Local Cytotoxic Effects in Cobra Envenoming: A Pilot Study
Abstract
:1. Introduction
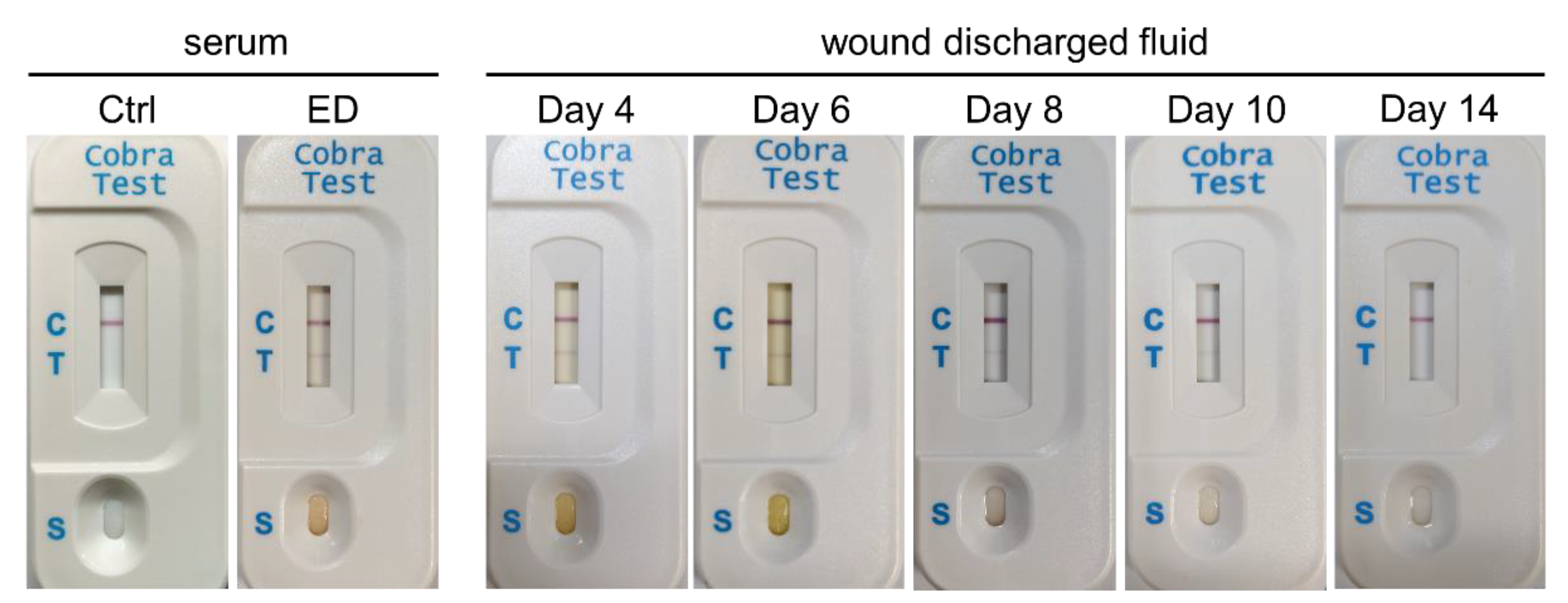
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Ethics Statement
4.3. Sandwich Enzyme-Linked Immunosorbent Assay (Sandwich ELISA)
4.4. Cases Presentation and Sample Collection
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montecucco, C.; Gutiérrez, J.M.; Lomonte, B. Cellular pathology induced by snake venom phospholipase A2 myotoxins and neurotoxins: Common aspects of their mechanisms of action. Cell. Mol. Life Sci. CMLS 2008, 65, 2897–2912. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Rucavado, A.; Chaves, F.; Díaz, C.; Escalante, T. Experimental pathology of local tissue damage induced by Bothrops asper snake venom. Toxicon Off. J. Int. Soc. Toxinol. 2009, 54, 958–975. [Google Scholar] [CrossRef]
- Williams, H.F.; Mellows, B.A.; Mitchell, R.; Sfyri, P.; Layfield, H.J.; Salamah, M.; Vaiyapuri, R.; Collins-Hooper, H.; Bicknell, A.B.; Matsakas, A.; et al. Mechanisms underpinning the permanent muscle damage induced by snake venom metalloprotease. PLoS Negl. Trop. Dis. 2019, 13, e0007041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, L.A.; Jorge, M.T.; Lebrão, M.L. Prognostic factors for local necrosis in Bothrops jararaca (Brazilian pit viper) bites. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 630–634. [Google Scholar] [CrossRef]
- Lin, J.H.; Liao, J.W.; Hung, D.Z. Comment on Clinical manifestations and treatments of Protobothrops mucrosquamatus bite and associated factors for wound necrosis and subsequent debridement and finger or toe amputation surgery. Clin. Toxicol. 2021, 59, 86–87. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Liu, B.S.; Chien, K.Y.; Chiang, L.C.; Huang, S.Y.; Sung, W.C.; Wu, W.G. Cobra venom proteome and glycome determined from individual snakes of Naja atra reveal medically important dynamic range and systematic geographic variation. J. Proteom. 2015, 128, 92–104. [Google Scholar] [CrossRef]
- Tan, K.Y.; Tan, C.H.; Fung, S.Y.; Tan, N.H. Venomics, lethality and neutralization of Naja kaouthia (monocled cobra) venoms from three different geographical regions of Southeast Asia. J. Proteom. 2015, 120, 105–125. [Google Scholar] [CrossRef]
- Whitaker, R.; Martin, G. Diversity and distribution of medically important snakes of India. In Clinical Toxinology in Asia Pacific and Africa; Springer: Berlin/Heidelberg, Germany, 2015; pp. 115–136. [Google Scholar]
- Tan, C.H.; Wong, K.Y.; Chong, H.P.; Tan, N.H.; Tan, K.Y. Proteomic insights into short neurotoxin-driven, highly neurotoxic venom of Philippine cobra (Naja philippinensis) and toxicity correlation of cobra envenomation in Asia. J. Proteom. 2019, 206, 103418. [Google Scholar] [CrossRef]
- Mukherjee, A.K. Species-specific and geographical variation in venom composition of two major cobras in Indian subcontinent: Impact on polyvalent antivenom therapy. Toxicon Off. J. Int. Soc. Toxinol. 2020, 188, 150–158. [Google Scholar] [CrossRef]
- Liu, C.C.; Chou, Y.S.; Chen, C.Y.; Liu, K.L.; Huang, G.J.; Yu, J.S.; Wu, C.J.; Liaw, G.W.; Hsieh, C.H.; Chen, C.K. Pathogenesis of local necrosis induced by Naja atra venom: Assessment of the neutralization ability of Taiwanese freeze-dried neurotoxic antivenom in animal models. PLoS Negl. Trop. Dis. 2020, 14, e0008054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, K.Y.; Tan, C.H.; Chanhome, L.; Tan, N.H. Comparative venom gland transcriptomics of Naja kaouthia (monocled cobra) from Malaysia and Thailand: Elucidating geographical venom variation and insights into sequence novelty. PeerJ 2017, 5, e3142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Hung, D.Z.; Liau, M.Y.; Lin-Shiau, S.Y. The clinical significance of venom detection in patients of cobra snakebite. Toxicon Off. J. Int. Soc. Toxinol. 2003, 41, 409–415. [Google Scholar] [CrossRef]
- Hung, D.-Z. Taiwan’s venomous snakebite: Epidemiological, evolution and geographic differences. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 96–101. [Google Scholar] [CrossRef]
- Regional Office for South-East Asia, WHO. Guidelines for the Management of Snakebites, 2nd ed.; WHO Regional Office for South-East Asia: New Delhi, India, 2016. [Google Scholar]
- Mao, Y.-C.; Liu, P.-Y.; Chiang, L.-C.; Lai, C.-S.; Lai, K.-L.; Ho, C.-H.; Wang, T.-H.; Yang, C.-C. Naja atra snakebite in Taiwan. Clin. Toxicol. 2018, 56, 273–280. [Google Scholar] [CrossRef]
- Hung, D.Z.; Lin, J.H.; Mo, J.F.; Huang, C.F.; Liau, M.Y. Rapid diagnosis of Naja atra snakebites. Clin. Toxicol. 2014, 52, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-H.; Sung, W.-C.; Liao, J.-W.; Hung, D.-Z. A rapid and international applicable diagnostic device for cobra (genus Naja) snakebites. Toxins 2020, 12, 572. [Google Scholar] [CrossRef]
- Lin, C.C.; Chaou, C.H.; Tseng, C.Y. An investigation of snakebite antivenom usage in Taiwan. J. Formos. Med. Assoc. Taiwan Yi Zhi 2016, 115, 672–677. [Google Scholar] [CrossRef] [Green Version]
- Wong, O.F.; Lam, T.S.; Fung, H.T.; Choy, C.H. Five-year experience with Chinese cobra (Naja atra)—Related injuries in two acute hospitals in Hong Kong. Hong Kong Med. J. Xianggang Yi Xue Za Zhi 2010, 16, 36–43. [Google Scholar]
- Su, H.Y.; Wang, M.J.; Li, Y.H.; Tang, C.N.; Tsai, M.J. Can surgical need in patients with Naja atra (Taiwan or Chinese cobra) envenomation be predicted in the emergency department? Hong Kong Med. J. Xianggang Yi Xue Za Zhi 2016, 22, 435–444. [Google Scholar] [CrossRef] [Green Version]
- Rivel, M.; Solano, D.; Herrera, M.; Vargas, M.; Villalta, M.; Segura, Á.; Arias, A.S.; León, G.; Gutiérrez, J.M. Pathogenesis of dermonecrosis induced by venom of the spitting cobra, Naja nigricollis: An experimental study in mice. Toxicon Off. J. Int. Soc. Toxinol. 2016, 119, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Tasoulis, T.; Isbister, G.K. A Review and Database of Snake Venom Proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.; Cristofori-Armstrong, B.; Rash, L.D.; Hodgson, W.C.; Isbister, G.K. Defining the role of post-synaptic α-neurotoxins in paralysis due to snake envenoming in humans. Cell. Mol. Life Sci. CMLS 2018, 75, 4465–4478. [Google Scholar] [CrossRef]
- Kessler, P.; Marchot, P.; Silva, M.; Servent, D. The three-finger toxin fold: A multifunctional structural scaffold able to modulate cholinergic functions. J. Neurochem. 2017, 142, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Petras, D.; Sanz, L.; Segura, A.; Herrera, M.; Villalta, M.; Solano, D.; Vargas, M.; Leon, G.; Warrell, D.A.; Theakston, R.D.; et al. Snake venomics of African spitting cobras: Toxin composition and assessment of congeneric cross-reactivity of the pan-African EchiTAb-Plus-ICP antivenom by antivenomics and neutralization approaches. J. Proteome Res. 2011, 10, 1266–1280. [Google Scholar] [CrossRef]
- Liu, C.C.; You, C.H.; Wang, P.J.; Yu, J.S.; Huang, G.J.; Liu, C.H.; Hsieh, W.C.; Lin, C.C. Analysis of the efficacy of Taiwanese freeze-dried neurotoxic antivenom against Naja kaouthia, Naja siamensis and Ophiophagus hannah through proteomics and animal model approaches. PLoS Negl. Trop. Dis. 2017, 11, e0006138. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.; Chandra, G. Sequence analysis and phylogenetic study of some toxin proteins of snakes and related non-toxin proteins of chordates. Bioinformation 2013, 9, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.S.; Wu, W.G.; Lin, M.H.; Li, C.H.; Jiang, B.R.; Wu, S.C.; Leng, C.H.; Sung, W.C. Identification of Immunoreactive Peptides of Toxins to Simultaneously Assess the Neutralization Potency of Antivenoms against Neurotoxicity and Cytotoxicity of Naja atra Venom. Toxins 2017, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Lo, T.-B.; Chen, Y.-H.; Lee, C.-Y. Chemical Studies of Formosan Cobra (Naja naja atra) Venom. Part I. Chromatographic Separation of Crude Venom on CM-Sepadex and Preliminary Characterization of its Components. J. Chin. Chem. Soc. 1966, 13, 25–37. [Google Scholar] [CrossRef]
- Lai, M.; Wen, C.; Lee, C. Local lesions caused by cardiotoxin isolated from Formosan cobra venom. Taiwan Yi Xue Hui Za Zhi. J. Formos. Med. Assoc. 1972, 71, 328–332. [Google Scholar]
- Tseng, L.; Chiu, T.; Lee, C. Absorption and distribution of 131I-labeled cobra venom and its purified toxins. Toxicol. Appl. Pharmacol. 1968, 12, 526–535. [Google Scholar] [CrossRef]
- Guo, M.P.; Wang, Q.C.; Liu, G.F. Pharmacokinetics of cytotoxin from Chinese cobra (Naja naja atra) venom. Toxicon Off. J. Int. Soc. Toxinol. 1993, 31, 339–343. [Google Scholar] [CrossRef]
- Tjong, S.-C.; Wu, P.-L.; Wang, C.-M.; Huang, W.-N.; Ho, N.-L.; Wu, W.-g. Role of Glycosphingolipid Conformational Change in Membrane Pore Forming Activity of Cobra Cardiotoxin. Biochemistry 2007, 46, 12111–12123. [Google Scholar] [CrossRef]
- Konshina, A.G.; Boldyrev, I.A.; Utkin, Y.N.; Omel’kov, A.V.; Efremov, R.G. Snake cytotoxins bind to membranes via interactions with phosphatidylserine head groups of lipids. PLoS ONE 2011, 6, e19064. [Google Scholar] [CrossRef] [Green Version]
- Forouhar, F.; Huang, W.-N.; Liu, J.-H.; Chien, K.-Y.; Wu, W.-g.; Hsiao, C.-D. Structural basis of membrane-induced cardiotoxin A3 oligomerization. J. Biol. Chem. 2003, 278, 21980–21988. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.P.; Lai, C.S.; Lin, S.D. Management of poisonous snake bites in southern Taiwan. Kaohsiung J. Med. Sci. 2007, 23, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Taiwan National Poison Control Center. The Flowchart of Management of Six Medically Importantly Venomous Snakebites in Taiwan. Available online: http://www.pcc-vghtpe.tw/antidote/page_list.asp?cid=10 (accessed on 23 March 2021).
- Chen, C.-K.; Lin, C.-C.; Shih, F.-Y.; Chaou, C.-H.; Lin, J.C.-C.; Lai, T.-I.; Tseng, C.-Y.; Fang, C.-C. Population-based study of venomous snakebite in Taiwan. J. Acute Med. 2015, 5, 38–42. [Google Scholar] [CrossRef]
- Dennis, E.A.; Darke, P.L.; Deems, R.A.; Kensil, C.R.; Plückthun, A. Cobra venom phospholipase A2: A review of its action toward lipid/water interfaces. Mol. Cell. Biochem. 1981, 36, 37–45. [Google Scholar] [CrossRef]
- Mukherjee, A.K. Correlation between the phospholipids domains of the target cell membrane and the extent of Naja kaouthia PLA(2)-induced membrane damage: Evidence of distinct catalytic and cytotoxic sites in PLA(2) molecules. Biochim. Biophys. Acta 2007, 1770, 187–195. [Google Scholar] [CrossRef]
- Dutta, S.; Sinha, A.; Dasgupta, S.; Mukherjee, A.K. Binding of a Naja naja venom acidic phospholipase A2 cognate complex to membrane-bound vimentin of rat L6 cells: Implications in cobra venom-induced cytotoxicity. Biochim. Biophys. Acta (BBA)-Biomembr. 2019, 1861, 958–977. [Google Scholar] [CrossRef] [PubMed]
- Hiu, J.J.; Yap, M.K.K. Cytotoxicity of snake venom enzymatic toxins: Phospholipase A2 and l-amino acid oxidase. Biochem. Soc. Trans. 2020, 48, 719–731. [Google Scholar] [CrossRef] [Green Version]
- Kriengkrairut, S.; Othong, R. Bacterial infection secondary to Trimeresurus species bites: A retrospective cohort study in a university hospital in Bangkok. Emerg. Med. Australas. EMA 2021, 33, 1006–1012. [Google Scholar] [CrossRef]
- Huang, L.-W.; Wang, J.-D.; Huang, J.-A.; Hu, S.-Y.; Wang, L.-M.; Tsan, Y.-T. Wound infections secondary to snakebite in central Taiwan. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 272–276. [Google Scholar] [CrossRef] [Green Version]
- Yeh, H.; Gao, S.Y.; Lin, C.C. Wound Infections from Taiwan Cobra (Naja atra) Bites: Determining Bacteriology, Antibiotic Susceptibility, and the Use of Antibiotics-A Cobra BITE Study. Toxins 2021, 13, 183. [Google Scholar] [CrossRef]
- Eckmann, C.; Montravers, P. Current management of necrotizing soft-tissue infections. Curr. Opin. Infect. Dis. 2021, 34, 89–95. [Google Scholar] [CrossRef]
- Lin, C.C.; Chen, Y.C.; Goh, Z.N.L.; Seak, C.K.; Seak, J.C.; Shi-Ying, G.; Seak, C.J.; Spot, I. Wound Infections of Snakebites from the Venomous Protobothrops mucrosquamatus and Viridovipera stejnegeri in Taiwan: Bacteriology, Antibiotic Susceptibility, and Predicting the Need for Antibiotics-A BITE Study. Toxins 2020, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-H.; Lo, C.-M.; Chuang, S.-H.; Chiang, C.-H.; Wang, S.-D.; Lin, T.-Y.; Liao, J.-W.; Hung, D.-Z. Collocation of avian and mammal antibodies to develop a rapid and sensitive diagnostic tool for Russell’s Vipers Snakebite. PLoS Negl. Trop. Dis. 2020, 14, e0008701. [Google Scholar] [CrossRef]


| Cases | |||||
|---|---|---|---|---|---|
| Venom or Toxin Measurement | # M/55 | M/68 | M/82 | # F/68 | |
| whole venom | S | 25.1 | 197.1 | 6.2 | 91.6 |
| T | 64.3 | 185.9 | 88.2 | -* | |
| W | 94 | 252.3 | 82.6 | -* | |
| CTX A3 | S | 6.4 | 1535.7 | 204.7 | 1013.7 |
| T | 65.2 | 1541.4 | 14.2 | -* | |
| W | 90.3 | 9124 | 470.2 | -* | |
| sNTX | S | 13.4 | 1958.3 | 958.3 | 91.1 |
| T | 12.4 | 10.3 | 18.2 | -* | |
| W | 4.5 | 238.3 | 131.6 | -* | |
| ICT-Cobra | S | P | P | P | P |
| W | P | P | P | P | |
| days in hospital | 16 | 24 | 15 | 45 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.-H.; Sung, W.-C.; Mu, H.-W.; Hung, D.-Z. Local Cytotoxic Effects in Cobra Envenoming: A Pilot Study. Toxins 2022, 14, 122. https://doi.org/10.3390/toxins14020122
Lin J-H, Sung W-C, Mu H-W, Hung D-Z. Local Cytotoxic Effects in Cobra Envenoming: A Pilot Study. Toxins. 2022; 14(2):122. https://doi.org/10.3390/toxins14020122
Chicago/Turabian StyleLin, Jing-Hua, Wang-Chou Sung, Han-Wei Mu, and Dong-Zong Hung. 2022. "Local Cytotoxic Effects in Cobra Envenoming: A Pilot Study" Toxins 14, no. 2: 122. https://doi.org/10.3390/toxins14020122
APA StyleLin, J.-H., Sung, W.-C., Mu, H.-W., & Hung, D.-Z. (2022). Local Cytotoxic Effects in Cobra Envenoming: A Pilot Study. Toxins, 14(2), 122. https://doi.org/10.3390/toxins14020122






