Antinociceptive Actions of Botulinum Toxin A1 on Immunogenic Hypersensitivity in Temporomandibular Joint of Rats
Abstract
:1. Introduction
2. Results
2.1. BoNT-A Effects on Rat Grimace Scale (RGS) and Spontaneous Nocifensive Responses (Flinching, Sctratching)
2.2. Effects of BoNT-A on Mechanically-Evoked Responses at the TMJ Area
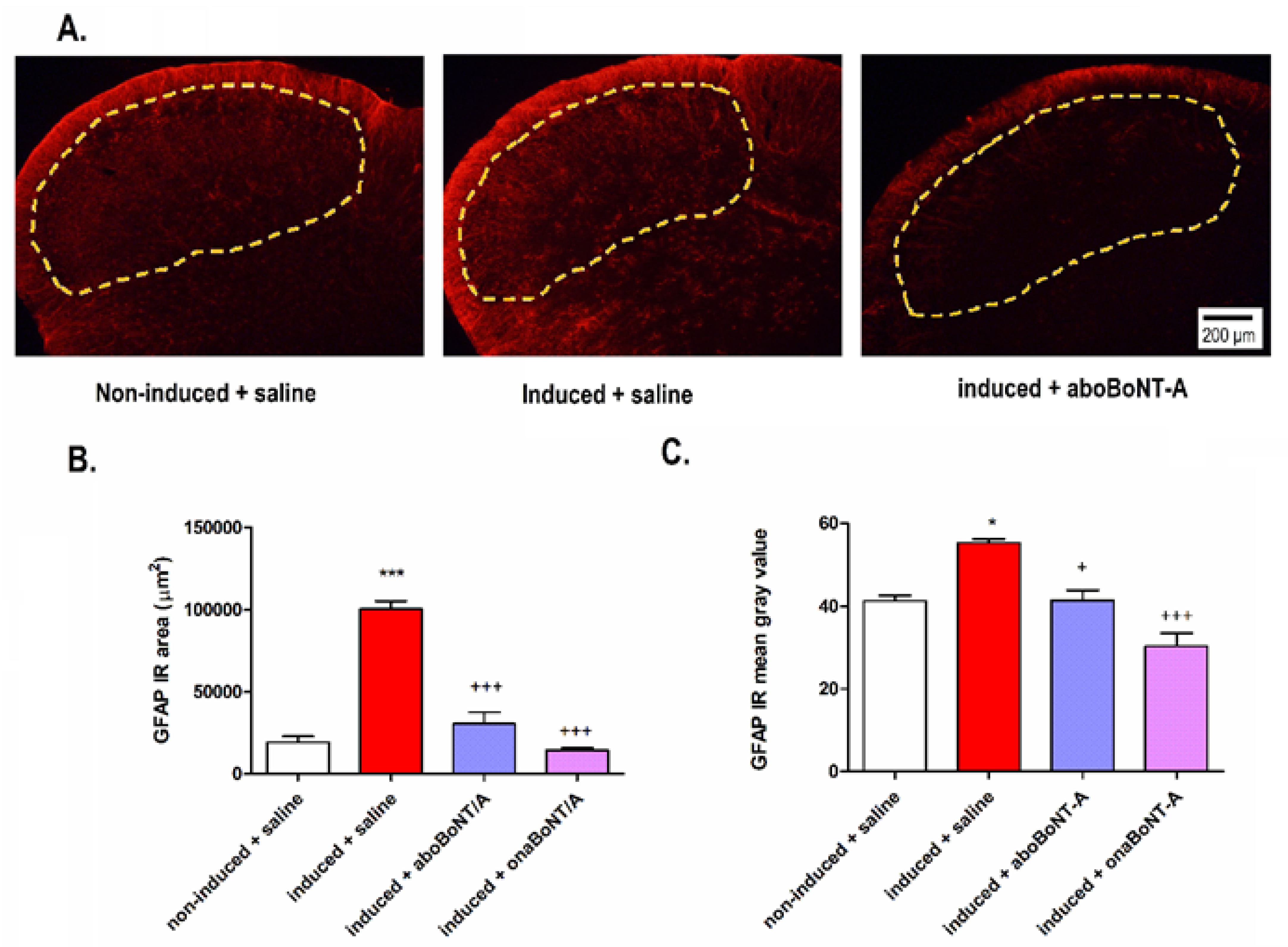
2.3. Effect of BoNT-A on Neuronal and Astrocyte Activation in the TNC
2.4. Immunohistochemical Localization of cSNAP-25 in the Brain
3. Discussion
3.1. Peripherally Injected BoNT-A Reduces Spontaneous and Mechanically Evoked Nocifensive Behaviors in Rats with PIH
3.2. Effects of BoNT-A on Nociceptive Neuronal and Glial Activation
3.3. Localization of cSNAP-25 in TNC
3.4. Preclinical Comparison of the Analgesic Efficacy of Different BoNT-A Pharmaceutical Preparations
3.5. Limitations
4. Materials and Methods
4.1. Persistent Immunogenic Hypersensitivity (PIH) in the TMJ
4.2. Study Design
4.3. Spontaneous and Mechanically Evoked Nocifensive Behaviors
4.3.1. Behavioral Nocifensive Responses
4.3.2. The Rat Grimace Scale (RGS)
4.3.3. Mechanical Sensitivity to von Frey Filaments
4.4. Immunohistochemistry
4.4.1. Localization of cSNAP-25 in the Brainstem
4.4.2. Quantification of Neuronal and Astrocyte Activation in the Brainstem
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alstergren, P.; Pigg, M.; Kopp, S. Clinical diagnosis of temporomandibular joint arthritis. J. Oral Rehabil. 2018, 45, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Catrina, A.I.; Alyamani, A.O.; Mustafa, H.; Alstergren, P. Deficient cytokine control modulates temporomandibular joint pain in rheumatoid arthritis. Eur. J. Oral Sci. 2015, 123, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, P.B.; Shah, D.S.; Ruparelia, K.; Sutaria, S.P.; Pathak, D. Bilateral TMJ involvement in rheumatoid arthritis. Case Rep. Dent. 2014, 2014, 262430. [Google Scholar] [CrossRef] [Green Version]
- Romero-Reyes, M.; Uyanik, J.M. Orofacial pain management: Current perspectives. J. Pain Res. 2014, 7, 99–115. [Google Scholar] [CrossRef] [Green Version]
- International Headache Society. International Classification of Orofacial Pain Committee International Classification of Orofacial Pain, 1st edition (ICOP). Cephalalgia 2020, 40, 129–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sodhi, A.; Naik, S.; Pai, A.; Anuradha, A. Rheumatoid arthritis affecting temporomandibular joint. Contemp. Clin. Dent. 2015, 6, 124–127. [Google Scholar] [CrossRef]
- Mogil, J.S. Animal models of pain: Progress and challenges. Nat. Rev. Neurosci. 2009, 10, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Lora, V.R.M.; Clemente-Napimoga, J.T.; Ballassini, H.; Gomes-Macedo, C.; De la Torre Canales, G.; Rizzatti-Barbosa, C.M. Botulinum toxin type A reduces inflammatory hypernociception induced by arthritis in the temporomadibular joint of rats. Toxicon 2017, 129, 52–57. [Google Scholar] [CrossRef]
- Leuchtweis, J.; Segond von Banchet, G.; Eitner, A.; Ebbinghaus, M.; Schaible, H.-G. Pain-related behaviors associated with persistence of mechanical hyperalgesia after antigen-induced arthritis in rats. Pain 2020, 161, 1571–1583. [Google Scholar] [CrossRef]
- Bonfante, R.; Napimoga, M.H.; Macedo, C.G.; Abdalla, H.B.; Pieroni, V.; Clemente-Napimoga, J.T. The P2X7 receptor, cathepsin S and fractalkine in the trigeminal subnucleus caudalis signal persistent hypernociception in temporomandibular rat joints. Neuroscience 2018, 391, 120–130. [Google Scholar] [CrossRef]
- de Sousa, L.M.; dos Santos Alves, J.M.; da Silva Martins, C.; Pereira, K.M.A.; Goes, P.; Gondim, D.V. Immunoexpression of canonical Wnt and NF-κB signaling pathways in the temporomandibular joint of arthritic rats. Inflamm. Res. 2019, 68, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Roveroni, R.C.; Parada, C.A.; Cecília, M.; Veiga, F.A.; Tambeli, C.H. Development of a behavioral model of TMJ pain in rats: The TMJ formalin test. Pain 2001, 94, 185–191. [Google Scholar] [CrossRef]
- Atkinson, S.M.; Nansen, A. Pharmacological value of murine delayed-type hypersensitivity arthritis: A robust mouse model of rheumatoid arthritis in C57BL/6 mice. Basic Clin. Pharmacol. Toxicol. 2017, 120, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Billiau, A.; Matthys, P. Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J. Leukoc. Biol. 2001, 70, 849–860. [Google Scholar] [CrossRef]
- Bach-Rojecky, L.; Lacković, Z. Central origin of the antinociceptive action of botulinum toxin type A. Pharmacol. Biochem. Behav. 2009, 94, 234–238. [Google Scholar] [CrossRef] [Green Version]
- Favre-Guilmard, C.; Chabrier, P.-E.; Kalinichev, M. Bilateral analgesic effects of abobotulinumtoxinA (Dysport®) following unilateral administration in the rat. Eur. J. Pain 2017, 21, 927–937. [Google Scholar] [CrossRef]
- Matak, I.; Bach-Rojecky, L.; Filipović, B.; Lacković, Z. Behavioral and immunohistochemical evidence for central antinociceptive activity of botulinum toxin A. Neuroscience 2011, 186, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Rapp, D.E.; Turk, K.W.; Bales, G.T.; Cook, S.P. Botulinum toxin type a inhibits calcitonin gene-related peptide release from isolated rat bladder. J. Urol. 2006, 175, 1138–1142. [Google Scholar] [CrossRef]
- Cui, M.; Khanijou, S.; Rubino, J.; Aoki, K.R. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain 2004, 107, 125–133. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Yoshimura, N.; Huang, C.-C.; Wu, M.; Chiang, P.-H.; Chancellor, M.B. Intravesical botulinum toxin A administration inhibits COX-2 and EP4 expression and suppresses bladder hyperactivity in cyclophosphamide-induced cystitis in rats. Eur. Urol. 2009, 56, 159–167. [Google Scholar] [CrossRef]
- Rojewska, E.; Piotrowska, A.; Popiolek-Barczyk, K.; Mika, J. Botulinum toxin type A—A modulator of spinal neuron–glia interactions under neuropathic pain conditions. Toxins 2018, 10, 145. [Google Scholar] [CrossRef] [Green Version]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods used to evaluate pain behaviors in rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef] [Green Version]
- Romero-Reyes, M.; Akerman, S.; Nguyen, E.; Vijjeswarapu, A.; Hom, B.; Dong, H.-W.; Charles, A.C. Spontaneous behavioral responses in the orofacial region: A model of trigeminal pain in mouse. Headache J. Head Face Pain 2013, 53, 137–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vos, B.P.; Hans, G.; Adriaensen, H. Behavioral assessment of facial pain in rats: Face grooming patterns after painful and non-painful sensory disturbances in the territory of the rats infraorbital nerve. Pain 1998, 76, 173–178. [Google Scholar] [CrossRef]
- Ekman, P.; Friesen, W.V. Facial Action Coding System: A Technique for the Measurement of Facial Movement; Consulting Psychologists Press: Palo Alto, CA, USA, 1978. [Google Scholar]
- Langford, D.J.; Bailey, A.L.; Chanda, M.L.; Clarke, S.E.; Drummond, T.E.; Echols, S.; Glick, S.; Ingrao, J.; Klassen-Ross, T.; Lacroix-Fralish, M.L.; et al. Coding of facial expressions of pain in the laboratory mouse. Nat. Methods 2010, 7, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Clavelou, P.; Dallel, R.; Orliaguet, T.; Woda, A.; Raboisson, P. The orofacial formalin test in rats: Effects of different formalin concentrations. Pain 1995, 62, 295–301. [Google Scholar] [CrossRef]
- Quinteiro, M.S.; Napimoga, M.H.; Mesquita, K.P.; Clemente-Napimoga, J.T. The indirect antinociceptive mechanism of 15d-PGJ2 on rheumatoid arthritis-induced TMJ inflammatory pain in rats. Eur. J. Pain 2012, 16, 1106–1115. [Google Scholar] [CrossRef]
- Filipović, B.; Matak, I.; Bach-Rojecky, L.; Lacković, Z. Central action of peripherally applied botulinum toxin type A on pain and dural protein extravasation in rat model of trigeminal neuropathy. PLoS ONE 2012, 7, e29803. [Google Scholar] [CrossRef] [Green Version]
- Bullitt, E. Expression ofC-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J. Comp. Neurol. 1990, 296, 517–530. [Google Scholar] [CrossRef]
- Drinovac, V.; Bach-Rojecky, L.; Matak, I.; Lacković, Z. Involvement of μ-opioid receptors in antinociceptive action of botulinum toxin type A. Neuropharmacology 2013, 70, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Matak, I.; Rossetto, O.; Lacković, Z. Botulinum toxin type A selectivity for certain types of pain is associated with capsaicin-sensitive neurons. Pain 2014, 155, 1516–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matak, I.; Tékus, V.; Bölcskei, K.; Lacković, Z.; Helyes, Z. Involvement of substance P in the antinociceptive effect of botulinum toxin type A: Evidence from knockout mice. Neuroscience 2017, 358, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Exposto, F.G.; Udagawa, G.; Naganawa, T.; Svensson, P. Comparison of masseter muscle referred sensations after mechanical and glutamate stimulation. Pain 2018, 159, 2649–2657. [Google Scholar] [CrossRef]
- Masuda, M.; Iida, T.; Exposto, F.; Baad-Hansen, L.; Kawara, M.; Komiyama, O.; Svensson, P. Referred pain and sensations evoked by standardized palpation of the masseter muscle in healthy participants. J. Oral Facial Pain Headache 2018, 32, 159–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, R.-R.; Berta, T.; Nedergaard, M. Glia and pain: Is chronic pain a gliopathy? Pain 2013, 154, S10–S28. [Google Scholar] [CrossRef]
- Scholz, J.; Woolf, C.J. The neuropathic pain triad: Neurons, immune cells and glia. Nat. Neurosci. 2007, 10, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, X.; Zhang, C.; Zhang, Y.; Yao, W. An update on reactive astrocytes in chronic pain. J. Neuroinflamm. 2019, 16, 140. [Google Scholar] [CrossRef]
- Piotrowska, A.; Kwiatkowski, K.; Rojewska, E.; Makuch, W.; Mika, J. Maraviroc reduces neuropathic pain through polarization of microglia and astroglia—Evidence from in vivo and in vitro studies. Neuropharmacology 2016, 108, 207–219. [Google Scholar] [CrossRef]
- Marinelli, S.; Vacca, V.; Ricordy, R.; Uggenti, C.; Tata, A.M.; Luvisetto, S.; Pavone, F. the analgesic effect on neuropathic pain of retrogradely transported botulinum neurotoxin A involves Schwann cells and astrocytes. PLoS ONE 2012, 7, e47977. [Google Scholar] [CrossRef] [Green Version]
- Lacković, Z.; Filipović, B.; Matak, I.; Helyes, Z. Activity of botulinum toxin type A in cranial dura: Implications for treatment of migraine and other headaches. Br. J. Pharmacol. 2016, 173, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Aoki, K.R. Pharmacology and immunology of botulinum toxin serotypes. J. Neurol. 2001, 248, I3–I10. [Google Scholar] [CrossRef] [PubMed]
- Casatti, C.A.; Frigo, L.; Bauer, J.A. Origin of sensory and autonomic innervation of the rat temporomandibular joint: A retrograde axonal tracing study with the fluorescent dye fast blue. J. Dent. Res. 1999, 78, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, F.; Rossi, C.; Gianfranceschi, L.; Rossetto, O.; Caleo, M. Long-distance retrograde effects of botulinum neurotoxin A. J. Neurosci. 2008, 28, 3689–3696. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F. Conversion ratio between Botox®, Dysport®, and Xeomin® in clinical practice. Toxins 2016, 8, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, T.J.; Dayan, S.H. Comparison and overview of currently available neurotoxins. J. Clin. Aesthet. Dermatol. 2014, 7, 31–39. [Google Scholar]
- Field, M.; Splevins, A.; Picaut, P.; van der Schans, M.; Langenberg, J.; Noort, D.; Foster, K. AbobotulinumtoxinA (Dysport®), OnabotulinumtoxinA (Botox®), and IncobotulinumtoxinA (Xeomin®) neurotoxin content and potential implications for duration of response in patients. Toxins 2018, 10, 535. [Google Scholar] [CrossRef] [Green Version]
- Wohlfarth, K.; Schwandt, I.; Wegner, F.; Jürgens, T.; Gelbrich, G.; Wagner, A.; Bogdahn, U.; Schulte-Mattler, W. Biological activity of two botulinum toxin type A complexes (Dysport and Botox) in volunteers: A double-blind, randomized, dose-ranging study. J. Neurol. 2008, 255, 1932–1939. [Google Scholar] [CrossRef]
- Kollewe, K.; Mohammadi, B.; Dengler, R.; Dressler, D. Hemifacial spasm and reinnervation synkinesias: Long-term treatment with either Botox or Dysport. J. Neural Transm. 2010, 117, 759–763. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthi, I.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Vet. Clin. Pathol. 2012, 41, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Sotocinal, S.G.; Sorge, R.E.; Zaloum, A.; Tuttle, A.H.; Martin, L.J.; Wieskopf, J.S.; Mapplebeck, J.C.S.; Wei, P.; Zhan, S.; Zhang, S.; et al. The Rat Grimace Scale: A partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain 2011, 7, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 5th ed.; Elsevier Academic: Burlington, MA, USA, 2005. [Google Scholar]
- Mills, C.; Leblond, D.; Joshi, S.; Zhu, C.; Hsieh, G.; Jacobson, P.; Meyer, M.; Decker, M. Estimating efficacy and drug ED50′s using von Frey thresholds: Impact of weber’s law and log transformation. J. Pain 2012, 13, 519–523. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Lora, V.R.M.; Dugonjić Okroša, A.; Matak, I.; Del Bel Cury, A.A.; Kalinichev, M.; Lacković, Z. Antinociceptive Actions of Botulinum Toxin A1 on Immunogenic Hypersensitivity in Temporomandibular Joint of Rats. Toxins 2022, 14, 161. https://doi.org/10.3390/toxins14030161
Muñoz-Lora VRM, Dugonjić Okroša A, Matak I, Del Bel Cury AA, Kalinichev M, Lacković Z. Antinociceptive Actions of Botulinum Toxin A1 on Immunogenic Hypersensitivity in Temporomandibular Joint of Rats. Toxins. 2022; 14(3):161. https://doi.org/10.3390/toxins14030161
Chicago/Turabian StyleMuñoz-Lora, Victor Ricardo Manuel, Ana Dugonjić Okroša, Ivica Matak, Altair Antoninha Del Bel Cury, Mikhail Kalinichev, and Zdravko Lacković. 2022. "Antinociceptive Actions of Botulinum Toxin A1 on Immunogenic Hypersensitivity in Temporomandibular Joint of Rats" Toxins 14, no. 3: 161. https://doi.org/10.3390/toxins14030161
APA StyleMuñoz-Lora, V. R. M., Dugonjić Okroša, A., Matak, I., Del Bel Cury, A. A., Kalinichev, M., & Lacković, Z. (2022). Antinociceptive Actions of Botulinum Toxin A1 on Immunogenic Hypersensitivity in Temporomandibular Joint of Rats. Toxins, 14(3), 161. https://doi.org/10.3390/toxins14030161




