The Influence of Micronutrient Trace Metals on Microcystis aeruginosa Growth and Toxin Production
Abstract
:1. Introduction
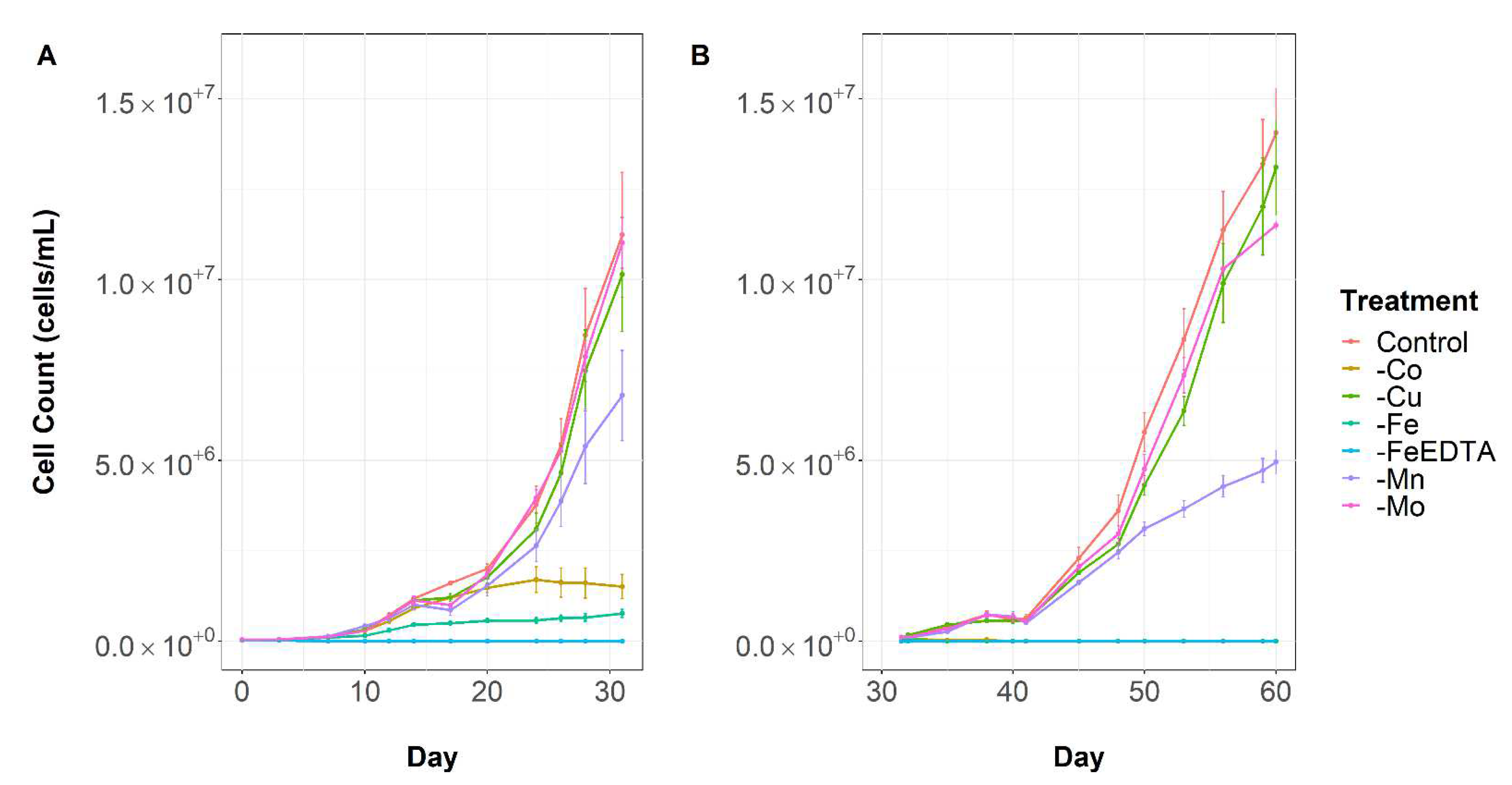
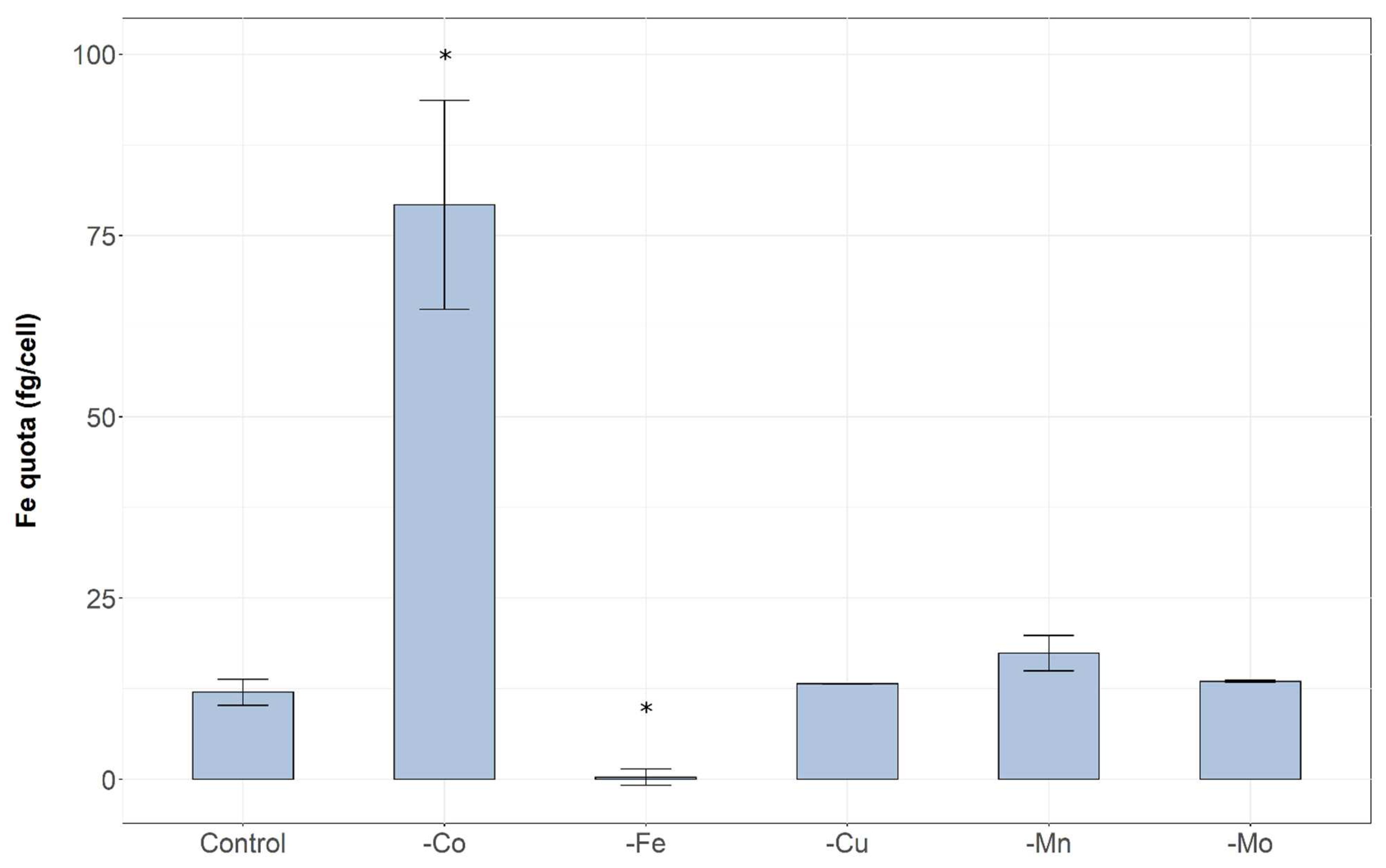
2. Results
3. Discussion
4. Materials and Methods
4.1. Microcystis Culturing Conditions
4.2. Culture Media
- (a)
- Control—filter sterilised MLA medium
- (b)
- MLA media without CoCl2·6H2O
- (c)
- MLA media without CuSO4·5H2O
- (d)
- MLA media without FeCl3·6H2O
- (e)
- MLA media without FeCl3·6H2O and Na2EDTA·2H2O
- (f)
- MLA media without MnCl2·4H2O
- (g)
- MLA media without Na2MoO4·2H2O
4.3. Sampling
4.4. Solution Nutrient Determination
4.5. Intracellular Iron Sample Preparation and Analysis
4.6. Microcystin-LR Method
4.7. Cell Volume
4.8. Growth Rate
4.9. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sciuto, K.; Moro, I. Cyanobacteria: The Bright and Dark Sides of a Charming Group. Biodivers. Conserv. 2015, 24, 711–738. [Google Scholar] [CrossRef]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and Harmful Algal Blooms: A Scientific Consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dignum, M.; Matthijs, H.C.P.; Pel, R.; Laanbroek, H.J.; Mur, L.R. Nutrient Limitation of Freshwater Cyanobacteria. In Harmful Cyanobacteria. Aquatic Ecology Series; Huisman, J., Matthijs, H.C.P., Visser, P.M., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 3, pp. 65–86. [Google Scholar] [CrossRef]
- Paerl, H.W.; Fulton, R.S. Ecology of Harmful Algae; Graneli, E., Turner, J., Eds.; Springer: New York, NY, USA, 2006; pp. 95–111. [Google Scholar] [CrossRef]
- North, R.L.; Guildford, S.J.; Smith, R.E.H.; Havens, S.M.; Twiss, M.R. Evidence for Phosphorus, Nitrogen, and Iron Colimitation of Phytoplankton Communities in Lake Erie. Limnol. Oceanogr. 2007, 52, 315–328. [Google Scholar] [CrossRef] [Green Version]
- Paerl, H.W.; Otten, T.G. Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Mitrovic, S.M. Phytoplankton Co-Limitation by Nitrogen and Phosphorus in a Shallow Reservoir: Progressing from the Phosphorus Limitation Paradigm. Hydrobiologia 2014, 744, 255–269. [Google Scholar] [CrossRef]
- Facey, J.A.; Michie, L.E.; King, J.J.; Hitchcock, J.N.; Apte, S.C.; Mitrovic, S.M. Severe Cyanobacterial Blooms in an Australian Lake; Causes and Factors Controlling Succession Patterns. Harmful Algae 2022, 117, 102284. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.S.; Vasconcelos, M.T. Cyanobacteria Metal Interactions: Requirements, Toxicity, and Ecological Implications. Crit. Rev. Microbiol. 2006, 32, 127–137. [Google Scholar] [CrossRef]
- Downs, T.M.; Schallenberg, M.; Burns, C.W. Responses of Lake Phytoplankton to Micronutrient Enrichment: A Study in Two New Zealand Lakes and an Analysis of Published Data. Aquat. Sci. 2008, 70, 347–360. [Google Scholar] [CrossRef]
- Facey, J.A.; Rogers, T.A.; Apte, S.C.; Mitrovic, S.M. Micronutrients as Growth Limiting Factors in Cyanobacterial Blooms; a Survey of Freshwaters in South East Australia. Aquat. Sci. 2021, 83, 28. [Google Scholar] [CrossRef]
- Facey, J.A.; King, J.J.; Apte, S.C.; Mitrovic, S.M. Assessing the Importance of Cobalt as a Micronutrient for Freshwater Cyanobacteria. J. Phycol. 2022, 58, 71–79. [Google Scholar] [CrossRef]
- Huertas, M.J.; López-Maury, L.; Giner-Lamia, J.; Sánchez-Riego, A.M.; Florencio, F.J. Metals in Cyanobacteria: Analysis of the Copper, Nickel, Cobalt and Arsenic Homeostasis Mechanisms. Life 2014, 4, 865–886. [Google Scholar] [CrossRef] [Green Version]
- Raven, J.A.; Evans, M.C.W.; Korb, R.E. The Role of Trace Metals in Photosynthetic Electron Transport in O-2-Evolving Organisms. Photosynth. Res. 1999, 60, 111–149. [Google Scholar] [CrossRef]
- Cavet, J.S.; Borrelly, G.P.M.; Robinson, N.J. Zn, Cu and Co in Cyanobacteria: Selective Control of Metal Availability. FEMS Microbiol. Rev. 2003, 27, 165–181. [Google Scholar] [CrossRef] [Green Version]
- Sunda, W.G. Trace Metals and Harmful Algal Blooms. In Ecology of Harmful Algae; Granéli, E., Turner, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 203–214. [Google Scholar] [CrossRef]
- Facey, J.A.; Apte, S.C.; Mitrovic, S.M. A Review of the Effect of Trace Metals on Freshwater Cyanobacterial Growth and Toxin Production. Toxins 2019, 11, 643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrede, T.; Tranvik, L.J. Iron Constraints on Planktonic Primary Production in Oligotrophic Lakes. Ecosystems 2006, 9, 1094–1105. [Google Scholar] [CrossRef] [Green Version]
- Wever, A.D.; Muylaert, K.; Langlet, D.; Alleman, L.; Descy, J.P.; André, L.; Cocquyt, C.; Vyverman, W. Differential Response of Phytoplankton to Additions of Nitrogen, Phosphorus and Iron in Lake Tanganyika. Freshw. Biol. 2008, 53, 264–277. [Google Scholar] [CrossRef]
- Auclair, J.C. Implications of Increased UV-B Induced Photoreduction: Iron(II) Enrichment Stimulates Picocyanobacterial Growth and the Microbial Food Web in Clear-Water Acidic Canadian Shield Lakes. Can. J. Fish. Aquat. Sci. 1995, 52, 1782–1788. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Xu, H.; Wells, M.; Tefsen, B.; Qin, B. Effect of Micronutrients on Algae in Different Regions of Taihu, a Large, Spatially Diverse, Hypereutrophic Lake. Water Res. 2019, 151, 500–514. [Google Scholar] [CrossRef]
- Lukac, M.; Aegerter, R. Influence of Trace Metals on Growth and Toxin Production of Microcystis aeruginosa. Toxicon 1993, 31, 293–305. [Google Scholar] [CrossRef]
- Li, H.; Murphy, T.; Guo, J.; Parr, T.; Nalewajko, C. Iron-Stimulated Growth and Microcystin Production of Microcystis Novacekii UAM 250. Limnologica 2009, 39, 255–259. [Google Scholar] [CrossRef] [Green Version]
- Polyak, Y.; Zaytseva, T.; Medvedeva, N. Response of Toxic Cyanobacterium Microcystis aeruginosa to Environmental Pollution. Water. Air. Soil Pollut. 2013, 224, 1494. [Google Scholar] [CrossRef]
- Fujii, M.; Dang, T.C.; Bligh, M.W.; Waite, T.D. Cellular Characteristics and Growth Behavior of Iron-Limited Microcystis aeruginosa in Nutrient-Depleted and Nutrient-Replete Chemostat Systems. Limnol. Oceanogr. 2016, 61, 2151–2164. [Google Scholar] [CrossRef]
- Molot, L.A.; Li, G.; Findlay, D.L.; Watson, S.B. Iron-Mediated Suppression of Bloom-Forming Cyanobacteria by Oxine in a Eutrophic Lake. Freshw. Biol. 2010, 55, 1102–1117. [Google Scholar] [CrossRef]
- Dengg, M.; Stirling, C.H.; Reid, M.R.; Verburg, P.; Armstrong, E.; Kelly, L.T.; Wood, S.A. Growth at the Limits: Comparing Trace Metal Limitation of a Freshwater Cyanobacterium (Dolichospermum lemmermannii) and a Freshwater Diatom (Fragilaria crotonensis). Sci. Rep. 2022, 12, 467. [Google Scholar] [CrossRef]
- Zurawell, R.W.; Chen, H.; Burke, J.M.; Prepas, E.E. Hepatotoxic Cyanobacteria: A Review of the Biological Importance of Microcystins in Freshwater Environments. J. Toxicol. Environ. Health Part B 2005, 8, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A Review of the Global Ecology, Genomics, and Biogeography of the Toxic Cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef] [Green Version]
- Mowe, M.A.D.; Mitrovic, S.M.; Lim, R.P.; Furey, A.; Yeo, D.C.J. Tropical cyanobacterial blooms: A review of prevalence, problem taxa, toxins and influencing environmental factors. J. Limnol. 2015, 74, 205–224. [Google Scholar] [CrossRef]
- Schatz, D.; Keren, Y.; Vardi, A.; Sukenik, A.; Carmeli, S.; Börner, T.; Dittmann, E.; Kaplan, A. Towards Clarification of the Biological Role of Microcystins, a Family of Cyanobacterial Toxins. Environ. Microbiol. 2007, 9, 965–970. [Google Scholar] [CrossRef]
- Zilliges, Y.; Kehr, J.C.; Meissner, S.; Ishida, K.; Mikkat, S.; Hagemann, M.; Kaplan, A.; Börner, T.; Dittmann, E. The Cyanobacterial Hepatotoxin Microcystin Binds to Proteins and Increases the Fitness of Microcystis under Oxidative Stress Conditions. PLoS ONE 2011, 6, e17615. [Google Scholar] [CrossRef] [Green Version]
- Neilan, B.A.; Pearson, L.A.; Muenchhoff, J.; Moffitt, M.C.; Dittmann, E. Environmental Conditions That Influence Toxin Biosynthesis in Cyanobacteria. Environ. Microbiol. 2013, 15, 1239–1253. [Google Scholar] [CrossRef]
- Bouaïcha, N.; Miles, C.O.; Beach, D.G.; Labidi, Z.; Djabri, A.; Benayache, N.Y.; Nguyen-Quang, T. Structural diversity, characterization and toxicology of microcystins. Toxins 2019, 11, 714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Giesy, J.P.; Adamovsky, O.; Svirčev, Z.; Meriluoto, J.; Codd, G.A.; Mijovic, B.; Shi, T.; Tuo, X.; Li, S.-C.; et al. Challenges of Using Blooms of Microcystis spp. in Animal Feeds: A Comprehensive Review of Nutritional, Toxicological and Microbial Health Evaluation. Sci. Total Environ. 2021, 764, 142319. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, T.; Kagalou, I.; Stalikas, C.; Pilidis, G.; Leonardos, I.D. Assessment of Microcystin Distribution and Biomagnification in Tissues of Aquatic Food Web Compartments from a Shallow Lake and Evaluation of Potential Risks to Public Health. Ecotoxicology 2012, 21, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.R.; Wilhelm, S.W.; Boyer, G.L. The Fate of Microcystins in the Environment and Challenges for Monitoring. Toxins 2014, 6, 3354–3387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facey, J.A.; Steele, J.R.; Violi, J.P.; Mitrovic, S.M.; Cranfield, C. An Examination of Microcystin-LR Accumulation and Toxicity Using Tethered Bilayer Lipid Membranes (TBLMs). Toxicon 2019, 158, 51–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial Toxins: Risk Management for Health Protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, J.S.M.; Giani, A. Microcystin Production and Regulation under Nutrient Stress Conditions in Toxic Microcystis Strains. Appl. Environ. Microbiol. 2014, 80, 5836–5843. [Google Scholar] [CrossRef]
- Wiedner, C.; Visser, P.M.; Fastner, J.; Metcalf, J.S.; Codd, G.A.; Mur, L.R. Effects of Light on the Microcystin Content of Microcystis Strain PCC 7806. Appl. Environ. Microbiol. 2003, 69, 1475–1481. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Sano, T.; Li, R.; Watanabe, M.M.; Liu, Y.; Kaya, K. Microcystin Production of Microcystis viridis (Cyanobacteria) under Different Culture Conditions. Phycol. Res. 1998, 46, 19–23. [Google Scholar] [CrossRef]
- Orr, P.T.; Jones, G.J. Relationship between Microcystin Production and Cell Division Rates in Nitrogen-Limited Microcystis aeruginosa Cultures. Limnol. Oceanogr. 1998, 43, 1604–1614. [Google Scholar] [CrossRef]
- Gouvêa, S.P.; Boyer, G.L.; Twiss, M.R. Influence of Ultraviolet Radiation, Copper, and Zinc on Microcystin Content in Microcystis aeruginosa (Cyanobacteria). Harmful Algae 2008, 7, 194–205. [Google Scholar] [CrossRef]
- Alexova, R.; Fujii, M.; Birch, D.; Cheng, J.; Waite, T.D.; Ferrari, B.C.; Neilan, B.A. Iron Uptake and Toxin Synthesis in the Bloom-Forming Microcystis aeruginosa under Iron Limitation. Environ. Microbiol. 2011, 13, 1064–1077. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.C.Y.; D’Agostino, P.M.; Poljak, A.; McDonald, J.; Bligh, M.W.; Waite, T.D.; Neilan, B.A. Physiological and Proteomic Responses of Continuous Cultures of Microcystis aeruginosa PCC 7806 to Changes in Iron Bioavailability and Growth Rate. Appl. Environ. Microbiol. 2016, 82, 5918–5929. [Google Scholar] [CrossRef] [Green Version]
- Sevilla, E.; Martin-Luna, B.; Vela, L.; Bes, M.T.; Fillat, M.F.; Peleato, M.L. Iron Availability Affects McyD Expression and Microcystin-LR Synthesis in Microcystis aeruginosa PCC7806. Environ. Microbiol. 2008, 10, 2476–2483. [Google Scholar] [CrossRef]
- Amé, M.V.; Wunderlin, D.A. Effects of Iron, Ammonium and Temperature on Microcystin Content by a Natural Concentrated Microcystis aeruginosa Population. Water. Air. Soil Pollut. 2005, 168, 235–248. [Google Scholar] [CrossRef]
- Utkilen, H.; Gjolme, N. Iron-Stimulated Toxin Production in Microcystis aeruginosa. Appl. Environ. Microbiol. 1995, 61, 797–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harland, F.M.J.; Wood, S.A.; Moltchanova, E.; Williamson, W.M.; Gaw, S. Phormidium Autumnale Growth and Anatoxin-a Production under Iron and Copper Stress. Toxins 2013, 5, 2504–2521. [Google Scholar] [CrossRef] [PubMed]
- Sherman, D.M.; Sherman, L.A. Effect of Iron Deficiency and Iron Restoration on Ultrastructure of Anacystis Nidulans. J. Bacteriol. 1983, 156, 393–401. [Google Scholar] [CrossRef] [Green Version]
- Schoffman, H.; Lis, H.; Shaked, Y.; Keren, N. Iron-Nutrient Interactions within Phytoplankton. Front. Plant Sci. 2016, 7, 1223. [Google Scholar] [CrossRef] [Green Version]
- Molot, L.A.; Watson, S.B.; Creed, I.F.; Trick, C.G.; Mccabe, S.K.; Verschoor, M.J.; Sorichetti, R.J.; Powe, C.; Venkiteswaran, J.J.; Schiff, S.L. A Novel Model for Cyanobacteria Bloom Formation: The Critical Role of Anoxia and Ferrous Iron. Freshw. Biol. 2014, 59, 1323–1340. [Google Scholar] [CrossRef]
- Bolch, C.J.S.; Blackburn, S.I. Isolation and Purification of Australian Isolates of the Toxic Cyanobacterium Microcystis aeruginosa Kütz. J. Appl. Phycol. 1996, 8, 5–13. [Google Scholar] [CrossRef]
- Lis, H.; Shaked, Y.; Kranzler, C.; Keren, N.; Morel, F.M.M. Iron Bioavailability to Phytoplankton: An Empirical Approach. ISME J. 2015, 9, 1003–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, W. Limiting Nutrient Elements in Filtered Lake Erie Water. Water Res. 1971, 5, 1031–1048. [Google Scholar] [CrossRef]
- Sunda, W.G.; Huntsman, S.A. Cobalt and Zinc Interreplacement in Marine Phytoplankton: Biological and Geochemical Implications. Limnol. Oceanogr. 1995, 40, 1404–1417. [Google Scholar] [CrossRef]
- Saito, M.A.; Moffett, J.W.; Chisholm, S.W.; Waterbury, J.B. Cobalt Limitation and Uptake in Prochlorococcus. Limnol. Oceanogr. 2002, 47, 1629–1636. [Google Scholar] [CrossRef]
- Rodriguez, I.B.; Ho, T.Y. Influence of Co and B12 on the Growth and Nitrogen Fixation of Trichodesmium. Front. Microbiol. 2015, 6, 623. [Google Scholar] [CrossRef] [PubMed]
- Helliwell, K.E.; Lawrence, A.D.; Holzer, A.; Kudahl, U.J.; Sasso, S.; Kräutler, B.; Scanlan, D.J.; Warren, M.J.; Smith, A.G. Cyanobacteria and Eukaryotic Algae Use Different Chemical Variants of Vitamin B12. Curr. Biol. 2016, 26, 999–1008. [Google Scholar] [CrossRef]
- Blaylock, A.D.; Davis, T.D.; Jolley, V.D.; Walser, R.H. Influence of Cobalt and Iron on Photosynthesis, Chlorophyll, and Nutrient Content in Rbgreening Chlorotic Tomatoes and Soybeans. J. Plant Nutr. 1986, 9, 823–838. [Google Scholar] [CrossRef]
- Wallace, A.; Abouzamzam, A.M. Low Levels, but Excesses, of Five Different Trace Elements, Singly and in Combination, on Interactions in Bush Beans Grown in Solution Culture. Soil Sci. 1989, 147, 439–441. [Google Scholar] [CrossRef]
- Gopal, R.; Dube, B.K.; Sinha, P.; Chatterjee, C. Cobalt Toxicity Effects on Growth and Metabolism of Tomato. Commun. Soil Sci. Plant Anal. 2003, 34, 619–628. [Google Scholar] [CrossRef]
- Latifi, A.; Ruiz, M.; Zhang, C.C. Oxidative Stress in Cyanobacteria. FEMS Microbiol. Rev. 2009, 33, 258–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfe-Simon, F.; Grzebyk, D.; Schofield, O.; Falkowski, P.G. The Role and Evolution of Superoxide Dismutases in Algae. J. Phycol. 2005, 41, 453–465. [Google Scholar] [CrossRef]
- Hernández-Prieto, M.A.; Schön, V.; Georg, J.; Barreira, L.; Varela, J.; Hess, W.R.; Futschik, M.E. Iron Deprivation in Synechocystis: Inference of Pathways, Non-Coding RNAs, and Regulatory Elements from Comprehensive Expression Profiling. G3 Genes Genomes Genet. 2012, 2, 1475–1495. [Google Scholar] [CrossRef] [Green Version]
- Salomon, E.; Keren, N. Manganese Limitation Induces Changes in the Activity and in the Organization of Photosynthetic Complexes in the Cyanobacterium Synechocystis Sp Strain PCC 6803. Plant Physiol. 2011, 155, 571–579. [Google Scholar] [CrossRef] [Green Version]
- Glass, J.B.; Wolfe-Simon, F.; Elser, J.J.; Anbar, A.D. Molybdenum-Nitrogen Co-Limitation in Freshwater and Coastal Heterocystous Cyanobacteria. Limnol. Oceanogr. 2010, 55, 667–676. [Google Scholar] [CrossRef]
- Ter Steeg, P.F.; Hanson, P.J.; Paerl, H.W. Growth-Limiting Quantities and Accumulation of Molybdenum in Anabaena Oscillarioides (Cyanobacteria). Hydrobiologia 1986, 140, 143–147. [Google Scholar] [CrossRef]
- Burnat, M.; Diestra, E.; Esteve, I.; Solé, A. In Situ Determination of the Effects of Lead and Copper on Cyanobacterial Populations in Microcosms. PLoS ONE 2009, 4, e6204. [Google Scholar] [CrossRef]
- Sunda, W.G. Feedback Interactions between Trace Metal Nutrients and Phytoplankton in the Ocean. Front. Microbiol. 2012, 3, 204. [Google Scholar] [CrossRef] [Green Version]
- González, A.; Fillat, M.F.; Bes, M.-T.; Peleato, M.-L.; Sevilla, E. The Challenge of Iron Stress in Cyanobacteria. In Cyanobacteria; Tiwari, A., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Spät, P.; Klotz, A.; Rexroth, S.; Maček, B.; Forchhammer, K. Chlorosis as a Developmental Program in Cyanobacteria: The Proteomic Fundament for Survival and Awakening. Mol. Cell. Proteom. 2018, 17, 1650–1669. [Google Scholar] [CrossRef] [Green Version]
- Kraemer, S.M.; Duckworth, O.W.; Harrington, J.M.; Schenkeveld, W.D.C. Metallophores and Trace Metal Biogeochemistry. Aquat. Geochem. 2015, 21, 159–195. [Google Scholar] [CrossRef]
- Wilhelm, S.W.; Maxwell, D.P.; Trick, C.G. Growth, Iron Requirements, and Siderophore Production in Iron-Limited Synechococcus PCC 7002. Limnol. Oceanogr. 1996, 41, 89–97. [Google Scholar] [CrossRef]
- Martínez-Ruiz, E.B.; Martínez-Jerónimo, F. How Do Toxic Metals Affect Harmful Cyanobacteria? An Integrative Study with a Toxigenic Strain of Microcystis aeruginosa Exposed to Nickel Stress. Ecotoxicol. Environ. Saf. 2016, 133, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.R.; Baldwin, D.S.; Silvester, E. Proton and Iron Binding by the Cyanobacterial Toxin Microcystin-LR. Environ. Sci. Technol. 2013, 47, 5178–5184. [Google Scholar] [CrossRef] [PubMed]
- Humble, A.V.; Gadd, G.M.; Codd, G.A. Binding of Copper and Zinc to Three Cyanobacterial Microcystins Quantified by Differential Pulse Polarography. Water Res. 1997, 31, 1679–1686. [Google Scholar] [CrossRef]
- Saito, K.; Sei, Y.; Miki, S.; Yamaguchi, K. Detection of Microcystin-Metal Complexes by Using Cryospray Ionization-Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Toxicon 2008, 51, 1496–1498. [Google Scholar] [CrossRef]
- Turner, A.D.; Waack, J.; Lewis, A.; Edwards, C.; Lawton, L. Development and Single-Laboratory Validation of a UHPLC-MS/MS Method for Quantitation of Microcystins and Nodularin in Natural Water, Cyanobacteria, Shellfish and Algal Supplement Tablet Powders. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1074–1075, 111–123. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 17 October 2021).



| Nutrient/Salt | Final Concentration (mg/L) |
|---|---|
| K2HPO4 | 34.80 |
| NaNO3 | 170.00 |
| NaHCO3 | 16.80 |
| CaCl2 | 29.40 |
| MgSO4·7H2O | 49.10 |
| H3BO3 | 2.40 |
| CoCl2·6H2O | 0.01 |
| CuSO4·5H2O | 0.01 |
| FeCl3·6H2O | 1.58 |
| Na2EDTA·2H2O | 4.56 |
| MnCl2·4H2O | 0.36 |
| Na2MoO4·2H2O | 0.006 |
| ZnSO4·7H2O | 0.022 |
| Thiamine HCl | 0.10 |
| Biotin | 5 × 10−4 |
| Cyanocobalamin (B12) | 5 × 10−4 |
| Time (min) | A% | B% | μL/min |
|---|---|---|---|
| 0.00 | 98.0 | 2.0 | 600.0 |
| 0.50 | 75% | 25.0 | 600.0 |
| 1.50 | 75% | 25.0 | 600.0 |
| 3.00 | 60% | 40.0 | 600.0 |
| 4.00 | 50% | 50.0 | 600.0 |
| 4.10 | 5% | 95.0 | 600.0 |
| 4.50 | 5% | 95.0 | 600.0 |
| 5.00 | 98% | 2.0 | 600.0 |
| 100% | 0.0 | 600.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Facey, J.A.; Violi, J.P.; King, J.J.; Sarowar, C.; Apte, S.C.; Mitrovic, S.M. The Influence of Micronutrient Trace Metals on Microcystis aeruginosa Growth and Toxin Production. Toxins 2022, 14, 812. https://doi.org/10.3390/toxins14110812
Facey JA, Violi JP, King JJ, Sarowar C, Apte SC, Mitrovic SM. The Influence of Micronutrient Trace Metals on Microcystis aeruginosa Growth and Toxin Production. Toxins. 2022; 14(11):812. https://doi.org/10.3390/toxins14110812
Chicago/Turabian StyleFacey, Jordan A., Jake P. Violi, Josh J. King, Chowdhury Sarowar, Simon C. Apte, and Simon M. Mitrovic. 2022. "The Influence of Micronutrient Trace Metals on Microcystis aeruginosa Growth and Toxin Production" Toxins 14, no. 11: 812. https://doi.org/10.3390/toxins14110812





