Ultrasound-Guided OnabotulinumtoxinA Injections to Treat Oromandibular Dystonia in Cerebral Palsy
Abstract
:1. Introduction and Literature Review
2. Cases
2.1. Case 1
2.2. Case 2
2.3. Case 3
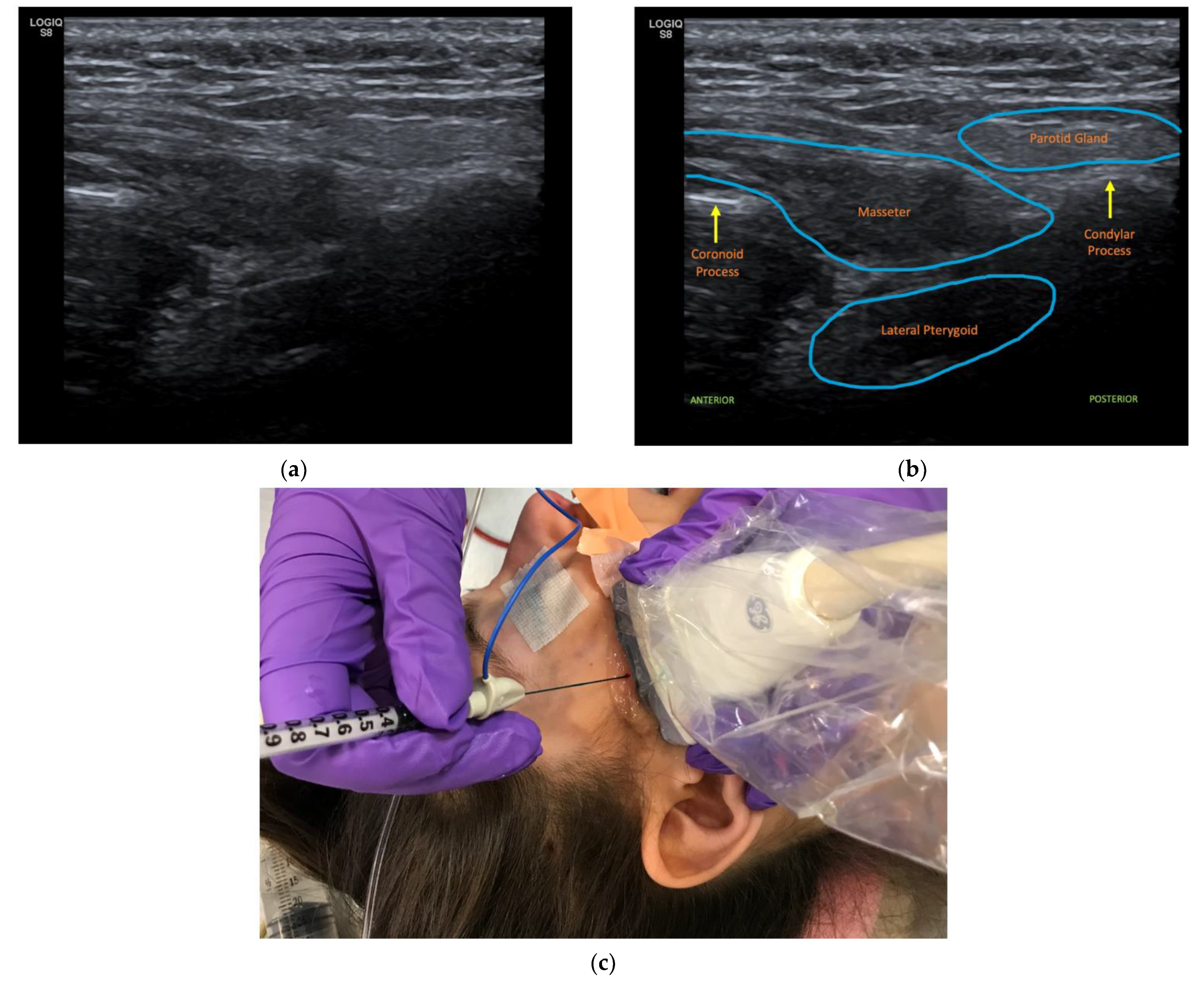
2.4. Case 4
2.5. Case 5
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenbaum, P.; Paneth, N.; Leviton, A.; Goldstein, M.; Bax, M.; Damiano, D.; Dan, B.; Jacobsson, B. A report: The definition and classification of cerebral palsy April 2006. Dev. Med. Child Neurol. 2007, 49, 8–14. [Google Scholar] [CrossRef]
- Papadimitriou, I.; Dalivigka, Z.; Outsika, C.; Scarmeas, N.; Pons, R. Dystonia assessment in children with cerebral palsy and periventricular leukomalacia. Eur. J. Paediatr. Neurol. 2021, 32, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Hassell, T.J.W.; Charles, D. Treatment of Blepharospasm and Oromandibular Dystonia with Botulinum Toxins. Toxins 2020, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Alomar, X.; Medrano, J.; Cabratosa, J.; Clavero, J.A.; Lorente, M.; Serra, I.; Monill, J.M.; Salvador, A. Anatomy of the Temporomandibular Joint. Semin. Ultrasound CT MRI 2007, 28, 170–183. [Google Scholar] [CrossRef]
- Jankovic, J. Primary and Secondary Generalized Dystonias. In Office Practice of Neurology, 2nd ed.; Samuels, M.A., Feske, S., Eds.; Churchill Linvingstone: London, UK, 2003; pp. 816–821. [Google Scholar]
- Arvedson, J.C. Feeding children with cerebral palsy and swallowing difficulties. Eur. J. Clin. Nutr. 2013, 67, S9–S12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sığan, S.N.; Uzunhan, T.A.; Aydınlı, N.; Eraslan, E.; Ekici, B.; Calışkan, M. Effects of oral motor therapy in children with cerebral palsy. Ann. Indian Acad. Neurol. 2013, 16, 342–346. [Google Scholar] [CrossRef]
- Karp, B.I.; Alter, K. Botulinum Toxin Treatment of Blepharospasm, Orofacial/Oromandibular Dystonia, and Hemifacial Spasm. Semin. Neurol. 2016, 36, 084–091. [Google Scholar] [CrossRef]
- Comella, C.L. Systematic review of botulinum toxin treatment for oromandibular dystonia. Toxicon 2018, 147, 96–99. [Google Scholar] [CrossRef]
- Jankovic, J.; Brin, M.F. Therapeutic Uses of Botulinum Toxin. N. Engl. J. Med. 1991, 324, 1186–1194. [Google Scholar] [CrossRef]
- Simpson, L.L. The origin, structure, and pharmacological activity of botulinum toxin. Pharmacol. Rev. 1981, 33, 155–158. [Google Scholar]
- Novak, I.; Morgan, C.; Fahey, M.; Finch-Edmondson, M.; Galea, C.; Hines, A.; Langdon, K.; Mc Namara, M.; Paton, M.C.; Popat, H.; et al. State of the Evidence Traffic Lights 2019: Systematic Review of Interventions for Preventing and Treating Children with Cerebral Palsy. Curr. Neurol. Neurosci. Rep. 2020, 20, 3. [Google Scholar] [CrossRef] [Green Version]
- Lumsden, D.E.; Crowe, B.; Basu, A.; Amin, S.; Devlin, A.; Dealwis, Y.; Kumar, R.; Lodh, R.; Lundy, C.T.; Mordekar, S.R.; et al. Pharmacological management of abnormal tone and movement in cerebral palsy. Arch. Dis. Child. 2019, 104, 775–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, K. Botulinum Neurotoxin Injection for the Treatment of Recurrent Temporomandibular Joint Dislocation with and without Neurogenic Muscular Hyperactivity. Toxins 2018, 10, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhidayasiri, R.; Cardoso, F.; Truong, D.D. Botulinum toxin in blepharospasm and oromandibular dystonia: Comparing different botulinum toxin preparations. Eur. J. Neurol. 2006, 13, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Ney, J.P.; Joseph, K.R. Neurologic uses of botulinum neurotoxin type A. Neuropsychiatr. Dis. Treat. 2007, 3, 785–798. [Google Scholar] [CrossRef] [Green Version]
- Ataran, R.; Bahramian, A.; Jamali, Z.; Pishahang, V.; Barzegani, H.S.; Sarbakhsh, P.; Yazdani, J. The Role of Botulinum Toxin A in Treatment of Temporomandibular Joint Disorders: A Review. J. Dent. 2017, 18, 157–164. [Google Scholar]
- Song, P.; Schwartz, J.; Blitzer, A. The emerging role of botulinum toxin in the treatment of temporomandibular disorders. Oral Dis. 2007, 13, 253–260. [Google Scholar] [CrossRef]
- Moscovich, M.; Chen, Z.P.; Rodriguez, R. Successful treatment of open jaw and jaw deviation dystonia with botulinum toxin using a simple intraoral approach. J. Clin. Neurosci. 2015, 22, 594–596. [Google Scholar] [CrossRef]
- Montastruc, J.; Marque, P.; Moulis, F.; Bourg, V.; Lambert, V.; Durrieu, G.; Montastruc, J.-L.; Montastruc, F. Adverse drug reactions of botulinum neurotoxin type A in children with cerebral palsy: A pharmaco-epidemiological study in VigiBase. Dev. Med. Child Neurol. 2017, 59, 329–334. [Google Scholar] [CrossRef]
- Quezada-Gaon, N.; Wortsman, X.; Peñaloza, O.; Carrasco, J.E. Comparison of clinical marking and ultrasound-guided injection of Botulinum type A toxin into the masseter muscles for treating bruxism and its cosmetic effects. J. Cosmet. Dermatol. 2016, 15, 238–244. [Google Scholar] [CrossRef]
- Chan, A.K.; Finlayson, H.; Mills, P.B. Does the method of botulinum neurotoxin injection for limb spasticity affect outcomes? A systematic review. Clin. Rehabil. 2017, 31, 713–721. [Google Scholar] [CrossRef]
- Grigoriu, A.-I.; Dinomais, M.; Remy-Neris, O.; Brochard, S. Impact of Injection-Guiding Techniques on the Effectiveness of Botulinum Toxin for the Treatment of Focal Spasticity and Dystonia: A Systematic Review. Arch. Phys. Med. Rehabil. 2015, 96, 2067–2078. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Rha, D.-W.; Yoo, J.K.; Park, E.S. Accuracy of Manual Needle Placement for Gastrocnemius Muscle in Children With Cerebral Palsy Checked Against Ultrasonography. Arch. Phys. Med. Rehabil. 2009, 90, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Chin, T.Y.; Nattrass, G.R.; Selber, P.; Graham, H.K. Accuracy of intramuscular injection of botulinum toxin A in juvenile cerebral palsy: A comparison between manual needle placement and placement guided by electrical stimulation. J. Pediatr. Orthop. 2005, 25, 286–291. [Google Scholar] [CrossRef] [PubMed]
| Patient Information | Baseline | Injections (Injection Number: Age, Total Units/LPt, (Units/kg/LPt)) | Results as Observed/Reported by Care Takers and Physician | Weight Trends (Injection Number: Weight) |
|---|---|---|---|---|
| Patient 1 Dx: mixed spastic/dystonic quadriplegic CP GMFCS: IV | General: Mouth open 75% of the time. Feeding: difficulty inserting spoon into mouth when feeding Speech: vocalizing monosyllabic sounds such as “ah, ah” Quality of Life: difficulty working with speech therapist | #1: 1 y 11 mo; 5 units /LPt (0.47 units/kg/LPt) #2: 2 y 2 mo; 10 units/ LPt (0.84 units/kg/LPt) #3: 3 y 7 mo; 10 units/ LPt (0.87 units/kg/LPt) | #1: Mouth open 40% of time, improved ease of feeding/clearing spoon when inserting into mouth #2: improved ability to close mouth, able to chew food and eat without breaks, ability to close mouth further improved by 50% #3: continued good mouth closure, improved speech, starting to close lips to swallow | #1: 10.4 kg #2: 11.6 kg #3: 10.5 kg Last Documented Age: 4 y 9 mo Weight: 16 kg |
| Patient 2 Dx: dyskinetic quadriplegic CP GMFCS: V | General: inability to control facial muscles, difficulty closing mouth, mouth remains open 80% of time but able to close mouth with masseter stimulation Feeding: difficulty chewing food Speech: has a few words, difficulty speaking Quality of Life: difficulty expressing emotions | #1: 9 y 0 mo; 10 units /LPt (0.625 units/kg/LPt) #2–7: 9 y 4 mo–11 y 1 mo; 10–15 units /LPt (0.63–0.75 units/kg/LPt) * #8: 11 y 6mo; 20 units/ LPt (1 unit/kg/LPt) #9: 11 y 10 mo; 20 units/LPt (0.99 units/kg/LPt) | # 1: starting to talk more, mouth appears more relaxed and can now close mouth volitionally # 2–8: improved ability to close mouth, improved quality and quantity of speech, takes less time to eat, mouth open 20% of time with further (60%) improvement after more injections, decreased tongue thrust from a severity of 9/10 to 2/10 on a Likert scale #9: difficulty closing mouth after longer than usual interval between injections (5 months), jaw is noted to be open throughout clinic visit | #1 16.1 kg #2–7: 15.85–19.95 kg #8: 19.5 kg #9: 20.3 kg Last Documented Age: 12 y 3 mo Weight: 38kg |
| Patient 3 Dx: mixed spastic/dystonic quadriplegic CP GMFCS: II | General: able to close mouth on command, but maintains it open for most of the exam Feeding: chokes and coughs when swallowing liquids while sitting Speech: difficult to understand, relies on gestures and signs to communicate Quality of Life: excessive drooling, saliva on clothes with bib change 15 times per day | #1: 7 y 2 mo; 20 units/ LPt (1.05 units/kg/LPt) #2: 7 y 5mo; 20 units/LPt (1.01 units/kg/LPt) #3: 7 y 9 mo; 20 units/LPt (0.96 units/kg/LPt) #4: 8 y 2 mo; 20/units/ LPt (0.9 units/kg/LPt) | #1: improved ability to close mouth and speak, appeared less frustrated with better behavior, bib change 5 times per day #2-3: improved ability to close mouth and speak #4: starting to use straw to drink thick liquids, injection effect lasting longer, and improved oral intake | #1: 18.9 kg #2: 19.7 kg #3: 20.9 kg #4: 22 kg Last Documented As per #4 |
| Patient 4 Dx: mixed spastic/dystonic quadriplegic CP GMFCS: IV | General: poor motor control of the mouth Feeding: eats soft diet and takes longer than typical to finish meals Speech: can speak 1–2 words together but difficult to articulate Quality of Life: excessive drooling | #1: 4 y 7 mo; 10 units/ LPt (0.63 units/kg/LPt) #2: 5 y 0 mo; 10 units/LPt (0.6 units/kg/LPt) | #1: improved chewing and overall eating, improved articulation, parents can understand speech 100% of the time and strangers can understand speech 60% of the time #2: improvement in chewing, swallowing, and amount of PO intake | #1: 14.1 kg #2: 14.6 kg Last Documented Age: 6 y 5 mo Weight: 16.3 kg |
| Patient 5 Dx: mixed spastic/dystonic quadriplegic CP GMFCS: V | General: frequent bruxism and mal-alignment of the jaw Feeding: difficulty with chewing and eating Quality of Life: pain with teeth grinding | #1 28 y/o; 20 units/LPt (0.44 units/kg/LPt) | #1: improved jaw alignment, improved ability to chew and eat, improved teeth approximation, able to keep food in mouth while eating, slight increase in drooling, pain resolved | #1: 45.4 kg Last Documented Age: 28 y/o., (3 months post injections) Weight: 45.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes, F.I.; Shoval, H.A.; Tenaglia, A.; Kim, H. Ultrasound-Guided OnabotulinumtoxinA Injections to Treat Oromandibular Dystonia in Cerebral Palsy. Toxins 2022, 14, 158. https://doi.org/10.3390/toxins14030158
Reyes FI, Shoval HA, Tenaglia A, Kim H. Ultrasound-Guided OnabotulinumtoxinA Injections to Treat Oromandibular Dystonia in Cerebral Palsy. Toxins. 2022; 14(3):158. https://doi.org/10.3390/toxins14030158
Chicago/Turabian StyleReyes, Fabiola I., Hannah A. Shoval, Amy Tenaglia, and Heakyung Kim. 2022. "Ultrasound-Guided OnabotulinumtoxinA Injections to Treat Oromandibular Dystonia in Cerebral Palsy" Toxins 14, no. 3: 158. https://doi.org/10.3390/toxins14030158
APA StyleReyes, F. I., Shoval, H. A., Tenaglia, A., & Kim, H. (2022). Ultrasound-Guided OnabotulinumtoxinA Injections to Treat Oromandibular Dystonia in Cerebral Palsy. Toxins, 14(3), 158. https://doi.org/10.3390/toxins14030158





