Comprehensive Assessment of the Time Course of Biomechanical, Electrophysiological and Neuro-Motor Effects after Botulinum Toxin Injections in Elbow Flexors of Chronic Stroke Survivors with Spastic Hemiplegia: A Cross Sectional Observation Study
Abstract
:1. Introduction
2. Results
2.1. Clinical Assessment of BoNT Effects
2.1.1. Elbow Flexor Spasticity
2.1.2. Muscle Strength—Maximum Voluntary Contraction (MVC) Tasks
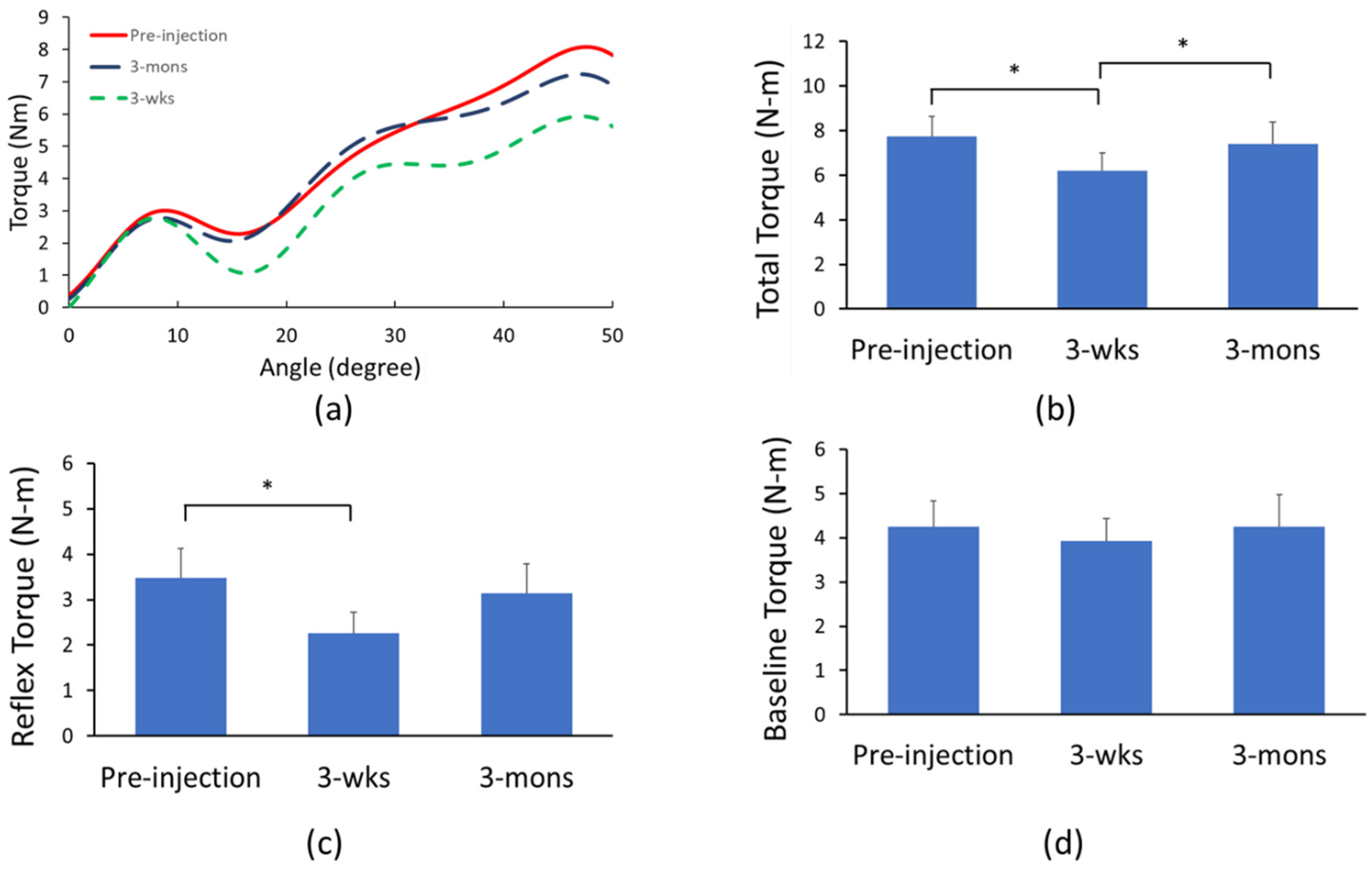
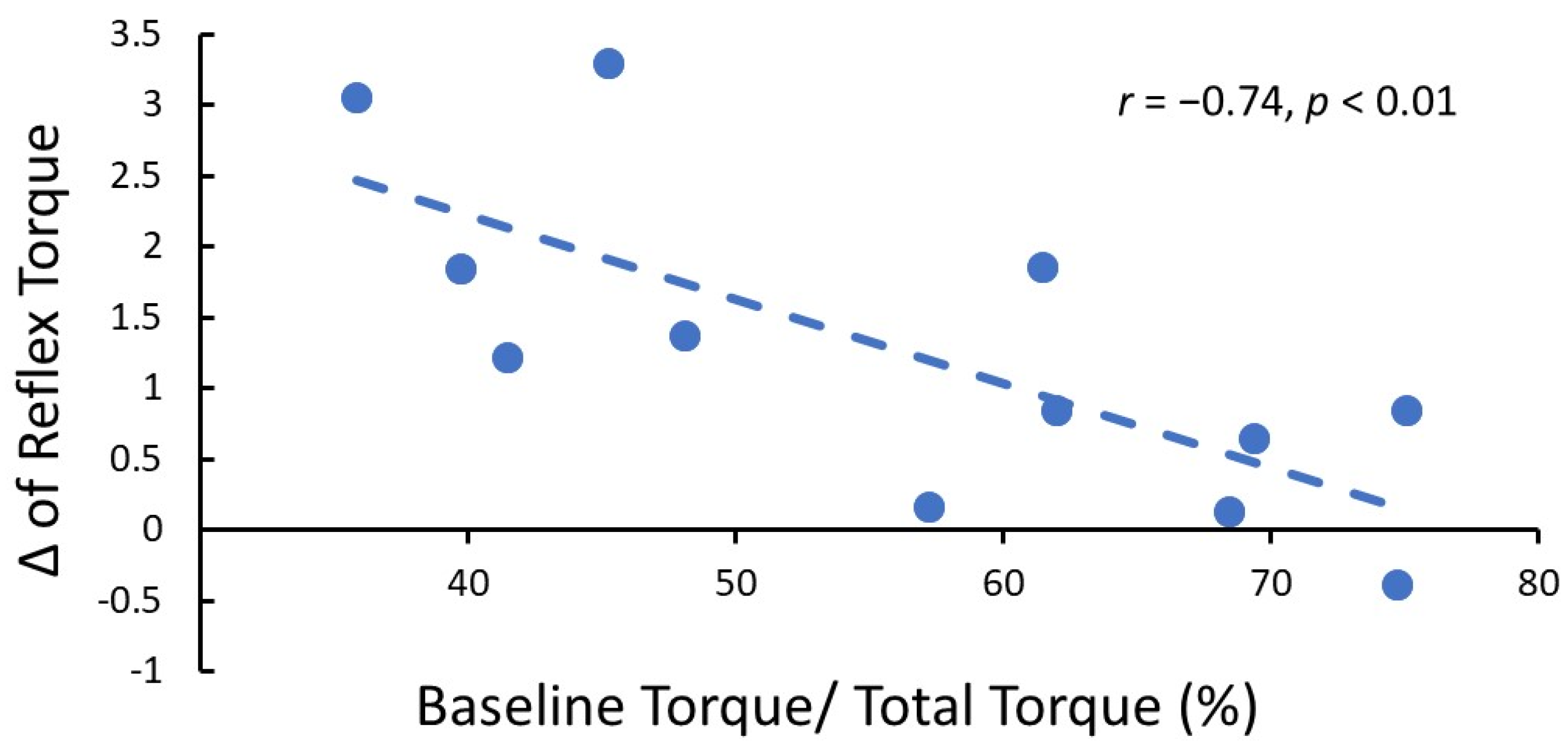
2.2. Biomechanical Assessment of BoNT Effects
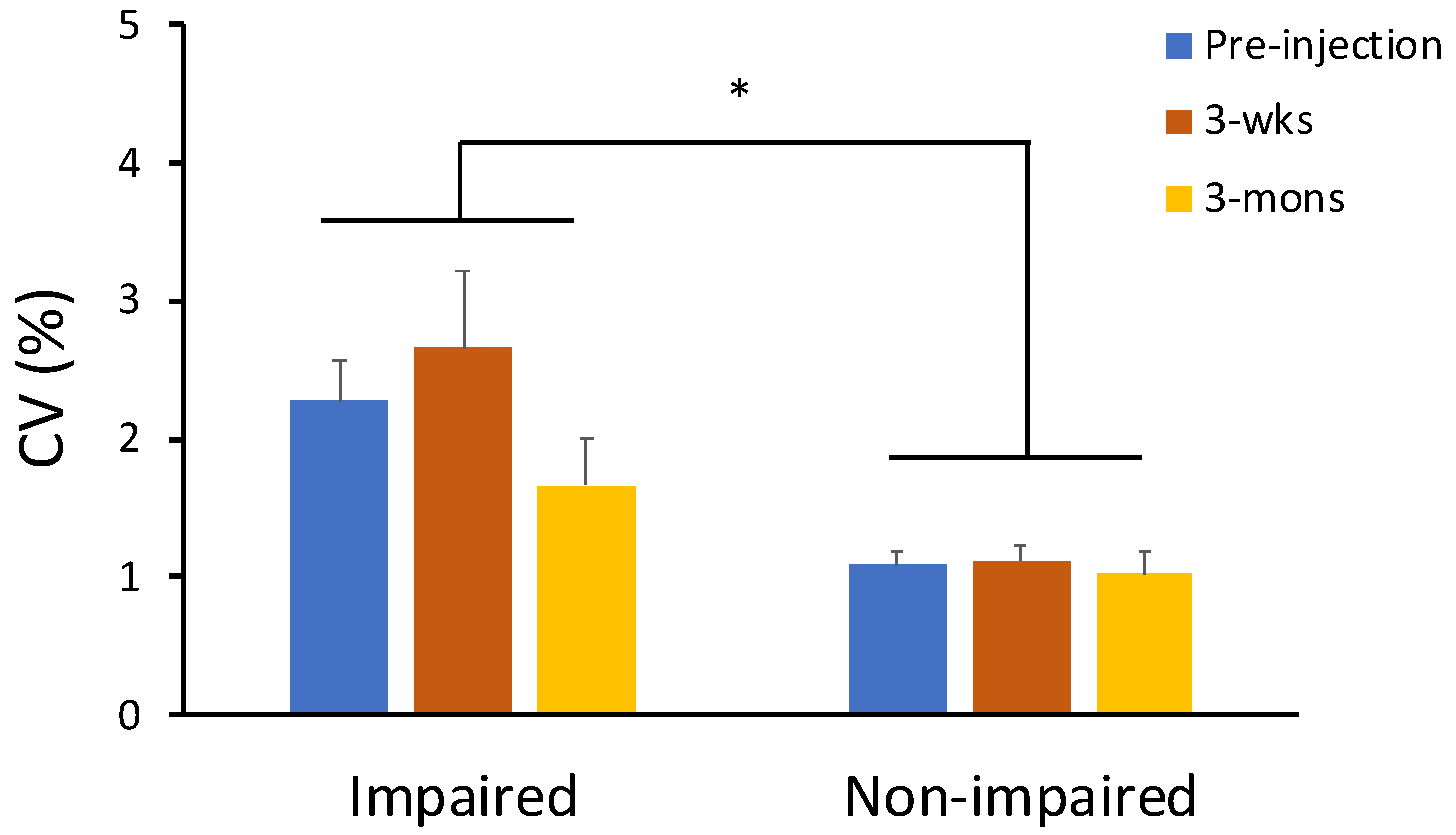
2.3. The Effect of BoNT on Neuro-Motor Performance
2.4. Neurophysiological Assessment of BoNT Effects—CMAP Amplitude
3. Discussion
3.1. BoNT Effects on Elbow Flexor Spastic Hypertonia
3.2. Effects on Neuro-Motor Control of Spastic Muscles
3.3. Clinical Implications
4. Conclusions
5. Methods
5.1. Participants
5.2. Experimental Set-Up
5.3. Experimental Protocol
5.4. Data Analysis
5.4.1. Neuro-Motor Performance
5.4.2. Resistance Torque
5.4.3. CMAP
5.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| BoNT | Botulinum neurotoxin |
| CMAP | compound muscle action potential |
| MAS | modified Ashworth scale |
| MVC | Maximum Voluntary Contraction |
| CV | coefficient of variation |
References
- Nam, K.E.; Lim, S.H.; Kim, J.S.; Hong, B.Y.; Jung, H.Y.; Lee, J.K.; Yoo, S.D.; Pyun, S.B.; Lee, K.M.; Lee, K.J.; et al. When does spasticity in the upper limb develop after a first stroke? A nationwide observational study on 861 stroke patients. J. Clin. Neurosci. 2019, 66, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeld, D.K.; Eek, E.U.; Svensson, A.K.; Holmqvist, L.W.; von Arbin, M.H. Spasticity after stroke: Its occurrence and association with motor impairments and activity limitations. Stroke 2004, 35, 134–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorňák, T.; Justanová, M.; Konvalinková, R.; Říha, M.; Mužík, J.; Hoskovcová, M.; Srp, M.; Navrátilová, D.; Otruba, P.; Gál, O.; et al. Prevalence and evolution of spasticity in patients suffering from first-ever stroke with carotid origin: A prospective, longitudinal study. Eur. J. Neurol. 2019, 26, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Pundik, S.; McCabe, J.; Skelly, M.; Tatsuoka, C.; Daly, J.J. Association of spasticity and motor dysfunction in chronic stroke. Ann. Phys. Rehabil. Med. 2018, 62, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Francisco, G.E. New insights into the pathophysiology of post-stroke spasticity. Front. Hum. Neurosci. 2015, 9, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, P. Pathophysiology of spasticity. J Neurol. Neurosurg. Psychiatry 1994, 57, 773–777. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Chen, Y.T.; Francisco, G.E.; Zhou, P.; Rymer, W.Z. A Unifying Pathophysiological Account for Post-stroke Spasticity and Disordered Motor Control. Front. Neurol. 2019, 10, 468. [Google Scholar] [CrossRef]
- Lance, J.W. Symposium synopsis. In Spasticity: Disordered Motor Control; Feldman, R.G., Young, R.R., Koella, W.P., Eds.; Year Book Medical Publishers: Chicago, IL, USA, 1980; pp. 485–494. [Google Scholar]
- Li, S.; Francisco, G.E.; Rymer, W.Z. A New Definition of Poststroke Spasticity and the Interference of Spasticity with Motor Recovery from Acute to Chronic Stages. Neurorehabil. Neural Repair 2021, 35, 601–610. [Google Scholar] [CrossRef]
- Mottram, C.J.; Suresh, N.L.; Heckman, C.J.; Gorassini, M.A.; Rymer, W.Z. Origins of abnormal excitability in biceps brachii motoneurons of spastic-paretic stroke survivors. J. Neurophysiol. 2009, 102, 2026–2038. [Google Scholar] [CrossRef] [Green Version]
- Mottram, C.J.; Wallace, C.L.; Chikando, C.N.; Rymer, W.Z. Origins of spontaneous firing of motor units in the spastic-paretic biceps brachii muscle of stroke survivors. J. Neurophysiol. 2010, 104, 3168–3179. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.H.; Francisco, G.E.; Zhou, P.; Rymer, W.Z.; Li, S. Spasticity, weakness, force variability, and sustained spontaneous motor unit discharges of resting spastic-paretic biceps brachii muscles in chronic stroke. Muscle Nerve 2013, 48, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.H.; Francisco, G.E.; Li, S. Botulinum Toxin (BT) injection improves voluntary motor control in selected patients with post-stroke spasticity. Neural Regen. Res. 2012, 7, 1436–1439. [Google Scholar] [PubMed]
- Seo, N.J.; Rymer, W.Z.; Kamper, D.G. Delays in grip initiation and termination in persons with stroke: Effects of arm support and active muscle stretch exercise. J. Neurophysiol. 2009, 101, 3108–3115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlyle, J.K.; Mochizuki, G. Influence of post-stroke spasticity on EMG-force coupling and force steadiness in biceps brachii. J. Electromyogr. Kinesiol. 2018, 38, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Lodha, N.; Naik, S.K.; Coombes, S.A.; Cauraugh, J.H. Force control and degree of motor impairments in chronic stroke. Clin. Neurophysiol. 2010, 121, 1952–1961. [Google Scholar] [CrossRef]
- Chen, Y.T.; Zhang, C.; Liu, Y.; Magat, E.; Verduzco-Gutierrez, M.; Francisco, G.E.; Zhou, P.; Zhang, Y.; Li, S. The Effects of Botulinum Toxin Injections on Spasticity and Motor Performance in Chronic Stroke with Spastic Hemiplegia. Toxins 2020, 12, 492. [Google Scholar] [CrossRef]
- Simpson, D.M.; Hallett, M.; Ashman, E.J.; Comella, C.L.; Green, M.W.; Gronseth, G.S.; Armstrong, M.J.; Gloss, D.; Potrebic, S.; Jankovic, J.; et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 1818–1826. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, A.; Smith, H.S. Botulinum toxins: Mechanisms of action, antinociception and clinical applications. Toxicology 2013, 306, 124–146. [Google Scholar] [CrossRef]
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum neurotoxins: Biology, pharmacology, and toxicology. Pharmacol. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef]
- Jankovic, J. Botulinum toxin: State of the art. Mov. Disord. 2017, 32, 1131–1138. [Google Scholar] [CrossRef]
- de Paiva, A.; Meunier, F.A.; Molgó, J.; Aoki, K.R.; Dolly, J.O. Functional repair of motor endplates after botulinum neurotoxin type A poisoning: Biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc. Natl. Acad. Sci. USA 1999, 96, 3200–3205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francisco, G.E.; Li, S. Spasticity. In Braddom Physical Medicine and Rehabilitation, 6th ed.; Cifu, D.X., Ed.; Elsevier: Philadelphia, PA, USA, 2020. [Google Scholar]
- Moeini-Naghani, I.; Hashemi-Zonouz, T.; Jabbari, B. Botulinum Toxin Treatment of Spasticity in Adults and Children. Semin. Neurol. 2016, 36, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Andringa, A.; van de Port, I.; van Wegen, E.; Ket, J.; Meskers, C.; Kwakkel, G. Effectiveness of botulinum toxin treatment for upper limb spasticity after stroke over different ICF domains: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2019, 100, 1703–1725. [Google Scholar] [CrossRef] [PubMed]
- Schmit, B.D.; Dhaher, Y.; Dewald, J.P.; Rymer, W.Z. Reflex torque response to movement of the spastic elbow: Theoretical analyses and implications for quantification of spasticity. Ann. Biomed. Eng. 1999, 27, 815–829. [Google Scholar] [CrossRef]
- Kamper, D.G.; Rymer, W.Z. Quantitative features of the stretch response of extrinsic finger muscles in hemiparetic stroke. Muscle Nerve 2000, 23, 954–961. [Google Scholar] [CrossRef]
- Li, S.; Shin, H.; Zhou, P.; Li, X. Different Effects of Cold Stimulation on Reflex and Non-Reflex Components of Poststroke Spastic Hypertonia. Front. Neurol. 2017, 8, 169. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-T.; Li, S.; Magat, E.; Zhou, P.; Li, S. Motor Overflow and Spasticity in Chronic Stroke Share a Common Pathophysiological Process: Analysis of Within-Limb and Between-Limb EMG-EMG Coherence. Front. Neurol. 2018, 9, 795. [Google Scholar] [CrossRef] [Green Version]
- Pingel, J.; Wienecke, J.; Lorentzen, J.; Nielsen, J.B. Botulinum toxin injection causes hyper-reflexia and increased muscle stiffness of the triceps surae muscle in the rat. J. Neurophysiol. 2016, 116, 2615–2623. [Google Scholar] [CrossRef]
- Miscio, G.; Del Conte, C.; Pianca, D.; Colombo, R.; Panizza, M.; Schieppati, M.; Pisano, F. Botulinum toxin in post-stroke patients: Stiffness modifications and clinical implications. J. Neurol. 2004, 251, 189–196. [Google Scholar] [CrossRef]
- Afsharipour, B.; Chandra, S.; Li, G.; Rymer, W.Z.; Suresh, N.L. Characterization of Differences in the Time Course of Reflex and Voluntary Responses Following Botulinum Toxin Injections in Chronic Stroke Survivors. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1642–1650. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.T.; Liu, Y.; Magat, E.; Gutierrez-Verduzco, M.; Francisco, G.E.; Zhou, P.; Li, S.; Zhang, Y. Improving Botulinum Toxin Efficiency in Treating Post-Stroke Spasticity Using 3D Innervation Zone Imaging. Int. J. Neural Syst. 2021, 31, 2150007. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Gäverth, J.; Herman, P.A. Changes in the Neural and Non-neural Related Properties of the Spastic Wrist Flexors after Treatment with Botulinum Toxin A in Post-stroke Subjects: An Optimization Study. Front. Bioeng. Biotechnol. 2018, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Afsharipour, B.; Rymer, W.Z.; Suresh, N.L. Precise quantification of the time course of voluntary activation capacity following Botulinum toxin injections in the biceps brachii muscles of chronic stroke survivors. J. Neuroeng. Rehabil. 2020, 17, 102. [Google Scholar] [CrossRef] [PubMed]
- Thilmann, A.F.; Fellows, S.J.; Garms, E. The mechanism of spastic muscle hypertonus. Variation in reflex gain over the time course of spasticity. Brain 1991, 114, 233–244. [Google Scholar]
- Lee, H.M.; Huang, Y.Z.; Chen, J.J.J.; Hwang, I.S. Quantitative analysis of the velocity related pathophysiology of spasticity and rigidity in the elbow flexors. J. Neurol. Neurosurg. Psychiatry 2002, 72, 621–629. [Google Scholar] [CrossRef] [Green Version]
- Lieber, R.L.; Friden, J. Spasticity causes a fundamental rearrangement of muscle-joint interaction. Muscle Nerve 2002, 25, 265–270. [Google Scholar] [CrossRef]
- Raghavan, P.; Lu, Y.; Mirchandani, M.; Stecco, A. Human Recombinant Hyaluronidase Injections for Upper Limb Muscle Stiffness in Individuals with Cerebral Injury: A Case Series. EBioMedicine 2016, 9, 306–313. [Google Scholar] [CrossRef] [Green Version]
- Thacker, B.E.; Tomiya, A.; Hulst, J.B.; Suzuki, K.P.; Bremner, S.N.; Gastwirt, R.F.; Greaser, M.L.; Lieber, R.L.; Ward, S.R. Passive mechanical properties and related proteins change with botulinum neurotoxin a injection of normal skeletal muscle. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2012, 30, 497–502. [Google Scholar] [CrossRef] [Green Version]
- Kaya, C.S.; Yılmaz, E.O.; Akdeniz-Doğan, Z.D.; Yucesoy, C.A. Long-Term Effects with Potential Clinical Importance of Botulinum Toxin Type-A on Mechanics of Muscles Exposed. Front. Bioeng. Biotechnol. 2020, 8, 738. [Google Scholar] [CrossRef]
- Ward, S.R.; Minamoto, V.B.; Suzuki, K.P.; Hulst, J.B.; Bremner, S.N.; Lieber, R.L. Recovery of rat muscle size but not function more than 1 year after a single botulinum toxin injection. Muscle Nerve 2018, 57, 435–441. [Google Scholar] [CrossRef]
- Binder-Markey, B.I.; Murray, W.M.; Dewald, J.P.A. Passive Properties of the Wrist and Fingers Following Chronic Hemiparetic Stroke: Interlimb Comparisons in Persons with and without a Clinical Treatment History that Includes Botulinum Neurotoxin. Front. Neurol. 2021, 12, 687624. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, C.; Ispoglou, S.; Helliwell, B.; Hicklin, D.; Sturman, S.; Pandyan, A. Can the early use of botulinum toxin in post stroke spasticity reduce contracture development? A randomised controlled trial. Clin. Rehabil. 2020, 35, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.F.; Jones, K.E.; Wolpert, D.M. The scaling of motor noise with muscle strength and motor unit number in humans. Exp. Brain Res. 2004, 157, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Gracies, J.M.; Park, S.B.; Lee, K.H.; Lee, J.Y.; Shin, J.H. Botulinum Toxin Injections and Electrical Stimulation for Spastic Paresis Improve Active Hand Function Following Stroke. Toxins 2018, 10, 426. [Google Scholar] [CrossRef] [Green Version]
- Preissner, K.S. The effects of serial casting on spasticity: A literature review. Occup. Ther. Health Care 2002, 14, 99–106. [Google Scholar] [CrossRef]
- Booth, B.J.; Doyle, M.; Montgomery, J. Serial casting for the management of spasticity in the head-injured adult. Phys. Ther. 1983, 63, 1960–1966. [Google Scholar] [CrossRef]
- Mortenson, P.A.; Eng, J.J. The use of casts in the management of joint mobility and hypertonia following brain injury in adults: A systematic review. Phys. Ther. 2003, 83, 648–658. [Google Scholar] [CrossRef] [Green Version]
- Pohl, M.; Mehrholz, J.; Ruckriem, S. The influence of illness duration and level of consciousness on the treatment effect and complication rate of serial casting in patients with severe cerebral spasticity. Clin. Rehabil. 2003, 17, 373–379. [Google Scholar] [CrossRef]
- Bovend’Eerdt, T.J.; Newman, M.; Barker, K.; Dawes, H.; Minelli, C.; Wade, D.T. The effects of stretching in spasticity: A systematic review. Arch. Phys. Med. Rehabil. 2008, 89, 1395–1406. [Google Scholar] [CrossRef]
- Lannin, N.A.; Novak, I.; Cusick, A. A systematic review of upper extremity casting for children and adults with central nervous system motor disorders. Clin. Rehabil. 2007, 21, 963–976. [Google Scholar] [CrossRef]
- Farina, S.; Migliorini, C.; Gandolfi, M.; Bertolasi, L.; Casarotto, M.; Manganotti, P.; Fiaschi, A.; Smania, N. Combined effects of botulinum toxin and casting treatments on lower limb spasticity after stroke. Funct. Neurol. 2008, 23, 87–91. [Google Scholar] [PubMed]
- Li, S.; Francisco, G.E. The Use of Botulinum Toxin for Treatment of Spasticity. Handb. Exp. Pharmacol. 2021, 263, 127–146. [Google Scholar] [CrossRef] [PubMed]
- Seo, N.J.; Fischer, H.W.; Bogey, R.A.; Rymer, W.Z.; Kamper, D.G. Effect of a serotonin antagonist on delay in grip muscle relaxation for persons with chronic hemiparetic stroke. Clin. Neurophysiol. 2011, 122, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Bhadane, M.Y.; Gao, F.; Francisco, G.E.; Zhou, P.; Li, S. Correlation of resting elbow angle with spasticity in chronic stroke survivors. Front. Neurol. 2015, 6, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Liu, J.; Bhadane, M.; Zhou, P.; Rymer, W.Z. Activation deficit correlates with weakness in chronic stroke: Evidence from evoked and voluntary EMG recordings. Clin. Neurophysiol. 2014, 125, 2413–2417. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, S.; Li, X.; Klein, C.; Rymer, W.Z.; Zhou, P. Suppression of stimulus artifact contaminating electrically evoked electromyography. NeuroRehabilitation 2014, 34, 381–389. [Google Scholar] [CrossRef] [Green Version]


| ID | Age | Gender | History of Stroke (Mons) | Paretic Side | Dominant Side | Elbow Flexor MAS (Pre-Injection) | Elbow Flexor MAS (3-Wks) | Elbow Flexor MAS (3-Mons) | Lesion Type |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 60 | M | 23 | Left | Right | 2 | 2 | 2 | Hemorrhagic |
| 2 | 52 | F | 104 | Left | Right | 2 | 1+ | 1+ | Ischemic |
| 3 | 40 | F | 77 | Right | Right | 2 | 1+ | 2 | Ischemic |
| 4 | 53 | M | 68 | Left | Right | 2 | 1+ | 2 | Hemorrhagic |
| 5 | 49 | M | 89 | Left | Right | 2 | 2 | 2 | Hemorrhagic |
| 6 | 63 | M | 137 | Left | Right | 2 | 1+ | 1+ | Ischemic |
| 7 | 49 | M | 7 | Right | Right | 2 | 1+ | 2 | Ischemic |
| 8 | 40 | M | 39 | Right | Right | 2 | 1+ | 1+ | Hemorrhagic |
| 9 | 68 | F | 7 | Left | Right | 2 | 2 | 2 | Ischemic |
| 10 | 65 | M | 29 | Left | Right | 2 | 1+ | 2 | Hemorrhagic |
| 11 | 39 | F | 76 | Right | Right | 2 | 1 | 2 | Hemorrhagic |
| 12 | 46 | F | 117 | Right | Right | 3 | 2 | 2 | Ischemic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-T.; Liu, Y.; Zhang, C.; Magat, E.; Zhou, P.; Zhang, Y.; Li, S. Comprehensive Assessment of the Time Course of Biomechanical, Electrophysiological and Neuro-Motor Effects after Botulinum Toxin Injections in Elbow Flexors of Chronic Stroke Survivors with Spastic Hemiplegia: A Cross Sectional Observation Study. Toxins 2022, 14, 104. https://doi.org/10.3390/toxins14020104
Chen Y-T, Liu Y, Zhang C, Magat E, Zhou P, Zhang Y, Li S. Comprehensive Assessment of the Time Course of Biomechanical, Electrophysiological and Neuro-Motor Effects after Botulinum Toxin Injections in Elbow Flexors of Chronic Stroke Survivors with Spastic Hemiplegia: A Cross Sectional Observation Study. Toxins. 2022; 14(2):104. https://doi.org/10.3390/toxins14020104
Chicago/Turabian StyleChen, Yen-Ting, Yang Liu, Chuan Zhang, Elaine Magat, Ping Zhou, Yingchun Zhang, and Sheng Li. 2022. "Comprehensive Assessment of the Time Course of Biomechanical, Electrophysiological and Neuro-Motor Effects after Botulinum Toxin Injections in Elbow Flexors of Chronic Stroke Survivors with Spastic Hemiplegia: A Cross Sectional Observation Study" Toxins 14, no. 2: 104. https://doi.org/10.3390/toxins14020104




