–33.92 | 2 | 0.04 ± 0.19 | 0.87 ± 0.66 | 0.30–1.00 | 9 | 5.61 ± 8.96 | 24.92 ± 5.33 | 15.33–24.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llorens Castelló, P.; Sacco, M.A.; Aquila, I.; Moltó Cortés, J.C.; Juan García, C. Evaluation of Zearalenones and Their Metabolites in Chicken, Pig and Lamb Liver Samples. Toxins 2022, 14, 782. https://doi.org/10.3390/toxins14110782
Llorens Castelló P, Sacco MA, Aquila I, Moltó Cortés JC, Juan García C. Evaluation of Zearalenones and Their Metabolites in Chicken, Pig and Lamb Liver Samples. Toxins. 2022; 14(11):782. https://doi.org/10.3390/toxins14110782
Chicago/Turabian StyleLlorens Castelló, Paula, Matteo Antonio Sacco, Isabella Aquila, Juan Carlos Moltó Cortés, and Cristina Juan García. 2022. "Evaluation of Zearalenones and Their Metabolites in Chicken, Pig and Lamb Liver Samples" Toxins 14, no. 11: 782. https://doi.org/10.3390/toxins14110782
APA StyleLlorens Castelló, P., Sacco, M. A., Aquila, I., Moltó Cortés, J. C., & Juan García, C. (2022). Evaluation of Zearalenones and Their Metabolites in Chicken, Pig and Lamb Liver Samples. Toxins, 14(11), 782. https://doi.org/10.3390/toxins14110782








