Local Differences in the Toxin Amount and Composition of Tetrodotoxin and Related Compounds in Pufferfish (Chelonodon patoca) and Toxic Goby (Yongeichthys criniger) Juveniles
Abstract
:1. Introduction
2. Results
2.1. Composition of TTX and 5,6,11-TrideoxyTTX in the Pufferfish
2.2. Composition of TTX and 5,6,11-TrideoxyTTX in the Toxic Goby
2.3. Difference in the TTX Concentration of the Pufferfish and Toxic Goby
2.4. Planocera multitentaculata-Specific Sequence (COI) from Intestinal Contents of TTX-Bearing Fish
2.4.1. The Pufferfish, Chelonodon patoca
2.4.2. The Toxic Goby, Yongeichthys criniger
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. TTX-Bearing Fish
5.2. LC–MS/MS Analysis
5.3. Planocera multitentaculata-Specific PCR Analysis and Sequencing Analysis
5.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noguchi, T.; Ebesu, J.S.M. Puffer poisoning: Epidemiology and treatment. Toxin Rev. 2001, 20, 1–10. [Google Scholar] [CrossRef]
- Tahara, Y. Studies on the pufferfish toxin. J. Pharm. Soc. Jpn. 1909, 29, 587–625. [Google Scholar]
- Tsuda, K.; Ikuma, S.; Kawamura, M.; Tachikawa, R.; Sakai, K.; Tamura, C.; Amakasu, D. Tetrodotoxin. VII. On the structures of tetrodotoxin and its derivatives. Chem. Pharm. Bull. 1964, 12, 1357–1374. [Google Scholar] [CrossRef] [Green Version]
- Woodward, R.B. The structure of tetrodotoxin. Pure Appl. Chem. 1964, 9, 49–74. [Google Scholar] [CrossRef]
- Goto, T.; Kishi, Y.; Takahashi, S.; Hirata, Y. Tetrodotoxin. Tetrahedron 1965, 21, 2059–2088. [Google Scholar] [CrossRef]
- Mosher, H.S.; Fuhrman, G.J.; Fuhrman, F.A.; Fischer, H.G. Tarichatoxin-tetrodotoxin, a potent neurotoxin. Science 1964, 144, 1100–1110. [Google Scholar] [CrossRef]
- Noguchi, T.; Arakawa, O.; Takatani, T. TTX accumulation in pufferfish. Comp. Biochem. Physiol. D 2006, 1, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Arakawa, O. Tetrodotoxin—Distribution and accumulation in aquatic organisms, and cases of human intoxication. Mar. Drugs 2008, 6, 220–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katikou, P.; Gokbulut, C.; Kosker, A.R.; Campàs, M.; Ozogul, F. An updated review of tetrodotoxin and its peculiarities. Mar. Drugs 2022, 20, 47. [Google Scholar] [CrossRef]
- Magarlamov, T.Y.; Melnikova, D.I.; Chernyshev, A.V. Tetrodotoxin-producing bacteria: Detection, distribution and migration of the toxin in aquatic systems. Toxins 2017, 9, 166. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, L.; Xia, G.; Zhang, J.; Nie, Y.; Hu, J.; Wang, S.; Zhang, R. A new tetrodotoxin-producing actinomycete, Nocardiopsis dassonvillei, isolated from the ovaries of puffer fish Fugu rubripes. Toxicon 2005, 45, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Magarlamov, T.Y.; Beleneva, I.A.; Chernyshev, A.V.; Kuhlevsky, A.D. Tetrodotoxin-producing Bacillus sp. from the ribbon worm (Nemertea) Cephalothrix simula (Iwata, 1952). Toxicon 2014, 85, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Arakawa, O.; Takatani, T.; Tachibana, K.; Yagi, M.; Tanigawa, A.; Noguchi, T. Toxification of cultured puffer fish Takifugu rubripes by feeding on tetrodotoxin-containing diet. Nippon Suisan Gakkaishi 2005, 71, 815–820. [Google Scholar] [CrossRef] [Green Version]
- Itoi, S.; Kozaki, A.; Komori, K.; Tsunashima, T.; Noguchi, S.; Kawane, M.; Sugita, H. Toxic Takifugu pardalis eggs found in Takifugu niphobles gut: Implications for TTX accumulation in the pufferfish. Toxicon 2015, 108, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Food Safety Commission of Japan. The liver of the aquacultured Japanese pufferfish (natural toxins): Summary. Food Saf. 2017, 5, 169–170. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.J.; Yu, R.C.; Luo, X.; Zhou, M.J.; Lin, X.T. Toxin-screening and identification of bacteria isolated from highly toxic marine gastropod Nassarius semiplicatus. Toxicon 2008, 52, 55–61. [Google Scholar] [CrossRef]
- Yang, G.; Xu, J.; Liang, S.; Ren, D.; Yan, X.; Bao, B. A novel TTX-producing Aeromonas isolated from the ovary of Takifugu obscurus. Toxicon 2010, 56, 324–329. [Google Scholar] [CrossRef]
- Kajihara, H.; Sun, S.-C.; Chernyshev, A.V.; Chen, H.-X.; Ito, K.; Asakawa, M.; Maslakova, S.A.; Norenburg, J.L.; Strand, M.; Sundberg, P.; et al. Taxonomic identity of a tetrodotoxin-accumulating ribbon-worm Cephalothrix simula (Nemertea: Palaeonemertea): A species artificially introduced from the Pacific to Europe. Zool. Sci. 2013, 30, 985–997. [Google Scholar] [CrossRef]
- Itoi, S.; Ueda, H.; Yamada, R.; Takei, M.; Sato, T.; Oshikiri, S.; Wajima, Y.; Ogata, R.; Oyama, H.; Shitto, T.; et al. Including planocerid flatworms in the diet effectively toxifies the pufferfish, Takifugu niphobles. Sci. Rep. 2018, 8, 12302. [Google Scholar] [CrossRef]
- Okabe, T.; Oyama, H.; Kashitani, M.; Ishimaru, Y.; Suo, R.; Sugita, H.; Itoi, S. Toxic flatworm egg plates serve as a possible source of tetrodotoxin for pufferfish. Toxins 2019, 11, 402. [Google Scholar] [CrossRef] [Green Version]
- Itoi, S.; Sato, T.; Takei, M.; Yamada, R.; Ogata, R.; Oyama, H.; Teranishi, S.; Kishiki, A.; Wada, T.; Noguchi, K.; et al. The planocerid flatworm is a main supplier of toxin to tetrodotoxin-bearing fish juveniles. Chemosphere 2020, 249, 126217. [Google Scholar] [CrossRef]
- Okabe, T.; Saito, R.; Yamamoto, K.; Watanabe, R.; Kaneko, Y.; Yanaoka, M.; Furukoshi, S.; Yasukawa, S.; Ito, M.; Oyama, H.; et al. The role of toxic planocerid flatworm larvae on tetrodotoxin accumulation in marine bivalves. Aquat. Toxicol. 2021, 237, 105908. [Google Scholar] [CrossRef]
- Kato, K. Polycladida of Japan. J. Sigenkagaku Kenkyusyo 1944, 1, 257–318. [Google Scholar]
- Teshirogi, W.; Ishida, S.; Jatani, K. On the early development of some Japanese polyclads. Rep. Fukaura Mar. Biol. Lab. Hirosaki Univ. 1981, 9, 2–31. [Google Scholar]
- Asano, Y.; Yoshida, A.; Isozaki, N.; Ishida, S. Production of intestine-specific monoclonal antibody and interspecific cross-reaction in triclads and polyclads. Belg. J. Zool. 2001, 131 (Suppl. 1), 137–141. [Google Scholar]
- Ueda, H.; Itoi, S.; Sugita, H. TTX-bearing planocerid flatworm (Platyhelminthes: Acotylea) in the Ryukyu Islands, Japan. Mar. Drugs 2018, 16, 37. [Google Scholar] [CrossRef] [Green Version]
- Kashitani, M.; Okabe, T.; Oyama, H.; Noguchi, K.; Yamazaki, H.; Suo, R.; Mori, T.; Sugita, H.; Itoi, S. Taxonomic distribution of tetrodotoxin in acotylean flatworms (Polycladida: Platyhelminthes). Mar. Biotechnol. 2020, 22, 805–811. [Google Scholar] [CrossRef]
- Suo, R.; Kashitani, M.; Oyama, H.; Adachi, M.; Nakahigashi, R.; Sakakibara, R.; Nishikawa, T.; Sugita, H.; Itoi, S. First detection of tetrodotoxins in the cotylean flatworm Prosthiostomum trilineatum. Mar. Drugs 2021, 19, 40. [Google Scholar] [CrossRef]
- Suo, R.; Tanaka, M.; Oyama, H.; Kojima, Y.; Adachi, M.; Nishikawa, T.; Sugita, H.; Itoi, S. Tetrodotoxins in the flatworm Planocera multitentaculata; Nihon University Department of Marine Science and Resources: Fujisawa, Kanagawa, Japan, 2022; manuscript in preparation. [Google Scholar]
- Noguchi, T.; Kao, H.; Hashimoto, Y. Toxicity of the goby, Gobius criniger. Bull. Jap. Soc. Sci. Fish. 1971, 37, 642–647. [Google Scholar] [CrossRef] [Green Version]
- Matsui, T.; Hamada, S.; Yamamori, K. Local variation of toxicity of the puffer fish Fugu niphobles. Bull. Jpn. Soc. Sci. Fish. 1982, 48, 1179. [Google Scholar] [CrossRef] [Green Version]
- Kanoh, S.; Noguchi, T.; Kamimura, S.; Hashimoto, K. A survey of toxicity of the pufferfish, Fugu pardalis, inhabiting the Sanriku Coast. J. Food Hyg. Soc. Japan 1984, 25, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Saito, T.; Sugiura, T. Studies on toxicity of a goby, Yongeichthys criniger, from Iriomote Island. Bull. Inst. Ocean. Res. Develop. Tokai Univ. 1997, 18, 35–41. [Google Scholar]
- Salvitti, L.; Wood, S.A.; Taylor, D.I.; McNabb, P.; Cary, S.C. First identification of tetrodotoxin (TTX) in the flatworm Stylochoplana sp.; a source of TTX for the sea slug Pleurobranchaea maculata. Toxicon 2015, 95, 23–29. [Google Scholar] [CrossRef]
- Hanifin, C.T.; Brodie, E.D., Jr.; Brodie, E.D., III. Phenotypic mismatches reveal escape from arms-race coevolution. PLoS Biol. 2008, 6, e60. [Google Scholar] [CrossRef]
- Gall, B.G.; Stokes, A.N.; Pett, J.J.; Spivey, K.L.; French, S.S.; Brodie, E.D., III; Brodie, E.D., Jr. Tetrodotoxin concentrations within a clutch and across embryonic development in eggs of the rough-skinned newts (Taricha granulosa). Toxicon 2014, 90, 249–254. [Google Scholar] [CrossRef]
- Mebs, D.; Yotsu-Yamashita, M. Acquiring toxicity of a newt, Cynops orientalis. Toxicon 2021, 198, 32–35. [Google Scholar] [CrossRef]
- Kudo, Y.; Yamashita, Y.; Mebs, D.; Cho, Y.; Konoki, K.; Yasumoto, T.; Yotsu-Yamashita, M. C5-C10 directly bonded tetrodotoxin analogues: Possible biosynthetic precursors of tetrodotoxin from newts. Angew. Chem. Int. Ed. Engl. 2014, 53, 14546–14549. [Google Scholar] [CrossRef]
- Ueyama, N.; Sugimoto, K.; Kudo, Y.; Onodera, K.I.; Cho, Y.; Konoki, K.; Nishikawa, T.; Yotsu-Yamashita, M. Spiro bicyclic guanidino compounds from pufferfish: Possible biosynthetic intermediates of tetrodotoxin in marine environments. Chemistry 2018, 24, 7250–7258. [Google Scholar] [CrossRef]
- Yamamori, K.; Kono, M.; Furukawa, K.; Matsui, T. The toxification of juvenile cultured kusafugu Takifugu niphobles by oral administration of crystalline tetrodotoxin. J. Food Hyg. Soc. Jpn. 2004, 45, 73–75. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.; Yotsu-Yamashita, M. Distribution of tetrodotoxin, saxitoxin, and their analogs among tissues of the puffer fish Fugu pardalis. Toxicon 2006, 48, 980–987. [Google Scholar] [CrossRef]
- Rodriguez, P.; Alfonso, A.; Vale, C.; Alfonso, C.; Vale, P.; Tellez, A.; Botana, L.M. First toxicity report of tetrodotoxin and 5,6,11-trideoxyTTX in the trumpet shell Charonia lampas lampas in Europe. Anal. Chem. 2008, 80, 5622–5629. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, J.S.; Yotsu-Yamashita, M. LC/MS analysis of tetrodotoxin and its deoxy analogs in the marine puffer fish Fugu niphobles from the southern coast of Korea, and in the brackishwater puffer fishes Tetraodon nigroviridis and Tetraodon biocellatus from Southeast Asia. Mar. Drugs 2010, 8, 1049–1058. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Abe, Y.; Kudo, Y.; Ritson-Williams, R.; Paul, V.J.; Konoki, K.; Cho, Y.; Adachi, M.; Imazu, T.; Nishikawa, T.; et al. First identification of 5,11-dideoxytetrodotoxin in marine animals, and characterization of major fragment ions of tetrodotoxin and its analogs by high resolution ESI-MS/MS. Mar. Drugs 2013, 11, 2799–2813. [Google Scholar] [CrossRef] [Green Version]
- Vlasenko, A.E.; Kuznetsov, V.G.; Malykin, G.V.; Pereverzeva, A.O.; Velansky, P.V.; Yakovlev, K.V.; Magarlamov, T.Y. Tetrodotoxins secretion and voltage-gated sodium channel adaptation in the ribbon worm Kulikovia alborostrata (Takakura, 1898) (Nemertea). Toxins 2021, 13, 606. [Google Scholar] [CrossRef]
- Asakawa, M.; Ito, M.; Kajihara, H. Highly toxic ribbon worm Cephalothrix simula containing tetrodotoxin in Hiroshima Bay, Hiroshima Prefecture, Japan. Toxins 2013, 5, 376–395. [Google Scholar] [CrossRef]
- Kudo, Y.; Chiba, C.; Konoki, K.; Cho, Y.; Yotsu-Yamashita, M. Dietary administration of tetrodotoxin and its putative biosynthetic intermediates to the captive-reared non-toxic Japanese fire-bellied newt, Cynops pyrrhogaster. Toxicon 2017, 137, 78–82. [Google Scholar] [CrossRef]
- Vlasenko, A.E.; Magarlamov, T.Y. Tetrodotoxin and its analogues in Cephalothrix cf. simula (Nemertea: Palaeonemertea) from the Sea of Japan (Peter the Great Gulf): Intrabody distribution and secretions. Toxins 2020, 12, 745. [Google Scholar] [CrossRef]
- Malykin, G.V.; Chernyshev, A.V.; Magarlamov, T.Y. Intrabody tetrodotoxin distribution and possible hypothesis for its migration in ribbon worms Cephalothrix cf. simula (Palaeonemertea, Nemertea). Mar. Drugs 2021, 19, 494. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Nagaoka, Y.; Muramoto, K.; Cho, Y.; Konoki, K. Pufferfish saxitoxin and tetrodotoxin binding protein (PSTBP) analogues in the blood plasma of the pufferfish Arothron nigropunctatus, A. hispidus, A. manilensis, and Chelonodon patoca. Mar. Drugs 2018, 16, 224. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, T.; Nagashima, Y.; Kusuhara, H.; Sugiyama, Y.; Ishizaki, S.; Shimakura, K.; Shiomi, K. Involvement of carrier-mediated transport system in uptake of tetrodotoxin into liver tissue slices of puffer fish Takifugu rubripes. Toxicon 2007, 50, 173–179. [Google Scholar] [CrossRef]
- Adachi, M.; Sakakibara, R.; Satake, Y.; Isobe, M.; Nishikawa, T. Synthesis of 5,6,11-trideoxytetrodotoxin. Chem. Lett. 2014, 43, 1719–1721. [Google Scholar] [CrossRef]
- Palumbi, S.R.; Martin, A.; Romano, S.; McMillan, W.O.; Stice, L.; Grabowski, G. The Simple Fool’s Guide to PCR, Version 2.0; Department of Zoology and Kewalo Marine Laboratory, University of Hawaii: Honolulu, HI, USA, 1991. [Google Scholar]
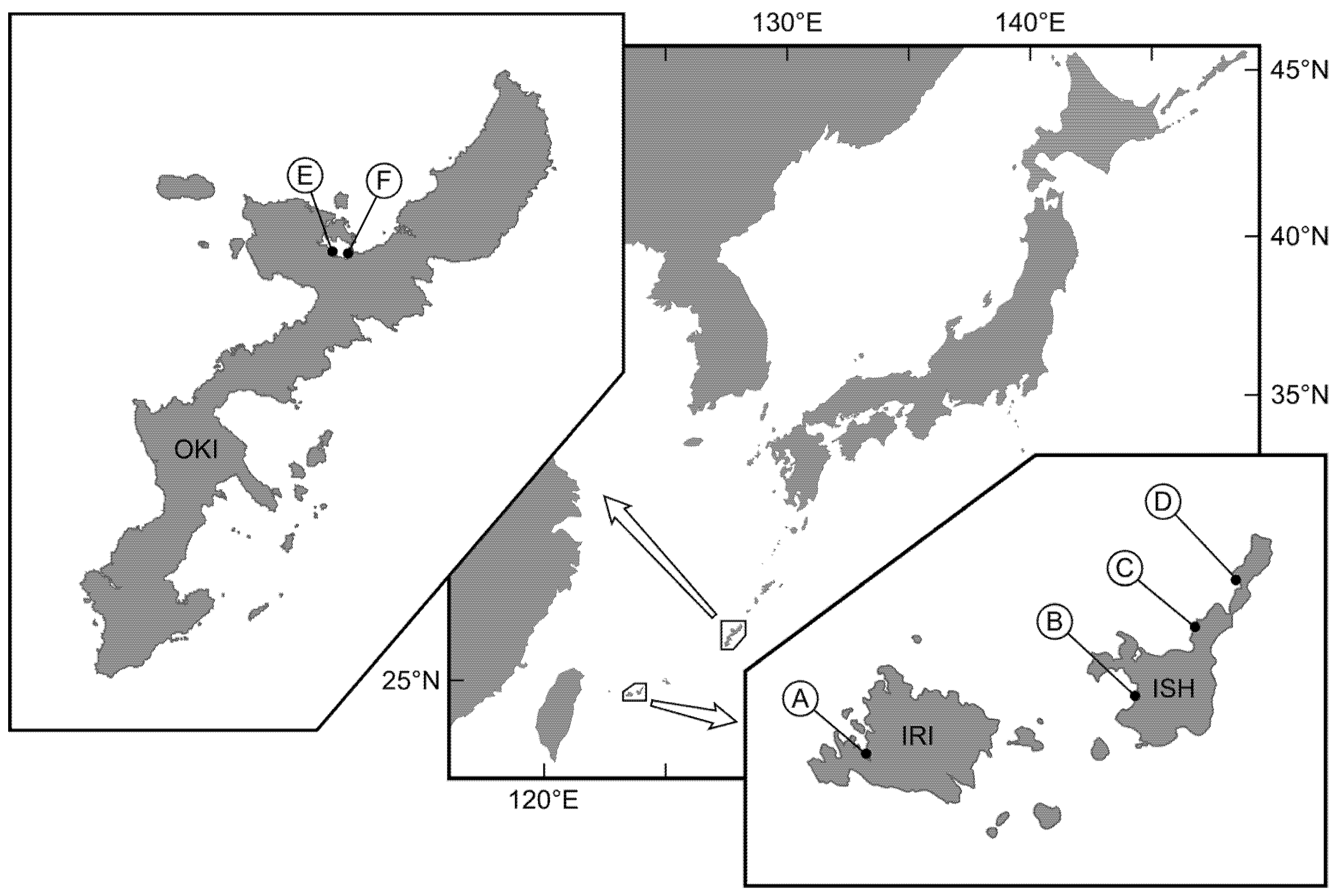




| Species | Year | Month | Locality | BW (g) | TTX Concentration (μg/g) | TDT Concentration (μg/g) | TTX/TDT Ratio | Sequence of Toxic Flatworm (%) |
|---|---|---|---|---|---|---|---|---|
| Chelonodon patoca | 2019 | June | IRI (A) *1 | 0.99 ± 0.48 | 51.4 ± 16.1 | 25.0 ± 24.4 | 3.4 ± 1.4 | 0 |
| ISI (B) *2 | 0.94 ± 0.32 | 30.9 ± 7.4 | 6.3 ± 2.3 | 5.6 ± 2.3 | 0 | |||
| OKI (F) *3 | 0.12 ± 0.03 | 60.4 ± 10.0 | 37.0 ± 12.0 | 1.8 ± 0.6 | 10 | |||
| 2020 | July | OKI (E) | 1.13 ± 0.97 | 6.0 ± 2.2 | 2.5 ± 1.0 | 2.4 ± 0.5 | 70 | |
| OKI (F) *4 | 0.70 ± 0.58 | 5.2 ± 2.0 | 1.8 ± 0.8 | 3.1 ± 1.3 | 90 | |||
| 2021 | May | ISI (B) *5 | 0.17 ± 0.08 | 59.8 ± 9.4 | 16.3 ± 4.3 | 3.8 ± 0.7 | 0 | |
| June | ISI (B) *6 | 0.48 ± 0.15 | 9.8 ± 1.9 | 2.2 ± 0.3 | 4.6 ± 1.2 | 0 | ||
| Yongeichthys criniger | 2019 | June | IRI (A) *1 | 0.82 ± 0.30 | 218.7 ± 137.5 | 270.7 ± 132.2 | 0.8 ± 0.2 | 40 |
| ISI (B) *2 | 0.59 ± 0.26 | 57.1 ± 16.1 | 83.9 ± 40.7 | 0.7 ± 0.2 | 60 | |||
| ISI (C) | 0.79 ± 0.31 | 50.8 ± 35.0 | 33.6 ± 14.6 | 1.7 ± 0.8 | 40 | |||
| OKI (F) *3 | 0.70 ± 0.10 | 226.5 ± 95.4 | 427.0 ± 56.3 | 0.5 ± 0.2 | 0 | |||
| 2020 | July | OKI (F) *4 | 1.35 ± 0.48 | 99.5 ± 40.3 | 153.5 ± 54.0 | 0.6 ± 0.1 | 70 | |
| 2021 | May | ISI (B) *5 | 0.34 ± 0.09 | 98.6 ± 54.7 | 206.0 ± 52.3 | 0.5 ± 0.2 | 0 | |
| ISI (D) | 0.52 ± 0.24 | 185.0 ± 29.7 | 109.4 ± 38.3 | 1.9 ± 0.6 | 0 | |||
| June | ISI (B) *6 | 0.61 ± 0.23 | 40.6 ± 7.6 | 64.1 ± 27.0 | 0.7 ± 0.2 | 0 | ||
| ISI (C) | 0.60 ± 0.19 | 107.9 ± 33.1 | 104.3 ± 36.3 | 1.1 ± 0.3 | 0 | |||
| ISI (D) | 0.96 ± 0.21 | 192.5 ± 39.1 | 107.4 ± 20.3 | 1.8 ± 0.2 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, M.; Furukawa, R.; Yasukawa, S.; Sato, M.; Oyama, H.; Okabe, T.; Suo, R.; Sugita, H.; Takatani, T.; Arakawa, O.; et al. Local Differences in the Toxin Amount and Composition of Tetrodotoxin and Related Compounds in Pufferfish (Chelonodon patoca) and Toxic Goby (Yongeichthys criniger) Juveniles. Toxins 2022, 14, 150. https://doi.org/10.3390/toxins14020150
Ito M, Furukawa R, Yasukawa S, Sato M, Oyama H, Okabe T, Suo R, Sugita H, Takatani T, Arakawa O, et al. Local Differences in the Toxin Amount and Composition of Tetrodotoxin and Related Compounds in Pufferfish (Chelonodon patoca) and Toxic Goby (Yongeichthys criniger) Juveniles. Toxins. 2022; 14(2):150. https://doi.org/10.3390/toxins14020150
Chicago/Turabian StyleIto, Masaaki, Risako Furukawa, Shino Yasukawa, Masaya Sato, Hikaru Oyama, Taiki Okabe, Rei Suo, Haruo Sugita, Tomohiro Takatani, Osamu Arakawa, and et al. 2022. "Local Differences in the Toxin Amount and Composition of Tetrodotoxin and Related Compounds in Pufferfish (Chelonodon patoca) and Toxic Goby (Yongeichthys criniger) Juveniles" Toxins 14, no. 2: 150. https://doi.org/10.3390/toxins14020150
APA StyleIto, M., Furukawa, R., Yasukawa, S., Sato, M., Oyama, H., Okabe, T., Suo, R., Sugita, H., Takatani, T., Arakawa, O., Adachi, M., Nishikawa, T., & Itoi, S. (2022). Local Differences in the Toxin Amount and Composition of Tetrodotoxin and Related Compounds in Pufferfish (Chelonodon patoca) and Toxic Goby (Yongeichthys criniger) Juveniles. Toxins, 14(2), 150. https://doi.org/10.3390/toxins14020150





