Fungal Naphthalenones; Promising Metabolites for Drug Discovery: Structures, Biosynthesis, Sources, and Pharmacological Potential
Abstract
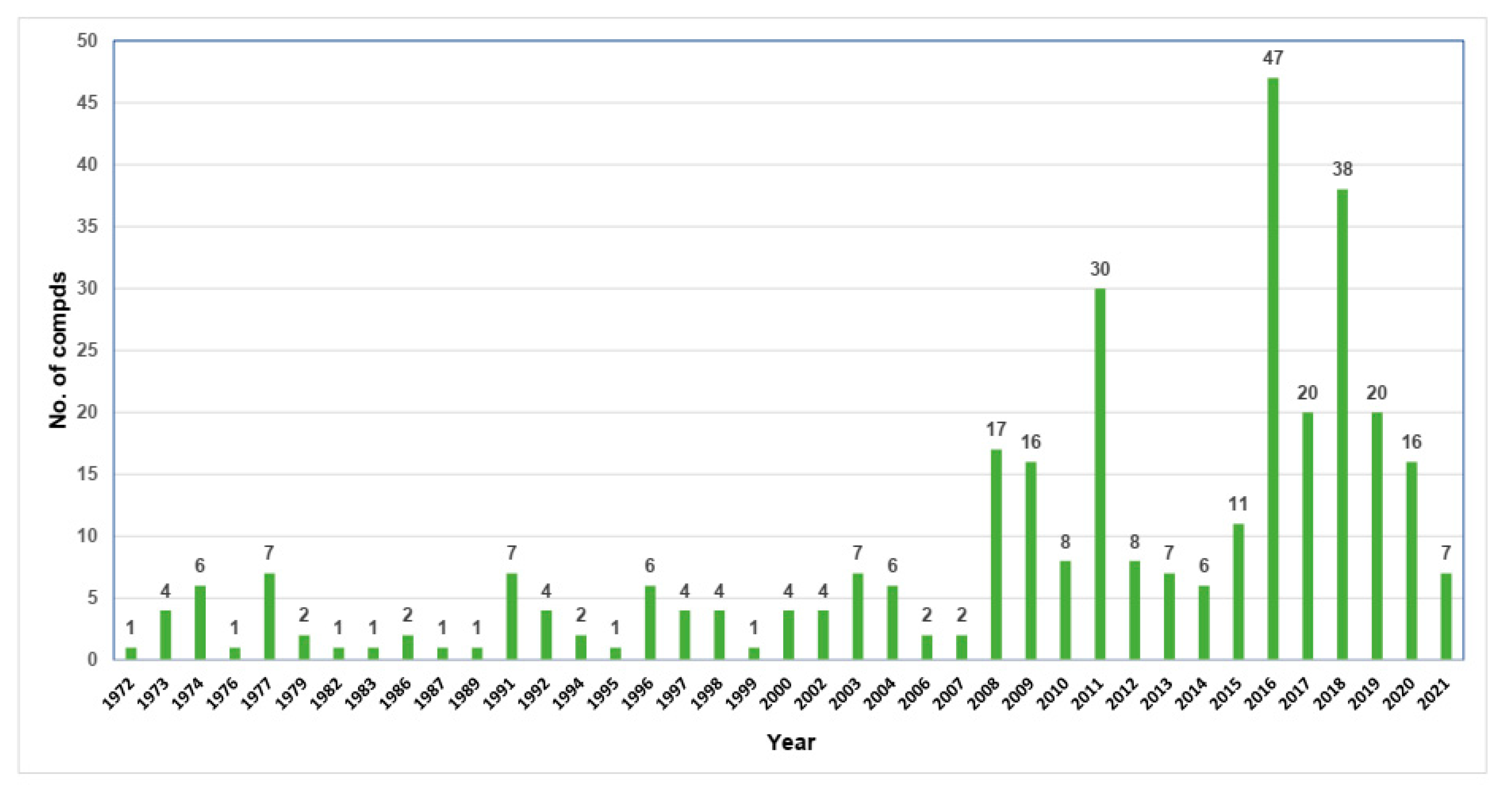
:1. Introduction
Biosynthesis of Naphthalenones
2. Bioactivities of Naphthalenones
2.1. Phytotoxic and Nematocidal Activities
2.2. Antimicrobial, Antimycobacterial, and Anti-Plasmodial Activities
2.3. Cytotoxic Activity
2.4. Antioxidant Activity
2.5. Serotonin Antagonistic Activity
2.6. Antiviral Activity
2.7. Melanin Synthesis Inhibitory Activity
2.8. Enzymes Inhibitory Activity
2.9. Anti-Inflammatory Activity
2.10. Neuroprotective Activity
2.11. Other Activities
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hawksworth, D.L.; Lücking, R. Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Altyar, A.E.; Mohamed, S.G.A.; Mohamed, G.A. Genus Thielavia: Phytochemicals, industrial importance and biological relevance. Nat. Prod. Res. 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Mohamed, S.G.A.; Sindi, I.A.; Mohamed, G.A. Biologically active secondary metabolites and biotechnological applications of species of the family Chaetomiaceae (Sordariales): An updated review from 2016 to 2021. Mycol. Prog. 2021, 20, 595–639. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, S.G.A.; Altyar, A.E.; Mohamed, G.A. Natural Products of the Fungal Genus Humicola: Diversity, Biological Activity, and Industrial Importance. Curr. Microbiol. 2021, 78, 2488–2509. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Sirwi, A.; Eid, B.G.; Mohamed, S.G.A.; Mohamed, G.A. Bright Side of Fusarium oxysporum: Secondary Metabolites Bioactivities and Industrial Relevance in Biotechnology and Nanotechnology. J. Fungi 2021, 7, 943. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Mohamed, G.A.; Kamal, H.M.; Mohamed, S.G.; Khedr, A.I. Terretonins from Aspergillus Genus: Structures, Biosynthesis, Bioactivities, and Structural Elucidation. Mini-Rev. Org. Chem. 2022, 19, 257–269. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R.M. Untapped potential of marine-associated Cladosporium species: An overview on secondary metabolites, biotechnological relevance, and biological activities. Mar. Drugs 2021, 19, 645. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K. The role of fungus in bioactive compound production and nanotechnology. In Role of Plant Growth Promoting Microorganisms in Sustainable Agriculture and Nanotechnology; Woodhead Publishing: Cambridge, UK, 2019; p. 145. [Google Scholar]
- Ibrahim, S.R.; Mohamed, G.A. Naphthylisoquinoline alkaloids potential drug leads. Fitoterapia 2015, 106, 194–225. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Ibrahim, S.R.M.; El Agamy, D.S.; Elsaed, W.M.; Sirwi, A.; Asfour, H.Z.; Koshak, A.E.; Elhady, S.S. Terretonin As A new protective agent against sepsis-induced qcute lung injury: Impact on SIRT1/Nrf2/NF-κBp65/NLRP3 signaling. Biology 2021, 10, 1219. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Elkhayat, E.; Mohamed, G.A.A.; Fat’Hi, S.M.; Ross, S.A. Fusarithioamide A, a new antimicrobial and cytotoxic benzamide derivative from the endophytic fungus Fusarium chlamydosporium. Biochem. Biophys. Res. Commun. 2016, 479, 211–216. [Google Scholar] [CrossRef]
- Ibrahim, P.S.R.; Mohamed, G.; Ahmed, H. Aegyoxepane: A New Oxepane Derivative from the Fungus Aspergillus aegyptiacus. Lett. Org. Chem. 2016, 13, 560–565. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.R.M.; Mohamed, G.A. Naturally occurring naphthalenes: Chemistry, biosynthesis, structural elucidation, and biological activities. Phytochem. Rev. 2016, 15, 279–295. [Google Scholar] [CrossRef]
- Ibrahim, P.S.R.; Mohamed, G.A.; Ross, S. Integracides F and G: New tetracyclic triterpenoids from the endophytic fungus Fusarium sp. Phytochem. Lett. 2016, 15, 125–130. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Abdallah, H.M.; Mohamed, G.A.; Ross, S.A. Integracides H-J: New tetracyclic triterpenoids from the endophytic fungus Fusarium sp. Fitoterapia 2016, 112, 161–167. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; Khedr, A.M.I. γ-Butyrolactones from Aspergillus species: Structures, biosynthesis, and biological activities. Nat. Prod. Commun. 2017, 12, 791–800. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.R.; Mohamed, G.A.; Al Haidari, R.A.; El-Kholy, A.A.; Zayed, M.F.; Khayat, M.T. Biologically active fungal depsidones: Chemistry, biosynthesis, structural characterization, and bioactivities. Fitoterapia 2018, 129, 317–365. [Google Scholar] [CrossRef]
- Ibrahim, S.R.; Mohamed, G.A.; Al Haidari, R.; Zayed, M.; El-Kholy, A.A.; Elkhayat, E.; Ross, S.A. Fusarithioamide B, a new benzamide derivative from the endophytic fungus Fusarium chlamydosporium with potent cytotoxic and antimicrobial activities. Bioorg. Med. Chem. 2018, 26, 786–790. [Google Scholar] [CrossRef]
- Ibrahim, P.S.R.; Abdallah, H.; Elkhayat, E.; Al Musayeib, N.M.; Asfour, H.Z.; Zayed, M.; Mohamed, G.A. Fusaripeptide A: New antifungal and anti-malarial cyclodepsipeptide from the endophytic fungus Fusarium sp. J. Asian Nat. Prod. Res. 2017, 20, 75–85. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Sirwi, A.; Eid, B.G.; Mohamed, S.G.A.; Mohamed, G.A. Fungal depsides naturally inspiring molecules: Biosynthesis, structural characterization, and biological activities. Metabolites 2021, 11, 683. [Google Scholar] [CrossRef]
- Barnes, E.C.; Jumpathong, J.; Lumyong, S.; Voigt, P.-D.D.K.; Hertweck, C. Daldionin, an Unprecedented Binaphthyl Derivative, and Diverse Polyketide Congeners from a Fungal Orchid Endophyte. Chem. A Eur. J. 2016, 22, 4551–4555. [Google Scholar] [CrossRef]
- Xu, D.; Xue, M.; Shen, Z.; Jia, X.; Hou, X.; Lai, D.; Zhou, L. Phytotoxic Secondary Metabolites from Fungi. Toxins 2021, 13, 261. [Google Scholar] [CrossRef]
- Andolfi, A.; Mugnai, L.; Luque, J.; Surico, G.; Cimmino, A.; Evidente, A. Phytotoxins Produced by Fungi Associated with Grapevine Trunk Diseases. Toxins 2011, 3, 1569–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langfelder, K.; Streibel, M.; Jahn, B.; Haase, G.; Brakhage, A.A. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet. Biol. 2003, 38, 143–158. [Google Scholar] [CrossRef]
- Watanabe, A.; Fujii, I.; Tsai, H.; Chang, Y.C.; Kwon-Chung, K.J.; Ebizuka, Y. Aspergillus fumigatus alb1 encodes naphthopyrone synthase when expressed in Aspergillus oryzae. FEMS Microbiol. Lett. 2000, 192, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.H.; Stipanovic, R.D. Melanin biosynthesis and the metabolism of flaviolin and 2-hydroxyjuglone inWangiella dermatitidis. Arch. Microbiol. 1985, 142, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Zhang, J.; Jiang, N.; Lu, Y.H.; Wang, L.; Xu, S.H.; Wang, W.; Zhang, G.F.; Xu, Q.; Ge, H.M.; et al. Immunosuppressive Polyketides from Mantis-AssociatedDaldinia eschscholzii. J. Am. Chem. Soc. 2011, 133, 5931–5940. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.G.; Wang, X.B.; Xu, Y.M.; U’Ren, J.M.; Arnold, A.E.; Kong, L.Y.; Gunatilaka, A.A. Delitschiapyrone A, a pyrone-naphthalenone adduct bearing a new pentacyclic ring system from the leaf-associated fungus Delitschia sp. FL1581. Org. Lett. 2014, 16, 5944–5947. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, L.-Q.; Long, H.-P.; Liu, J.; Jiang, Y.-P.; Xue, Y.; Wang, W.-X.; Tan, G.-S.; Gong, Z.-C.; Liu, J.-K. Xylarinaps A–E, five pairs of naphthalenone derivatives with neuroprotective activities from Xylaria nigripes. Phytochemistry 2021, 186, 112729. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Delavault, P.; Timko, M.P. Management of Infection by Parasitic Weeds: A Review. Plants 2020, 9, 1184. [Google Scholar] [CrossRef]
- Macías-Rubalcava, M.L.; Garrido-Santos, M.Y. Phytotoxic compounds from endophytic fungi. Appl. Microbiol. Biotechnol. 2022, 106, 931–950. [Google Scholar] [CrossRef]
- Iwasaki, S.; Muro, H.; Nozoe, S.; Okuda, S.; Sato, Z. Isolation of 3,4-dihydro-3,4,8-trihydroxy-1(2H)-naphthalenone and tenuazonic acid from Pyricularia oryzae cavara. Tetrahedron Lett. 1972, 13, 13–16. [Google Scholar] [CrossRef]
- Masi, M.; Meyer, S.; Górecki, M.; Mandoli, A.; Di Bari, L.; Pescitelli, G.; Cimmino, A.; Cristofaro, M.; Clement, S.; Evidente, A. Pyriclins A and B, two monosubstituted hex-4-ene-2,3-diols and other phytotoxic metabolites produced by Pyricularia grisea isolated from buffelgrass (Cenchrus ciliaris). Chirality 2017, 29, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Semar, M.; Anke, H.; Arendholz, W.-R.; Veiten, R.; Steglich, W. Lachnellins A, B, C, D, and Naphthalene-l,3,8-triol, Biologically Active Compounds from a Lachnellula Species (Ascomycetes). Z. Naturforsch. C J. Biosci. 1996, 51, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Ayer, W.A.; Trifonov, L.S.; Hutchison, L.J.; Chakravarty, P. Metabolites from a Wood-Inhabiting Cup Fungus, Urnula craterium. Nat. Prod. Lett. 2000, 14, 405–410. [Google Scholar] [CrossRef]
- Morita, T.; Aoki, H. Isosclerone, a New Metabolite of Sclerotinia sclerotiorum (LIB.) DE BARY. Agric. Biol. Chem. 1974, 38, 1501–1505. [Google Scholar] [CrossRef]
- Venkatasubbaiah, P.; Chilton, W.S. Phytotoxins produced by Tubakia dryina. Mycopathologia 1992, 120, 33–37. [Google Scholar] [CrossRef]
- Bürki, N.; Michel, A.; Tabacchi, R. Naphthalenones and isocoumarins of the fungus Ceratocystis fimbriata f. sp. platani. Mediterranna 2003, 14, 1–55. [Google Scholar] [CrossRef]
- Stierle, A.A.; Upadhyay, R.; Hershenhorn, J.; Strobel, G.A.; Molina, G. The phytotoxins ofMycosphaerella fijiensis, the causative agent of Black Sigatoka disease of bananas and plantains. Experientia 1991, 47, 853–859. [Google Scholar] [CrossRef]
- Gremaud, G.; Tabacchi, R. Relationship between the fungus Ceratocystis fimbriata coffea and the canker disease of the coffee tree. Phytochemistry 1996, 42, 1547–1549. [Google Scholar] [CrossRef]
- Evidente, A.; Sparapano, L.; Andolfi, A.; Bruno, G. Two naphthalenone pentaketides from liquid cultures of Phaeoacremonium aleophilum, a fungus associated with esca of grapevine. Phytopathol. Mediterr. 2000, 39, 162–168. [Google Scholar]
- Nakamura, T.; Supratman, U.; Harneti, D.; Maharani, R.; Koseki, T.; Shiono, Y. New compounds from Japanese oak wilt disease-associated fungus Raffaelea quercivora. Nat. Prod. Res. 2020, 35, 5304–5310. [Google Scholar] [CrossRef] [PubMed]
- Shan, R.; Stadler, M.; Anke, H.; Sterner, O. Naphthalenone and Phthalide Metabolites from Lachnum papyraceum. J. Nat. Prod. 1997, 60, 804–805. [Google Scholar] [CrossRef]
- Cimmino, A.; Villegas-Fernández, A.M.; Andolfi, A.; Melck, D.; Rubiales, D.; Evidente, A. Botrytone, a New Naphthalenone Pentaketide Produced by Botrytis fabae, the Causal Agent of Chocolate Spot Disease on Vicia faba. J. Agric. Food Chem. 2011, 59, 9201–9206. [Google Scholar] [CrossRef] [PubMed]
- Burruano, S.; Giambra, S.; Mondello, V.; Dellagreca, M.; Basso, S.; Tuzi, A.; Andolfi, A. Naphthalenone polyketides produced by Neofusicoccum parvum, a fungus associated with grapevine Botryosphaeria dieback. Phytopathol. Mediterr. 2016, 55, 197–206. [Google Scholar]
- Masi, M.; Nocera, P.; Zonno, M.C.; Tuzi, A.; Pescitelli, G.; Cimmino, A.; Boari, A.; Infantino, A.; Vurro, M.; Evidente, A. Lentiquinones A, B, and C, Phytotoxic Anthraquinone Derivatives Isolated from Ascochyta lentis, a Pathogen of Lentil. J. Nat. Prod. 2018, 81, 2700–2709. [Google Scholar] [CrossRef]
- Otomo, N.; Sato, H.; Sakamura, S. Novel phytotoxins produced by the causal fungus of the shoot blight of larches. Agric. Biol. Chem. 1983, 47, 1115–1119. [Google Scholar]
- Abad, P.; Gouzy, J.; Aury, J.M.; Castagnone-Sereno, P.; Danchin, E.G.J.; Deleury, E.; Perfus-Barbeoch, L.; Anthouard, V.; Artiguenave, F.; Blok, V.C.; et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Song, B. Natural nematicidal active compounds: Recent research progress and outlook. J. Integrat. Agricul. 2021, 20, 2015–2031. [Google Scholar]
- Dong, J.Y.; Song, H.C.; Li, J.H.; Tang, Y.S.; Sun, R.; Wang, L.; Zhou, Y.P.; Wang, L.M.; Shen, K.Z.; Wang, C.R.; et al. Ymf 1029A−E, Preussomerin Analogues from the Fresh-Water-Derived Fungus YMF 1.01029. J. Nat. Prod. 2008, 71, 952–956. [Google Scholar] [CrossRef]
- Zhu, Y.; Dong, J.; Wang, L.; Zhou, W.; Li, L.; He, H.; Liu, H.; Zhang, K. Screening and isolation of antinematodal metabolites againstBursaphelenchus xylophilus produced by fungi. Ann. Microbiol. 2008, 58, 375–380. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fair, R.J.; Tor, Y. Antibiotics and Bacterial Resistance in the 21st Century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inácio, M.L.; Silva, G.H.; Teles, H.L.; Trevisan, H.C.; Cavalheiro, A.J.; Bolzani, V.D.S.; Young, M.C.; Pfenning, L.H.; Araújo, R. Antifungal metabolites from Colletotrichum gloeosporioides, an endophytic fungus in Cryptocarya mandioccana Nees (Lauraceae). Biochem. Syst. Ecol. 2006, 34, 822–824. [Google Scholar] [CrossRef]
- Suleiman, M.; McGaw, L.; Naidoo, V.; Eloff, J. Detection of antimicrobial compounds by bioautography of different extracts of leaves of selected South African tree species. Afr. J. Tradit. Complement. Altern. Med. 2010, 7, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J.A.; Kwan, D. Metabolites from a Scytalidium Species. Can. J. Chem. 1973, 51, 3299–3301. [Google Scholar] [CrossRef]
- Li, Y.-X.; Himaya, S.; Dewapriya, P.; Kim, H.J.; Kim, S.-K. Anti-proliferative effects of isosclerone isolated from marine fungus Aspergillus fumigatus in MCF-7 human breast cancer cells. Process Biochem. 2014, 49, 2292–2298. [Google Scholar] [CrossRef]
- El-Elimat, T.; Raja, H.A.; Figueroa, M.; Swanson, S.M.; Iii, J.O.F.; Lucas, D.M.; Grever, M.R.; Wani, M.C.; Pearce, C.J.; Oberlies, N.H. Sorbicillinoid analogs with cytotoxic and selective anti-Aspergillus activities from Scytalidium album. J. Antibiot. 2014, 68, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Draeger, S.; Schulz, B.; Krohn, K.; Ahmed, I.; Hussain, H.; Yi, Y.; Li, L.; Zhang, W. Bioactive Aromatic Derivatives from Endophytic Fungus, Cytospora sp. Nat. Prod. Commun. 2011, 6, 661–666. [Google Scholar] [CrossRef] [Green Version]
- Pittayakhajonwut, P.; Sohsomboon, P.; Dramae, A.; Suvannakad, R.; Lapanun, S.; Tantichareon, M. Antimycobacterial Substances from Phaeosphaeria sp BCC8292. Planta Med. 2008, 74, 281–286. [Google Scholar] [CrossRef]
- Yuan, C.; Li, G.; Wu, C.-S.; Wang, H.-Y.; Zhao, Z.-T.; Lou, H.-X. A New Fatty Acid from the Endolichenic Fungus Massarina sp. Chem. Nat. Compd. 2015, 51, 415–417. [Google Scholar] [CrossRef]
- Kim, K.H.; Beemelmanns, C.; Murillo, C.; Guillén, A.; Umaña, L.; Tamayo-Castillo, G.; Kim, S.-N.; Clardy, J.; Cao, S. Naphthalenones and Isocoumarins from a Costa Rican Fungus Xylariaceae sp. CR1546C. J. Chem. Res. 2014, 38, 722–725. [Google Scholar] [CrossRef]
- Wu, J.-T.; Zheng, C.-J.; Zhang, B.; Zhou, X.-M.; Zhou, Q.; Chen, G.-Y.; Zeng, Z.-E.; Xie, J.-L.; Han, C.-R.; Lyu, J.-X. Two new secondary metabolites from a mangrove-derived fungus Cladosporium sp. JJM22. Nat. Prod. Res. 2018, 33, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Kongyen, W.; Rukachaisirikul, V.; Phongpaichit, S.; Sakayaroj, J. A new hydronaphthalenone from the mangrove-derived Daldinia eschscholtzii PSU-STD57. Nat. Prod. Res. 2015, 29, 1995–1999. [Google Scholar] [CrossRef] [PubMed]
- Prabpai, S.; Wiyakrutta, S.; Sriubolmas, N.; Kongsaeree, P. Antimycobacterial dihydronaphthalenone from the endophytic fungus Nodulisporium sp. of Antidesma ghaesembilla. Phytochem. Lett. 2015, 13, 375–378. [Google Scholar] [CrossRef]
- Sun, R.; Gao, Y.-X.; Shen, K.-Z.; Xu, Y.-B.; Wang, C.-R.; Liu, H.-Y.; Dong, J.-Y. Antimicrobial metabolites from the aquatic fungus Delitschia corticola. Phytochem. Lett. 2011, 4, 101–105. [Google Scholar] [CrossRef]
- Isaka, M.; Yangchum, A.; Rachtawee, P.; Khoyaiklang, P.; Boonyuen, N.; Lumyong, S. Dihydronaphthalenones from the endophytic fungus Botryosphaeria sp. BCC 8200. Phytochem. Lett. 2009, 2, 207–210. [Google Scholar] [CrossRef]
- Liu, C.H.; Meng, J.C.; Zou, W.X.; Huang, L.L.; Tang, H.Q.; Tan, R.X. Antifungal carbon skeleton from Keissleriella sp. Y4108, a marine filamentous fungus. Planta Med. 2002, 68, 363–365. [Google Scholar] [CrossRef]
- Shushni, M.A.M.; Mentel, R.; Lindequist, U.; Jansen, R. Balticols A-F, New Naphthalenone Derivatives with Antiviral Activity, from an Ascomycetous Fungus. Chem. Biodivers. 2009, 6, 127–137. [Google Scholar] [CrossRef]
- Sommart, U.; Rukachaisirikul, V.; Sukpondma, Y.; Phongpaichit, S.; Sakayaroj, J.; Kirtikara, K. Hydronaphthalenones and a Dihydroramulosin from the Endophytic Fungus PSU-N24. Chem. Pharm. Bull. 2008, 56, 1687–1690. [Google Scholar] [CrossRef] [Green Version]
- Trisuwan, K.; Khamthong, N.; Rukachaisirikul, V.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. Anthraquinone, Cyclopentanone, and Naphthoquinone Derivatives from the Sea Fan-Derived Fungi Fusarium spp. PSU-F14 and PSU-F135. J. Nat. Prod. 2010, 73, 1507–1511. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, L.; Niu, S.; Li, L.; Si, Y.; Liu, X.; Che, Y. Naphthalenones from a Perenniporia sp. Inhabiting the Larva of a Phytophagous Weevil, Euops chinesis. J. Nat. Prod. 2012, 75, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-H.; Lu, C.-H.; Zheng, Z.-H.; Shen, Y.-M. New Polyketides Isolated from Botryosphaeria australis Strain ZJ12-1A. Helvetica Chim. Acta 2011, 94, 897–902. [Google Scholar] [CrossRef]
- Kornsakulkarn, J.; Dolsophon, K.; Boonyuen, N.; Boonruangprapa, T.; Rachtawee, P.; Prabpai, S.; Kongsaeree, P.; Thongpanchang, C. Dihydronaphthalenones from endophytic fungus Fusarium sp. BCC14842. Tetrahedron 2011, 67, 7540–7547. [Google Scholar] [CrossRef]
- Amand, S.; Vallet, M.; Guedon, L.; Genta-Jouve, G.; Wien, F.; Mann, S.; Dupont, J.; Prado, S.; Nay, B. A Reactive Eremophilane and Its Antibacterial 2(1H)-Naphthalenone Rearrangement Product, Witnesses of a Microbial Chemical Warfare. Org. Lett. 2017, 19, 4038–4041. [Google Scholar] [CrossRef]
- Ai, W.; Lin, X.; Wang, Z.; Lü, X.; Mangaladoss, F.; Yang, X.; Zhou, X.; Tu, Z.; Liu, Y. Cladosporone A, a new dimeric tetralone from fungus Cladosporium sp. KcFL6’ derived of mangrove plant Kandelia candel. J. Antibiot. 2014, 68, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-L.; Li, X.-M.; Mándi, A.; Antus, S.; Li, X.; Zhang, P.; Liu, Y.; Kurtán, T.; Wang, B.-G. Characterization of Cladosporols from the Marine Algal-Derived Endophytic Fungus Cladosporium cladosporioides EN-399 and Configurational Revision of the Previously Reported Cladosporol Derivatives. J. Org. Chem. 2017, 82, 9946–9954. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Zheng, C.-J.; Tang, D.-Q.; Zhang, F.; Wang, H.-Y.; Chen, G.-Y. Two new secondary metabolites from a mangrove-derived fungus Cladosporium sp. JS1-2. J. Antibiot. 2019, 72, 779–782. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, L.; Kong, F.; Ma, Q.; Xie, Q.; Li, J.; Dai, H.; Guo, L.; Zhao, Y. Altertoxins with Quorum Sensing Inhibitory Activities from The Marine-Derived Fungus Cladosporium sp. KFD33. Mar. Drugs 2020, 18, 67. [Google Scholar] [CrossRef] [Green Version]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer. 2020. Available online: https://gco.iarc.fr/today (accessed on 25 December 2021).
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, H.-X.; Chen, Y.-C.; Sun, Z.-H.; Li, H.-H.; Li, S.-N.; Yan, M.-L.; Zhang, W.-M. Two New Metabolites from the Endophytic Fungus Alternaria sp. A744 Derived from Morinda officinalis. Molecules 2017, 22, 765. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, Y.; Yokoyama, E.; Takahashi, T.; Uzawa, J.; Morooka, N.; Tsunoda, H.; Tatsuno, T. Studies on the metabolites of Penicillium diversum var. aureum. I. Chem. Pharm. Bull. 1986, 34, 1497–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Amrania, M.; Ebadab, S.S.; Gadb, H.A.; Proksch, P. Pestalotiopamide E and pestalotiopin B from an endophytic fungus Aureobasidium pullulans isolated from Aloe vera leaves. Phytochem. Lett. 2016, 18, 95–98. [Google Scholar] [CrossRef]
- Tang, J.-W.; Wang, W.-G.; Li, A.; Yan, B.-C.; Chen, R.; Li, X.-N.; Du, X.; Sun, H.-D.; Pu, J.-X. Polyketides from the endophytic fungus Phomopsis sp. sh917 by using the one strain/many compounds strategy. Tetrahedron 2017, 73, 3577–3584. [Google Scholar] [CrossRef]
- Flores-Bocanegra, L.; Raja, H.A.; Bacon, J.W.; Maldonado, A.C.; Burdette, J.E.; Pearce, C.J.; Oberlies, N.H. Cytotoxic Naphthoquinone Analogues, Including Heterodimers, and Their Structure Elucidation Using LR-HSQMBC NMR Experiments. J. Nat. Prod. 2020, 84, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Sun, B.D.; Zhang, G.S.; Deng, H.; Wang, M.H.; Tan, X.M.; Zhang, X.Y.; Jia, H.M.; Zhang, H.W.; Zhang, T.; et al. Polyketides with different post-modifications from desert endophytic fungus Paraphoma sp. Nat. Prod. Res. 2017, 32, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, M.F.; Kurtán, T.; Mándi, A.; Müller, W.E.G.; Fouad, M.A.; Kamel, M.S.; Liu, Z.; Ebrahim, W.; Daletos, G.; Proksch, P. Induced secondary metabolites from the endophytic fungus Aspergillus versicolor through bacterial co-culture and OSMAC ap-proaches. Tetrahedron Lett. 2018, 59, 2647–2652. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Ding, J.; Ding, K.; Chen, D.; Cen, S.; Ge, M. Phomonaphthalenone A: A novel dihydronaphthalenone with anti-HIV activity from Phomopsis sp. HCCB04730. Phytochem. Lett. 2013, 6, 257–260. [Google Scholar] [CrossRef]
- Zhang, P.; Jia, C.; Lang, J.; Li, J.; Luo, G.; Chen, S.; Yan, S.; Liu, L. Mono- and Dimeric Naphthalenones from the Marine-Derived Fungus Leptosphaerulina chartarum 3608. Mar. Drugs 2018, 16, 173. [Google Scholar] [CrossRef] [Green Version]
- Zurlo, D.; Leone, C.; Assante, G.; Salzano, S.; Renzone, G.; Scaloni, A.; Foresta, C.; Colantuoni, V.; Lupo, A. Cladosporol a stimulates G1-phase arrest of the cell cycle by up-regulation of p21waf1/cip1 expression in human colon carcinoma HT-29 cells. Mol. Carcinog. 2013, 52, 1–17. [Google Scholar] [CrossRef]
- Zurlo, D.; Assante, G.; Moricca, S.; Colantuoni, V.; Lupo, A. Cladosporol A, a new peroxisome proliferator-activated receptor γ (PPARγ) ligand, inhibits colorectal cancer cells proliferation through β-catenin/TCF pathway inactivation. Biochim. Biophys. Acta 2014, 1840, 2361–2372. [Google Scholar] [CrossRef]
- Koul, M.; Kumar, A.; Deshidi, R.; Sharma, V.; Singh, R.D.; Singh, J.; Sharma, P.R.; Shah, B.A.; Jaglan, S.; Singh, S.; et al. Cladosporol A triggers apoptosis sensitivity by ROS-mediated autophagic flux in human breast cancer cells. BMC Cell Biol. 2017, 18, 26. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; He, X.; Liu, C.; Che, Q.; Zhu, T.; Gu, Q.; Li, D. Clindanones A and B and cladosporols F and G, polyketides from the deep-sea derived fungus Cladosporium cladosporioides HDN14-342. RSC Adv. 2016, 6, 76498–76504. [Google Scholar] [CrossRef]
- Fan, Z.; Sun, Z.-H.; Liu, H.; Chen, Y.-C.; Li, H.-H.; Zhang, W.-M. Perangustols A and B, a pair of new azaphilone epimers from a marine sediment-derived fungus Cladosporium perangustm FS62. J. Asian Nat. Prod. Res. 2016, 18, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Rukachaisirikul, V.; Sommart, U.; Phongpaichit, S.; Hutadilok-Towatana, N.; Rungjindamai, N.; Sakayaroj, J. Metabolites from the Xylariaceous Fungus PSU-A80. Chem. Pharm. Bull. 2007, 55, 1316–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Lateff, A.; König, G.M.; Fisch, K.M.; Höller, U.; Jones, P.G.; Wright, A.D. New Antioxidant Hydroquinone Derivatives from the Algicolous Marine Fungus Acremonium sp. J. Nat. Prod. 2002, 65, 1605–1611. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A.; El-Messery, S.M. In silico Modeling Studies of 5-HT2B Antagonistic Activity of 2-(2- phenylethyl)chromone Derivatives from Cucumis melo Seeds. Lett. Drug Des. Discov. 2016, 13, 840–844. [Google Scholar] [CrossRef] [Green Version]
- Costagliola, C.; Parmeggiani, F.; Semeraro, F.; Sebastiani, A. Selective Serotonin Reuptake Inhibitors: A Review of its Effects on Intraocular Pressure. Curr. Neuropharmacol. 2008, 6, 293–310. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Zhao, S.; Wu, Y.; Cao, H.; Xu, Y.; Liu, X.; Shui, W.; Cheng, J.; Zhao, S.; Shen, L.; et al. Identification of natural products as novel ligands for the human 5-HT2C receptor. Biophys. Rep. 2018, 4, 50–61. [Google Scholar] [CrossRef] [Green Version]
- Bös, M.; Canesso, R.; Inoue-Ohga, N.; Nakano, A.; Takehana, Y.; Sleight, A.J. O-Methylasparvenone, a nitrogen-free serotonin antagonist. Bioorg. Med. Chem. 1997, 5, 2165–2171. [Google Scholar] [CrossRef]
- Bell, A.A.; Wheeler, M.H. Biosynthesis and functions of fungal melanins. Annu. Rev. Phytopathol. 1986, 24, 411–451. [Google Scholar] [CrossRef]
- Lee, J.-K.; Jung, H.-M.; Kim, S.-Y. 1,8-Dihydroxynaphthalene (DHN)-Melanin Biosynthesis Inhibitors Increase Erythritol Production in Torula corallina, and DHN-Melanin Inhibits Erythrose Reductase. Appl. Environ. Microbiol. 2003, 69, 3427–3434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thines, E.; Anke, H.; Sterner, O. Scytalols A, B, C, and D and other modulators of melanin biosynthesis from Scytalidium sp. 36-93. J. Antibiot. 1998, 51, 387–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nong, X.-H.; Zheng, Z.-H.; Zhang, X.-Y.; Lu, X.-H.; Qi, S.-H. Polyketides from a Marine-Derived Fungus Xylariaceae sp. Mar. Drugs 2013, 11, 1718–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.; Zhang, H.; Liu, Y.; Gu, Q.; Xu, J.; Huang, X.; She, Z. Ethylnaphthoquinone derivatives as inhibitors of indoleamine-2, 3-dioxygenase from the mangrove endophytic fungus Neofusicoccum austral SYSU-SKS024. Fitoterapia 2018, 125, 281–285. [Google Scholar] [CrossRef]
- Xiao, W.-J.; Chen, H.-Q.; Wang, H.; Cai, C.-H.; Mei, W.-L.; Dai, H.-F. New secondary metabolites from the endophytic fungus Fusarium sp. HP-2 isolated from “Qi-Nan” agarwood. Fitoterapia 2018, 130, 180–183. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New Adventures of an Old Flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Yatoo, M.I.; Gopalakrishnan, A.; Saxena, A.; Parray, O.R.; ALAM Tufani, N.; Chakraborty, S.; Tiwari, R.; Dhama, K.; Iqbal, H. Anti-Inflammatory Drugs and Herbs with Special Emphasis on Herbal Medicines for Countering Inflammatory Diseases and Disorders—A Review. Recent Patents Inflamm. Allergy Drug Discov. 2018, 12, 39–58. [Google Scholar] [CrossRef]
- Fizeșan, I.; Rusu, M.E.; Georgiu, C.; Pop, A.; Ștefan, M.G.; Muntean, D.M.; Mirel, S.; Vostinaru, O.; Kiss, B.; & Popa, D.S. Antitussive, Antioxidant, and Anti-Inflammatory Effects of a Walnut (Juglans regia L.) Septum Extract Rich in Bioactive Compounds. Antioxidants 2021, 10, 119. [Google Scholar] [CrossRef]
- Liu, W.; Chen, S.; Li, J.; Yang, X.; Yan, C.; Liu, H. A new β-tetralonyl glucoside from the Santalum album derived endophytic fungus Colletotrichum sp. GDMU-1. Nat. Prod. Res. 2018, 33, 354–359. [Google Scholar] [CrossRef]
- Girich, E.V.; Yurchenko, A.N.; Smetanina, O.F.; Trinh, P.T.H.; Ngoc, N.T.D.; Pivkin, M.V.; Popov, R.S.; Pislyagin, E.A.; Menchinskaya, E.S.; Chingizova, E.A.; et al. Neuroprotective Metabolites from Vietnamese Marine Derived Fungi of Aspergillus and Penicillium Genera. Mar. Drugs 2020, 18, 608. [Google Scholar] [CrossRef]
- Zhao, D.-L.; Shao, C.-L.; Wang, C.-Y.; Wang, M.; Yang, L.-J.; Wang, C.-Y. Naphthalenones and Depsidones from a Sponge-Derived Strain of the Fungus Corynespora cassiicola. Molecules 2016, 21, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.H.; Zhang, G.; Yan, Q.X.; Zou, Z.B.; Xiao, H.X.; Xie, C.L.; Tang, X.X.; Luo, L.Z.; Yang, X.W. Cladosporactone A, a unique polyketide with 7-methylisochromen-3-one skeleton from the deep-sea-derived fungus Cladosporium cladosporioides. Chem. Biodivers. 2020, 17, e2000158. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.-J.; Xiao, J.-H. Secondary Metabolites from Higher Fungi: Discovery, Bioactivity, and Bioproduction. Adv. Biochem. Eng. Biotechnol. China I 2009, 113, 79–150. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167180. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Hussain, M.; Zhang, W.; Stadler, M.; Liu, X.; Xiang, M. Current insights into fungal species diversity and perspective on naming the environmental DNA sequences of fungi. Mycology 2019, 10, 127–140. [Google Scholar] [CrossRef] [Green Version]
- Hemingway, R.W.; McGraw, G.W.; Barras, S.J. Polyphenols in Ceratocystis minor infected Pinus taeda: Fungal metabolites, phloem and xylem phenols. J. Agric. Food Chem. 1977, 25, 717–723. [Google Scholar] [CrossRef]
- Stipanovic, R.D.; Bell, A.A. Pentaketide metabolites of Verticillium dahliae. II. Accumulation of naphthol derivatives by the aberrant-melanin mutant BRM-2. Mycologia 1977, 69, 164–172. [Google Scholar] [CrossRef]
- Ayer, W.A.; Browne, L.M.; Lin, G. Metabolites of Leptographium wageneri, the causative agent of black stain root disease of conifers. J. Nat. Prod. 1989, 52, 119–129. [Google Scholar] [CrossRef]
- Borgschulte, K.; Rebuffat, S.; Trowitzsch-Kienast, W.; Schomburg, D.; Pinon, J.; Bodo, B. Isolation and structure elucidation of hymatoxins B–E and other phytotoxins from Hypoxylon mammatum fungal pathogen of leuce poplars. Tetrahedron 1991, 47, 8351–8360. [Google Scholar] [CrossRef]
- Tabuchi, H.; Tajimi, A.; Ichihara, A. Phytotoxic metabolites isolated from Scolecotrichum graminis Fuckel. Biosci. Biotech. Biochem. 1994, 58, 1956–1959. [Google Scholar] [CrossRef]
- Trisuwan, K.; Rukachaisirikul, V.; Sukpondma, Y.; Preedanon, S.; Phongpaichit, S.; Rungjindamai, N.; Sakayaroj, J. Epoxydons and a pyrone from the marine-derived fungus Nigrospora sp. PSU-F5. J. Nat. Prod. 2008, 71, 1323–1326. [Google Scholar] [CrossRef]
- Arunpanichlert, J.; Rukachaisirikul, V.; Phongpaichit, S.; Supaphon, O.; Sakayaroj, J. Xylariphilone: A new azaphilone derivative from the seagrass-derived fungus Xylariales sp. PSU-ES163. Nat. Prod. Res. 2016, 30, 46–51. [Google Scholar] [CrossRef]
- Fan, C.; Zhou, G.; Wang, W.; Zhang, G.; Zhu, T.; Che, Q.; Li, D. Tetralone derivatives from a deep-sea derived fungus Cladosporium sp. HDN17-58. Nat. Prod. Commun. 2021, 16. [Google Scholar] [CrossRef]
- Sviridov, S.I. Secondary metabolites of Pyricularia oryzae. Chem. Nat. Compd. 1991, 27, 410–413. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Kramer, M.; Essmann, F.; Grond, S. A New Acetylenic compound and other bioactive metabolites from a shark gill-derived Penicillium strain. Rec. Nat. Prod. 2017, 11, 31–36. [Google Scholar]
- Quang, T.H.; Huong, P.T.M.; Ngan, N.T.T.; Hanh, T.T.H.; Cuong, N.X.; Nam, N.H.; Minh, C.V. Secondary metabolites from a marine sponge-associated fungus Xenomyrothecium sp. IMBC-FP2.11. Vietnam J. Chem. 2020, 58, 752–758. [Google Scholar]
- Iwasaki, S.; Muro, H.; Sasaki, K.; Nozoe, S.; Okuda, S.; Sato, Z. Isolations of phytotoxic substances produced by pyricularia oryzae cavara. Tetrahedron Lett. 1973, 14, 3537–3542. [Google Scholar] [CrossRef]
- Krohn, K.; Biele, C.; Drogies, K.H.; Steingrover, K.; Aust, H.J.; Draeger, S.; Schulz, B. Fusidilactones, a new group of polycyclic lactones from an endophyte, Fusidium sp. Eur. J. Org. Chem. 2002, 14, 2331–2336. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Masuma, R.; Kim, Y.-P.; Uchida, R.; Tomoda, H.; Omura, S. Taxonomy and secondary metabolites of Pseudobotrytis sp. FKA-25. Mycoscience 2004, 45, 9–16. [Google Scholar] [CrossRef]
- Mancilla, G.; Jiménez-Teja, D.; Femenía-Rios, M.; Macías-Sánchez, A.J.; Collado, I.G.; Hernández-Galán, R. Novel macrolide from wild strains of the phytopathogen Fungus Colletotrichum acutatum. Nat. Prod. Commun. 2009, 4, 395–398. [Google Scholar] [CrossRef] [Green Version]
- Ouchbani, T.; Zouihri, H.; Essassi, E.; Proksch, P.; Ng, S.W. (3R,4S)-3,4,8-Trihydroxy-1,2,3,4-tetrahydronaphthalen-1-one monohydrate from Embellisia eureka. Acta Cryst. 2012, E68, o1874. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubbaiah, P.; Chilton, W.S.J. Toxins produced by the Dogwood Anthracnose fungus Discula sp. J. Nat. Prod. 1991, 54, 1293–1297. [Google Scholar] [CrossRef]
- Höller, U.; Konig, G.M.; Wright, A.D. Three new metabolites from marine-derived fungi of the genera coniothyrium and microsphaeropsis. J. Nat. Prod. 1999, 62, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Chang, W.; Zhang, M.; Li, Y.; Li, W.; Shi, H.; Zheng, S.; Lou, H. Quinone derivatives isolated from the endolichenic fungus Phialocephala fortinii are Mdr1 modulators that combat azole resistance in Candida albicans. Sci. Rep. 2016, 6, 33687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza, E.M.C.; Da Silva, E.L.; Marinho, A.M.R.; Marinho, P.S.B. (4S)-4,8-dihydroxy-1-tetralone and other chemical constituents from Pestalotiopsis sp. EJC07, endophytic from Bauhinia guianensis. An. Acad. Bras. Ciênc. 2016, 88, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, Y.; Cheng, M.J.; Chang, H.S.; Wu, M.D.; Hsieh, S.Y.; Liu, T.W.; Lin, C.H.; Yuan, G.F.; Chen, I.S. Six new metabolites produced by Colletotrichum aotearoa 09F0161, an endophytic fungus isolated from Bredia oldhamii. Nat. Prod. Res. 2016, 30, 251–258. [Google Scholar] [CrossRef]
- Kokubun, T.; Veitch, N.C.; Bridge, P.D.; Simmonds, M.S.J. Dihydroisocoumarins and a tetralone from Cytospora eucalypticola. Phytchemistry 2003, 62, 779–782. [Google Scholar] [CrossRef]
- Gallo, M.B.C.; Cavalcanti, B.C.; Barros, F.W.; de Moraes, M.O.; Costa-Lotufo, L.V.; Pessoa, C.; Bastos, J.K.; Pupo, M.T. Chemical constituents of Papulaspora immersa, an endophyte from Smallanthus sonchifolius (Asteraceae), and their cytotoxic activity. Chem. Biodivers. 2010, 7, 2941–2950. [Google Scholar] [CrossRef]
- Evidente, A.; Punzo, B.; Andolfi, A.; Cimmino, A.; Melck, D.; Luque, J. Lipophilic phytotoxins produced by Neofusicoccum parvum, a grapevine canker agent. Phytopathol. Mediterr. 2010, 49, 74–79. [Google Scholar]
- El-Elimat, T.; Raja, H.A.; Figueroa, M.; Falkinham, J.O.; Oberlies, N.H. Isochromenones, isobenzofuranone, and tetrahydronaphthalenes produced by Paraphoma radicina, a fungus isolated from a freshwater habitat. Phytochemistry 2014, 104, 114–120. [Google Scholar] [CrossRef] [Green Version]
- Sadorn, K.; Saepua, S.; Boonyuen, N.; Boonruangprapa, T.; Rachtawee, P.; Pittayakhajonwut, P. Antimicrobial activity and cytotoxicity of xanthoquinodin analogs from the fungus Cytospora eugeniae BCC42696. Phytochemistry 2018, 151, 99–109. [Google Scholar] [CrossRef]
- Yang, H.X.; Peng, X.P.; Gao, H.; Zhang, H.M.; Wang, Z.R.; Li, G.; Lou, H.X. Pleosporalins H and I, two new heptaketides from the endophytic fungus Pleosporales sp. F46 by using OSMAC strategy. Nat. Prod. Res. 2021, 35, 13307–13313. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-Y.; Zhang, J.-X.; Ding, Q.-Y.; He, Z.-C.; Zhu, C.-Y.; Zhang, K.-Q.; Niu, X.-M. Metabolites from two dominant thermophilic fungal species Thermomyces lanuginosus and Scytalidium thermophilum. Chem. Biodivers. 2020, 17, e2000137. [Google Scholar]
- Sato, H.; Takishima, T.; Otomo, N.; Sakamura, S. Phytotoxins produced by the fungus of the larch shoot bligh. Nippon Nôgeikagaku Kaishi. 1982, 56, 649–653. [Google Scholar] [CrossRef]
- Luo, G.; Chen, S.; Yu, J.; Yuan, J.; Zheng, L.; Liu, L.; Chen, B.; Li, J. Naphthalenones and naphthols isolated from the Saussurea laniceps endophytic fungus Didymella glomerata X223. Chem. Biodivers. 2020, 17, e2000315. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.A.; Stipanovic, R.D.; Puhalla, J.E. Pentaketide metabolites of Verticillium dahliae: Identification of (+)-scytalone as a natural precursor to melanin. Tetrahedron 1976, 32, 1353–1356. [Google Scholar] [CrossRef]
- Liu, Y.; Stuhldreier, F.; Kurtan, T.; Mandi, A.; Arumugam, S.; Lin, W.; Stork, B.; Wesselborg, S.; Weber, H.; Henrich, B.; et al. Daldinone derivatives from the mangrove-derived endophytic fungus Annulohypoxylon sp. RSC Adv. 2017, 7, 5381–5393. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liang, J.H.; Zhao, J.C.; Wang, Y.L.; Dong, P.P.; Liu, X.G.; Zhang, T.Y.; Wu, Y.Y.; Shang, D.J.; Zhang, Y.X.; et al. Xylarianins A-D from the endophytic fungus Xylaria sp. SYPF 8246 as natural inhibitors of human carboxylesterase 2. Bioorg. Chem. 2018, 81, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Padumadasa, C.; Xu, Y.M.; Wijeratne, E.M.K.; Espinosa-Artiles, P.; U’Ren, J.M.; Arnold, A.E.; Gunatilaka, A.A.L. Cytotoxic and noncytotoxic metabolites from Teratosphaeria sp. FL2137, a fungus associated with Pinus clausa. J. Nat. Prod. 2018, 81, 616–624. [Google Scholar] [CrossRef]
- Bartman, C.D.; Campbell, I.M. Naphthalenone production in Aspergillus parvulus. Can. J. Microbiol. 1979, 25, 130–137. [Google Scholar] [CrossRef]
- Hao, G.; Qing-Hua, Z.; Miao-Miao, J.; Jin-Shan, T.; Cheng-Du, M.; Kui, H.; Michio, N.; Nai-Li, W.; Xin-Sheng, Y. Polyketides from a marine sponge-derived fungus Mycelia sterilia and proton-proton long-range coupling. Magn. Reson. Chem. 2008, 46, 1148–1152. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Chávez, J.; El-Elimat, T.; Gallagher, J.M.; Graf, T.N.; Fournier, J.; Panigrahi, G.K.; Deep, G.; Bunch, R.L.; Raja, H.A.; Oberlies, N.H. Delitpyrones: α-Pyrone derivatives from a freshwater Delitschia sp. Planta Med. 2019, 85, 62–71. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.-C.; Li, D.-L.; Chen, Y.-C.; Zhang, W.-M. A new isofuranonaphthalenone and benzopyrans from the endophytic fungus Nodulisporium sp. A4 from Aquilaria sinensis. Helv. Chim. Acta 2010, 93, 920–924. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Zhang, M.; Zhu, R.X.; Zhang, J.Z.; Xie, F.; Li, X.B.; Chang, W.Q.; Wang, X.N.; Zhao, Z.T.; Lou, H.X. Heptaketides from an endolichenic fungus Biatriospora sp. and their antifungal activity. J. Nat. Prod. 2016, 79, 2149–2157. [Google Scholar] [CrossRef] [PubMed]
- Ariefta, N.R.; Nikmawahda, H.T.; Koseki, T.; Shiono, Y. Fusopoltides B–E, new polyketides isolated from Fusarium solani B-18. Tetrahedron Lett. 2019, 60, 151361. [Google Scholar] [CrossRef]
- Niu, S.; Tang, X.X.; Fan, Z.; Xia, J.M.; Xie, C.L.; Yang, X.W. Fusarisolins A–E, polyketides from the marine-derived fungus Fusarium solani H918. Mar. Drugs 2019, 17, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itsuo, K.; Shunji, S.; Akihiko, M.; Akio, F. 5-Hydroxydihydrofusarubin, a Process for Its Preparation and Its Use as a Medicament. European Patent Application 0234431 A2, 2 September 1987. [Google Scholar]
- Wen, Y.; Lv, Y.; Hao, J.; Chen, H.; Huang, Y.; Liu, C.; Huang, H.; Ma, Y.; Yang, X. Two new compounds of Penicillium polonicum, an endophytic fungus from Camptotheca acuminata Decne. Nat. Prod. Res. 2020, 34, 1879–1883. [Google Scholar] [CrossRef]
- Stodůlková, E.; Man, P.; Kuzma, M.; Černý, J.; Císařová, I.; Kubátová, A.; Chudíčková, M.; Kolařík, M.; Flieger, M. A highly diverse spectrum of naphthoquinone derivatives produced by the endophytic fungus Biatriospora sp. CCF 4378. Folia Microbiol. (Praha) 2015, 60, 259–267. [Google Scholar] [CrossRef]
- Sakagami, Y.; Sano, A.; Marumo, S.; Yoshikawa, N.; Nakagawa, J. Cladosporol, a plant growth regurator produced by fungus Cladosporium cladosporioides. Symp. Chem. Nat. Prod. 1992, 134–141. [Google Scholar] [CrossRef]
- Sakagami, Y.; Sano, A.; Hara, O.; Mikawa, T.; Marumo, S. Cladosporol, β-l,3-glucan biosynthesis inhibitor, isolated from fungus, Cladosporium cladosporioides. Tetrahedron Lett. 1995, 36, 1469–1472. [Google Scholar] [CrossRef]
- Zurlo, D.; Ziccardi, P.; Votino, C.; Colangelo, T.; Cerchia, C.; Dal Piaz, F.; Dallavalle, S.; Moricca, S.; Novellino, E.; Lavecchia, A.; et al. The antiproliferative and proapoptotic effects of cladosporols A and B are related to their different binding mode as PPARγ ligands. Biochem. Pharmacol. 2016, 108, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Nasini, G.; Arnone, A.; Assante, G.; Bava, A.; Moricca, S.; Ragazzi, A. Secondary mould metabolites of Cladosporium tenuissimum, a hyperparasite of rust fungi. Phytochemistry 2004, 65, 2107–2111. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Yagi, A.; Akaishi, M.; Kirikoshi, R.; Takahashi, O.; Abe, T.; Chiba, S.; Takahashi, K.; Iwakura, N.; Namikoshi, M.; et al. Halogenated cladosporols produced by the sodium halide-supplemented fermentation of the plant-associated fungus Cladosporium sp. TMPU1621. Tetrahedron Lett. 2018, 59, 1913–1915. [Google Scholar] [CrossRef]
- Fan, Z.; Sun, Z.; Chen, Y.; Li, H.; Zhang, W. Cladosperanol A, a new dimeric tetralone from marine-derived fungus cladosporium perangustum FS62. Nat. Prod. Res. Dev. 2016, 28, 486–489. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, S.R.M.; Fadil, S.A.; Fadil, H.A.; Eshmawi, B.A.; Mohamed, S.G.A.; Mohamed, G.A. Fungal Naphthalenones; Promising Metabolites for Drug Discovery: Structures, Biosynthesis, Sources, and Pharmacological Potential. Toxins 2022, 14, 154. https://doi.org/10.3390/toxins14020154
Ibrahim SRM, Fadil SA, Fadil HA, Eshmawi BA, Mohamed SGA, Mohamed GA. Fungal Naphthalenones; Promising Metabolites for Drug Discovery: Structures, Biosynthesis, Sources, and Pharmacological Potential. Toxins. 2022; 14(2):154. https://doi.org/10.3390/toxins14020154
Chicago/Turabian StyleIbrahim, Sabrin R. M., Sana A. Fadil, Haifa A. Fadil, Bayan A. Eshmawi, Shaimaa G. A. Mohamed, and Gamal A. Mohamed. 2022. "Fungal Naphthalenones; Promising Metabolites for Drug Discovery: Structures, Biosynthesis, Sources, and Pharmacological Potential" Toxins 14, no. 2: 154. https://doi.org/10.3390/toxins14020154
APA StyleIbrahim, S. R. M., Fadil, S. A., Fadil, H. A., Eshmawi, B. A., Mohamed, S. G. A., & Mohamed, G. A. (2022). Fungal Naphthalenones; Promising Metabolites for Drug Discovery: Structures, Biosynthesis, Sources, and Pharmacological Potential. Toxins, 14(2), 154. https://doi.org/10.3390/toxins14020154







