Ultrasound Imaging of the Facial Muscles and Relevance with Botulinum Toxin Injections: A Pictorial Essay and Narrative Review
Abstract
:1. Introduction
2. Overview of the Superficial Facial Anatomy
3. Muscles of the Upper Face
3.1. Frontalis
3.1.1. Anatomy
3.1.2. Scanning Technique
3.1.3. Clinical Relevance
3.2. Temporalis
3.2.1. Anatomy
3.2.2. Scanning Technique
3.2.3. Clinical Relevance
3.3. Procerus
3.3.1. Anatomy
3.3.2. Scanning Technique
3.3.3. Clinical Relevance
3.4. Depressor Supercilii
3.4.1. Anatomy
3.4.2. Scanning Technique
3.4.3. Clinical Relevance
3.5. Corrugator Supercilii
3.5.1. Anatomy
3.5.2. Scanning Technique
3.5.3. Clinical Relevance
3.6. Orbicularis Oculi
3.6.1. Anatomy
3.6.2. Scanning Technique
3.6.3. Clinical Relevance
4. Muscles of the Middle Face
4.1. Nasalis
4.1.1. Anatomy
4.1.2. Scanning Technique
4.1.3. Clinical Relevance
4.2. Levator Labii Superioris Alaeque Nasi
4.2.1. Anatomy
4.2.2. Scanning Technique
4.2.3. Clinical Relevance
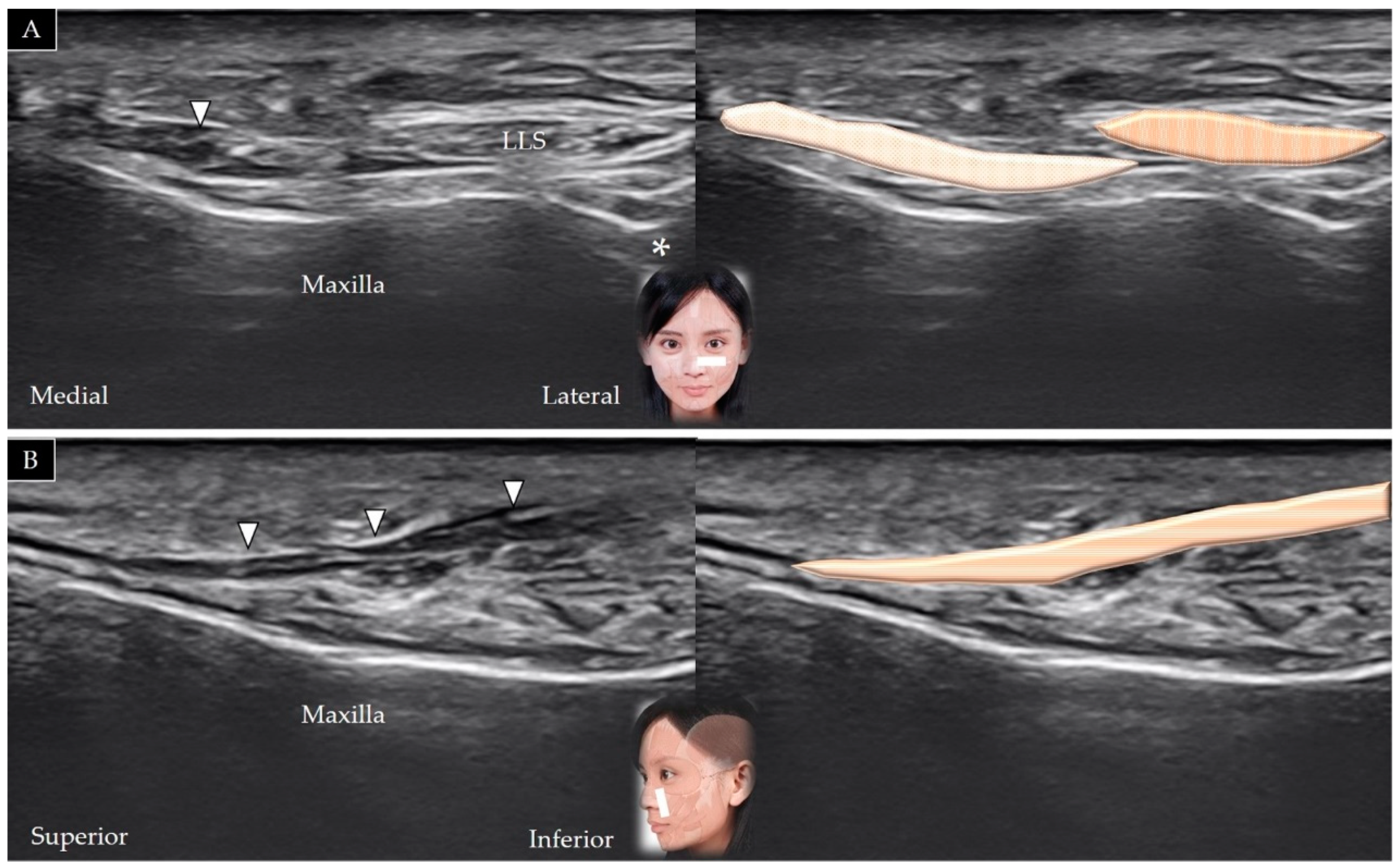
4.3. Levator Labii Superioris
4.3.1. Anatomy
4.3.2. Scanning Technique
4.3.3. Clinical Relevance
4.4. Levator Anguli Oris
4.4.1. Anatomy
4.4.2. Scanning Technique
4.4.3. Clinical Relevance
4.5. Zygomaticus Minor
4.5.1. Anatomy
4.5.2. Scanning Technique
4.5.3. Clinical Relevance
4.6. Zygomaticus Major
4.6.1. Anatomy
4.6.2. Scanning Technique
4.6.3. Clinical Relevance
4.7. Masseter
4.7.1. Anatomy
4.7.2. Scanning Technique
4.7.3. Clinical Relevance
5. Muscles of the Lower Face
5.1. Orbicularis Oris
5.1.1. Anatomy
5.1.2. Scanning Technique
5.1.3. Clinical Relevance
5.2. Buccinator
5.2.1. Anatomy
5.2.2. Scanning Technique
5.2.3. Clinical Relevance
5.3. Risorius
5.3.1. Anatomy
5.3.2. Scanning Technique
5.3.3. Clinical Relevance
5.4. Mentalis
5.4.1. Anatomy
5.4.2. Scanning Technique
5.4.3. Clinical Relevance
5.5. Depressor Labii Inferioris
5.5.1. Anatomy
5.5.2. Scanning Technique
5.5.3. Clinical Relevance
5.6. Depressor Anguli Oris
5.6.1. Anatomy
5.6.2. Scanning Technique
5.6.3. Clinical Relevance
6. Literature Review
6.1. Literature Search
6.2. Results
7. Future Perspectives and Limitations
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, P.H.; Chen, Y.J.; Chang, K.V.; Wu, W.T.; Ozcakar, L. Ultrasound measurements of superficial and deep masticatory muscles in various postures: Reliability and influencers. Sci. Rep. 2020, 10, 14357. [Google Scholar] [CrossRef] [PubMed]
- Mlosek, R.K.; Malinowska, S. Ultrasound image of the skin, apparatus and imaging basics. J. Ultrason 2013, 13, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Alfen, N.V.; Gilhuis, H.J.; Keijzers, J.P.; Pillen, S.; Van Dijk, J.P. Quantitative facial muscle ultrasound: Feasibility and reproducibility. Muscle Nerv. 2013, 48, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Volk, G.F.; Pohlmann, M.; Sauer, M.; Finkensieper, M.; Guntinas-Lichius, O. Quantitative ultrasonography of facial muscles in patients with chronic facial palsy. Muscle Nerv. 2014, 50, 358–365. [Google Scholar] [CrossRef]
- Abe, T.; Spitz, R.W.; Wong, V.; Viana, R.B.; Yamada, Y.; Bell, Z.W.; Chatakondi, R.N.; Loenneke, J.P. Assessments of Facial Muscle Thickness by Ultrasound in Younger Adults: Absolute and Relative Reliability. Cosmetics 2019, 6, 65. [Google Scholar] [CrossRef] [Green Version]
- Nasr, M.W.; Jabbour, S.F.; Sidaoui, J.A.; Haber, R.N.; Kechichian, E.G. Botulinum Toxin for the Treatment of Excessive Gingival Display: A Systematic Review. Aesthet. Surg. J. 2015, 36, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Hsu, A.K.; Frankel, A.S. Modification of Chin Projection and Aesthetics With OnabotulinumtoxinA Injection. JAMA Fac. Plast. Surg. 2017, 19, 522–527. [Google Scholar] [CrossRef]
- Walter, U.; Dressler, D. Ultrasound-guided botulinum toxin injections in neurology: Technique, indications and future perspectives. Expert Rev. Neurother. 2014, 14, 923–936. [Google Scholar] [CrossRef]
- Cilento, B.W. Superficial Musculoaponeurotic System (SMAS). In Encyclopedia of Otolaryngology, Head and Neck Surgery; Kountakis, S.E., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2616–2617. [Google Scholar]
- Whitney, Z.B.; Jain, M.; Zito, P.M. Anatomy, Skin, Superficial Musculoaponeurotic System (SMAS) Fascia. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Pessino, K.; Patel, J.; Patel, B.C. Anatomy, Head and Neck, Frontalis Muscle. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Renga, M. A personalized treatment approach of frontalis muscle with botulinum toxin A (Bont-A) related to functional anatomy: Case studies. J. Cosmet. Laser Ther. 2020, 22, 100–106. [Google Scholar] [CrossRef]
- Sedlmayr, J.C.; Kirsch, C.F.; Wisco, J.J. The human temporalis muscle: Superficial, deep, and zygomatic parts comprise one structural unit. Clin. Anat. 2009, 22, 655–664. [Google Scholar] [CrossRef]
- McGuigan, C.; O’Riordan, S.; Farrell, M.; Mitchell, B.; Hutchinson, M. Case report: Recurrent temporalis muscle swelling and headache. J. Neurol. 2003, 60, 724–725. [Google Scholar] [CrossRef] [PubMed]
- Juhász, M.L.; Marmur, E.S. Temporal fossa defects: Techniques for injecting hyaluronic acid filler and complications after hyaluronic acid filler injection. J. Cosmet. Dermatol. 2015, 14, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.M.; Drake, T.M.; Krishnamurthy, K. Anatomy, Head and Neck, Procerus Muscle. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Romano, S.; Colosimo, C. Procerus sign in progressive supranuclear palsy. J. Neurol. 2001, 57, 1928. [Google Scholar] [CrossRef] [Green Version]
- De Sanctis Pecora, C.; Shitara, D. Botulinum Toxin Type A to Improve Facial Symmetry in Facial Palsy: A Practical Guideline and Clinical Experience. Toxins 2021, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Cook, B.E., Jr.; Lucarelli, M.J.; Lemke, B.N. Depressor supercilii muscle: Anatomy, histology, and cosmetic implications. Ophthalm. Plast. Reconstruct. Surg. 2001, 17, 404–411. [Google Scholar] [CrossRef]
- Yu, M.; Wang, S.M. Anatomy, Head and Neck, Eye Corrugator Muscle. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Allam, A.E.; Khalil, A.A.F.; Eltawab, B.A.; Wu, W.T.; Chang, K.V. Ultrasound-Guided Intervention for Treatment of Trigeminal Neuralgia: An Updated Review of Anatomy and Techniques. Pain Res. Manag. 2018, 2018, 5480728. [Google Scholar] [CrossRef]
- Olver, J.M. Botulinum toxin A treatment of overactive corrugator supercilii in thyroid eye disease. J. Brit. J. Ophthalmol. 1998, 82, 528–533. [Google Scholar] [CrossRef]
- Tong, J.; Lopez, M.J.; Patel, B.C. Anatomy, Head and Neck, Eye Orbicularis Oculi Muscle. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Jeong, J.; Terence, G.; Kim, J. Understanding the Anatomy of the Transverse Nasalis Aponeurotic Fibers and Its Importance in Asian Rhinoplasty. Ann. Plast. Surg. 2018, 81, 516–522. [Google Scholar] [CrossRef]
- Hormazabal-Peralta, A.; Lee, K.-W.; Lee, H.-J.; Choi, Y.-J.; Hu, K.-S.; Kim, H.-J. Clinical anatomy considerations on the muscular and vascular components of the midface by ultrasonographic imaging. Clin. Anat. 2021, 34, 1142–1149. [Google Scholar] [CrossRef]
- Hur, M.S.; Hu, K.S.; Park, J.T.; Youn, K.H.; Kim, H.J. New anatomical insight of the levator labii superioris alaeque nasi and the transverse part of the nasalis. Surg. Radiol. Anat. SRA 2010, 32, 753–756. [Google Scholar] [CrossRef]
- Kane, M.A.C. The Effect of Botulinum Toxin Injections on the Nasolabial Fold. Plast. Reconstruct. Surg. 2003, 112, 66S–72S. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.; Lopez, M.J.; Rayi, A. Anatomy, Head and Neck, Eye Levator Labii Superioris Muscle. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Hwang, W.S.; Hur, M.S.; Hu, K.S.; Song, W.C.; Koh, K.S.; Baik, H.S.; Kim, S.T.; Kim, H.J.; Lee, K.J. Surface anatomy of the lip elevator muscles for the treatment of gummy smile using botulinum toxin. Angle Orthod. 2009, 79, 70–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dao, D.P.D.; Le, P.H. Anatomy, Head and Neck, Eye Levator Anguli Oris Muscle. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Hur, M.S.; Youn, K.H.; Kim, H.J. New Insight Regarding the Zygomaticus Minor as Related to Cosmetic Facial Injections. Clin. Anat. 2018, 31, 974–980. [Google Scholar] [CrossRef]
- Elvan, Ö.; Bobus Örs, A.; Tezer, M.S. Anatomical Evaluation of Zygomaticus Major Muscle with Relation to Orbicularis Oculi Muscle and Parotid Duct. J. Craniofac. Surg. 2020, 31, 1844–1847. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.V.; Wu, W.T.; Ozcakar, L. Ultrasound examination for facial asymmetry: Thermal injury of the zygomatic major muscle after ultherapy. Med. Ultrason 2021, 23, 368–369. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.P.; Peng, J.H. Complications of botulinum toxin injection for masseter hypertrophy: Incidence rate from 2036 treatments and summary of causes and preventions. J. Cosmet. Dermatol. 2018, 17, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Rathee, M. Anatomy, Head and Neck, Orbicularis Oris Muscle. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Volk, G.F.; Sauer, M.; Pohlmann, M.; Guntinas-Lichius, O. Reference values for dynamic facial muscle ultrasonography in adults. Muscle Nerv. 2014, 50, 348–357. [Google Scholar] [CrossRef]
- Guerrissi, J.O. Intraoperative injection of botulinum toxin A into orbicularis oculi muscle for the treatment of crow’s feet. Plast. Reconstruct.Surg. 2000, 105, 2219–2225. [Google Scholar] [CrossRef]
- Rathee, M.; Jain, P. Anatomy, Head and Neck, Buccinator Muscle. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Patel, P.N.; Owen, S.R.; Norton, C.P.; Emerson, B.T.; Bronaugh, A.B.; Ries, W.R.; Stephan, S.J. Outcomes of Buccinator Treatment with Botulinum Toxin in Facial Synkinesis. JAMA Fac. Plast. Surg. 2018, 20, 196–201. [Google Scholar] [CrossRef]
- Germann, A.M.; Al Khalili, Y. Anatomy, Head and Neck, Risorius Muscle. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Hur, M.S.; Kim, H.J.; Choi, B.Y.; Hu, K.S.; Kim, H.J.; Lee, K.S. Morphology of the mentalis muscle and its relationship with the orbicularis oris and incisivus labii inferioris muscles. J. Craniofac. Surg. 2013, 24, 602–604. [Google Scholar] [CrossRef]
- Gordon, K.; Cadera, W.; Hinton, G. Successful treatment of hereditary trembling chin with botulinum toxin. J. Child. Neurol. 1993, 8, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; We, Y.-J.; Lee, H.-J.; Lee, K.-W.; Gil, Y.-C.; Hu, K.-S.; Tansatit, T.; Kim, H.-J. Three-Dimensional Evaluation of the Depressor Anguli Oris and Depressor Labii Inferioris for Botulinum Toxin Injections. Aesth. Surg. J. 2020, 41, NP456–NP461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volk, G.F.; Wystub, N.; Pohlmann, M.; Finkensieper, M.; Chalmers, H.J.; Guntinas-Lichius, O. Quantitative ultrasonography of facial muscles. Muscle Nerv. 2013, 47, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Volk, G.F.; Leier, C.; Guntinas-Lichius, O. Correlation between electromyography and quantitative ultrasonography of facial muscles in patients with facial palsy. Muscle Nerv. 2016, 53, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Anandan, C.; Jankovic, J. Botulinum Toxin in Movement Disorders: An Update. Toxins 2021, 13, 42. [Google Scholar] [CrossRef]
















| Muscle | Origin | Insertion | Transducer Position |
|---|---|---|---|
| Frontalis | Galea aponeurotica | Orbicularis oculi | In the horizontal plane at one fingerbreadth cranial to the eyebrow (short axis view) |
| Temporalis | Parietal and sphenoid bones | Coronoid process of the mandible and retromolar fossa | Along the zygomatic arch in the horizontal plane, then moved cranially (short axis view) |
| Procerus | Fascia over the lower portion of the nasal bone | Lower forehead between bilateral eyebrows | In the horizontal plane on the lower portion of the forehead between the eyebrows |
| Depressor supercilii | Medial orbital rim | Medial wall of the bony orbit | In the horizontal plane on the middle one third of the eyebrow |
| Corrugator supercilii | Supraorbital ridge | Skin of the forehead near the eyebrow | In the horizontal plane on the middle one third of the eyebrow |
| Orbicularis oculi | Frontal bone, medial palpebral ligament and lacrimal bone | Lateral palpebral raphe | In the horizontal plane over the lateral orbital wall (short/oblique axis view) |
| Muscle | Origin | Insertion | Transducer Position |
|---|---|---|---|
| Nasalis | Maxilla | Nasal bone | In the oblique coronal plane along the nasal cartilage (short axis view) |
| Levator labii superioris alaeque nasi | Nasal bone | Nostril and upper lip | In the oblique horizontal plane passing the nasal crease (lateral to the nasalis) |
| Levator labii superioris | Medial infraorbital region | Upper lip | In the middle of the inferior orbital rim in the horizontal plane (short axis view) |
| Levator anguli oris | Maxilla | Modiolus | Same as the overlying levator labii superioris |
| Zygomaticus minor | Zygomatic bone | Upper lip | In the horizontal plane over the lateral inferior corner of the orbital rim (underneath the orbicularis oculi) |
| Zygomaticus major | Zygomatic bone | Upper lip | In the horizontal plane over the inferior lateral edge of the orbital rim (lateral to the zygomatic minor) |
| Masseter | Zygomatic arch | Mandibular ramus and angle | In the horizontal plane along the zygomatic arch, then moved caudally (short axis view) |
| Muscle | Origin | Insertion | Transducer Position |
|---|---|---|---|
| Orbicularis oris | Maxilla and mandible, perioral skin and muscles and modiolus | Skin and mucosa of the lip | In the horizontal plane over the lower philtrum and labiomandibular crease |
| Buccinator | Maxilla, buccinator ridge of mandible and pterygomandibular raphe | Modiolus | In the horizontal plane between the zygomatic arch and mandible (emerging under the masseter) |
| Risorius | Parotid fascia and buccal skin | Modiolus | In the horizontal plane (emerging from the masseter superficial fascia) |
| Mentalis | Anterior mandible | Chin | Over the midline of the chin (short axis view) |
| Depressor labii inferioris | Oblique line of the mandible | Lower lip | Over the midline of the chin (lateral to the mentalis) |
| Depressor anguli oris | Oblique line of the mandible | Modiolus | Over the midline of the chin (lateral to the depressor labii inferioris) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.-T.; Chang, K.-V.; Chang, H.-C.; Chen, L.-R.; Kuan, C.-H.; Kao, J.-T.; Wei, L.-Y.; Chen, Y.-J.; Han, D.-S.; Özçakar, L. Ultrasound Imaging of the Facial Muscles and Relevance with Botulinum Toxin Injections: A Pictorial Essay and Narrative Review. Toxins 2022, 14, 101. https://doi.org/10.3390/toxins14020101
Wu W-T, Chang K-V, Chang H-C, Chen L-R, Kuan C-H, Kao J-T, Wei L-Y, Chen Y-J, Han D-S, Özçakar L. Ultrasound Imaging of the Facial Muscles and Relevance with Botulinum Toxin Injections: A Pictorial Essay and Narrative Review. Toxins. 2022; 14(2):101. https://doi.org/10.3390/toxins14020101
Chicago/Turabian StyleWu, Wei-Ting, Ke-Vin Chang, Hsiang-Chi Chang, Lan-Rong Chen, Chen-Hsiang Kuan, Jung-Ting Kao, Ling-Ying Wei, Yunn-Jy Chen, Der-Sheng Han, and Levent Özçakar. 2022. "Ultrasound Imaging of the Facial Muscles and Relevance with Botulinum Toxin Injections: A Pictorial Essay and Narrative Review" Toxins 14, no. 2: 101. https://doi.org/10.3390/toxins14020101
APA StyleWu, W.-T., Chang, K.-V., Chang, H.-C., Chen, L.-R., Kuan, C.-H., Kao, J.-T., Wei, L.-Y., Chen, Y.-J., Han, D.-S., & Özçakar, L. (2022). Ultrasound Imaging of the Facial Muscles and Relevance with Botulinum Toxin Injections: A Pictorial Essay and Narrative Review. Toxins, 14(2), 101. https://doi.org/10.3390/toxins14020101







