A Sixty-Year Research and Development of Trichosanthin, a Ribosome-Inactivating Protein
Abstract
:1. Introduction
2. Trichosanthin as an Abortifacient
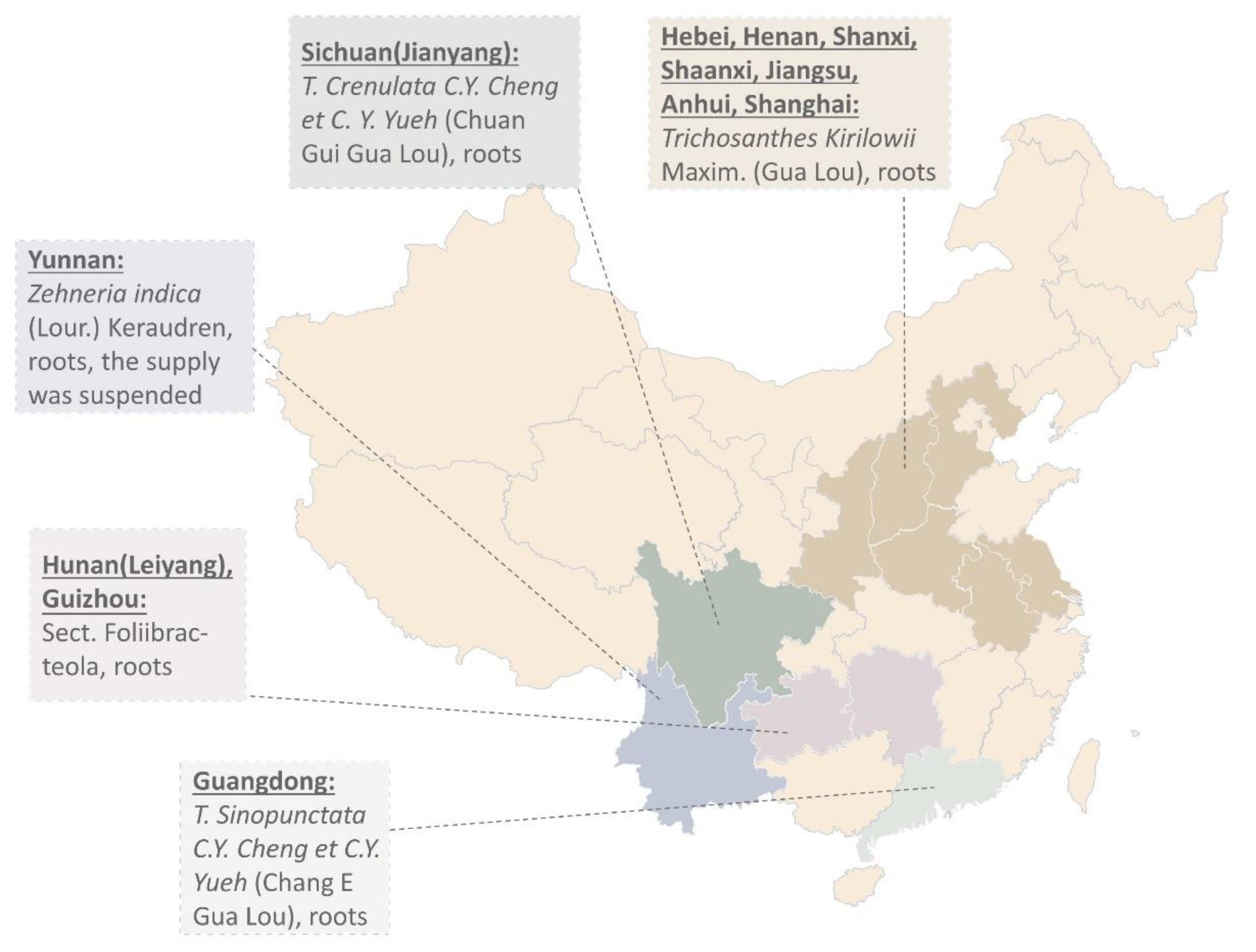
2.1. From Ancient Prescriptions to Crystallized Protein Powder
2.2. Clinical Application of Tian Hua Fen or TCS as an Abortifacient
2.3. Protein and DNA Sequences of TCS
3. Trichosanthin as an rRNA N-glycosylase
3.1. Mechanism of TCS as an rRNA N-glycosylase
3.2. Mechanism of TCS as Revealed by Structural Studies
3.3. Interaction of TCS with the Ribosome and the Recruitment by Ribosomal P-Protein
4. Trichosanthin as an Anti-HIV Agent
4.1. Anti-HIV Activity of TCS and Its Related Mechanism
4.2. Clinical Study of TCS on AIDS Patients
5. Trichosanthin as an Anti-Cancer Agent
5.1. The Entering of TCS into Cells
5.2. Anti-Cancer Properties of TCS
6. Engineering and TCS-Based Immunotoxins
7. Conclusion and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shaw, P.C.; Chan, W.L.; Yeung, H.W.; Ng, T.B. Minireview: Trichosanthin-a protein with multiple pharmacological properties. Life Sci. 1994, 55, 253–262. [Google Scholar] [CrossRef]
- Chang, M.C.; Saksena, S.K.; Lau, I.F.; Wang, Y.H. Induction of mid-term abortion by trichosanthin in laboratory animals. Contraception 1979, 19, 175–184. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, S. Trichosanthin, 2nd ed.; Ke Xue Chu Ban She: Beijing, China, 2000. [Google Scholar]
- Tang, S. Chongxiu Zhenghe Jingshi Zhenglei Beiyong Bencao; Hunan Ke Xue Ji Shu Chu Ban She: Changsha, China, 2014. [Google Scholar]
- Le, C.; Wei, J.; Lou, Z. Renewed report on study on the identification of Trichosanthin and its similar products. Acta Pharm. Sin. 1982, 17, 777–782. [Google Scholar]
- Le, C.; Lou, Z. Identification of Trichosanthin and its similar products. Acta Pharm. Sin. 1979, 14, 641–654. [Google Scholar]
- Wang, N.; Xu, G.; Jin, R.; Xu, L. Study on the identification of Trichosanthin. Zhongguo Yao Ke Da Xue Xue Bao 1983, 2, 48–76. [Google Scholar]
- Wang, Y. Trichosanthin, 1st ed.; Ke Xue Chu Ban She: Beijing, China, 1990. [Google Scholar]
- P., Z. A brief introduction of the use of Trichosanthin. Guang Dong Yi Xue 1974, 10, 16–24. [Google Scholar]
- Jin, Y.C. Intra-amniotic injection of crystal trichosanthin for induction of labour in second trimester pregnancy. Sheng Zhi Yu Bi Yun 1985, 5, 15–17, 20. [Google Scholar]
- Song, J.F.; Liu, T.; Shen, X.; Wu, G.D.; Xia, Q.C. Application of free-flow electrophoresis to the purification of trichosanthin from a crude product of acetone fractional precipitation. Electrophoresis 1998, 19, 1097–1103. [Google Scholar] [CrossRef]
- Jin, S.; Sun, X.; Wang, S.; Tian, G.; Gu, Z.; Qian, W.; Liu, Y.; She, W.; Qian, R.; Wang, Y. Chemistry of Trichosanthin I. physical and chemical properties of crystallized Trichosanthin. Hua Xue Xue Bao 1981, 39, 513–519. [Google Scholar]
- Jin, Y.; Zou, Y. Comparative analysis of intramuscular and cervical injection of crystallized trichosanthin in 200 cases of 10—14 weeks of pregnancy. Sheng Zhi Yu Bi Yun 1990, 10, 34–37. [Google Scholar]
- Mondal, A. A novel extraction of trichosanthin from Trichosanthes kirilowii roots using three-phase partitioning and its in vitro anticancer activity. Pharm Biol. 2014, 52, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.F.; Wu, X.; Li, Y.; Zhang, P.; Guo, X.; Lv, S. Application of crystallized Trichosanthin prescription to difficult induced abortion-preliminary analyses on 179 patients. Reprod. Contracept. 1985, 1, 10–14. [Google Scholar]
- Xu, M.F.; Jin, Y.C. Clinical trial of trichosanthin with or without dexamethasone in induction of abortion by four different routes of administration. Sheng Zhi Yu Bi Yun 1991, 11, 47–50. [Google Scholar]
- Katzin, L. Compound Q clinical tests begin. Am. J. Nurs. 1989, 89, 916. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.O.; Kaplan, L.D.; Gambertoglio, J.G.; Bredesen, D.; Arri, C.J.; Turin, L.; Kibort, T.; Williams, R.L.; Lifson, J.D.; Volberding, P.A. The safety and pharmacokinetics of GLQ223 in subjects with AIDS and AIDS-related complex: A phase I study. AIDS 1990, 4, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Byers, V.S.; Levin, A.S.; Waites, L.A.; Starrett, B.A.; Mayer, R.A.; Clegg, J.A.; Price, M.R.; Robins, R.A.; Delaney, M.; Baldwin, R.W. A phase I/II study of trichosanthin treatment of HIV disease. AIDS 1990, 4, 1189–1196. [Google Scholar] [CrossRef]
- Gatti, G.; Kahn, J.O.; Lifson, J.; Williams, R.; Turin, L.; Volberding, P.A.; Gambertoglio, J.G. Pharmacokinetics of GLQ223 in rats, monkeys, and patients with AIDS or AIDS-related complex. Antimicrob. Agents Chemother. 1991, 35, 2531–2537. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Zheng, Z.; Lu, D. Safety, efficacy and mechanism of trichosanthin therapy for AIDS. Shang Hai Mian Yi Xue Za Zhi 1992, 12, 116–120. [Google Scholar]
- Garcia, P.A.; Bredesen, D.E.; Vinters, H.V.; Graefin von Einsiedel, R.; Williams, R.L.; Kahn, J.O.; Byers, V.S.; Levin, A.S.; Waites, L.A.; Messing, R.O. Neurological reactions in HIV-infected patients treated with trichosanthin. Neuropathol. Appl. Neurobiol. 1993, 19, 402–405. [Google Scholar] [CrossRef]
- Kahn, J.O.; Gorelick, K.J.; Gatti, G.; Arri, C.J.; Lifson, J.D.; Gambertoglio, J.G.; Bostrom, A.; Williams, R. Safety, activity, and pharmacokinetics of GLQ223 in patients with AIDS and AIDS-related complex. Antimicrob. Agents Chemother. 1994, 38, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Jin, Y. Analysis of Trichosanthin Cervix injection to induce abortion and dexamethasone to reduce side effects. Xian Dai Fu Chan Ke Jin Zhan 1994, 3, 227–228. [Google Scholar]
- Zhang, S.; Jin, Y.; Yu, J.; Qi, W.; Liu, Y.; Xue, M.; Chen, M. Further study on the effect of trichosanthin induced Abortion on heart, liver and kidney function in pregnant women. Sheng Zhi Yu Bi Yun 1994, 14, 19–24. [Google Scholar]
- Byers, V.S.; Levin, A.S.; Malvino, A.; Waites, L.; Robins, R.A.; Baldwin, R.W. A phase II study of effect of addition of trichosanthin to zidovudine in patients with HIV disease and failing antiretroviral agents. AIDS Res. Hum. Retrovir. 1994, 10, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Chun, L.; Yucui, J. Clinical observation of the effect intracervical and intramuscular injection of Trichosanthin on tubal pregnancy. J. Shanghai Jiaotong Univ. (Med. Sci) 2000, 20, 447–449. [Google Scholar]
- Wang, J.; Jin, Y. Comparative study in three methods of medicinal abortion of mid-term pregenancy. Shang Hai Di Er Jun Yi Da Xue Xue Bao 2001, 21, 264–266. [Google Scholar]
- Leung, K.N.; Yeung, H.W.; Leung, S.O. The immunomodulatory and antitumor activities of trichosanthin-an abortifacient protein isolated from tian-hua-fen (Trichosanthes kirilowii). Asian Pac. J. Allergy Immunol. 1986, 4, 111–120. [Google Scholar]
- Chan, W.Y.; Ng, T.B.; Yeung, H.W. Trichosanthin as an abortifacient for terminating early pregnancy in mice. Int. J. Fertil. 1993, 38, 99–107. [Google Scholar]
- Chu, J.J.; Devall, A.J.; Beeson, L.E.; Hardy, P.; Cheed, V.; Sun, Y.; Roberts, T.E.; Ogwulu, C.O.; Williams, E.; Jones, L.L.; et al. Mifepristone and misoprostol versus misoprostol alone for the management of missed miscarriage (MifeMiso): A randomised, double-blind, placebo-controlled trial. Lancet 2020, 396, 770–778. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, R.-Q.; Gu, Z.-W.; Jin, S.-W.; Zhang, L.-Q.; Xia, Z.-X.; Tian, G.-Y.; Ni, C.-Z. Scientific evaluation of Tian Hua Fen (THF)-history, chemistry and application. Pure Appl. Chem. 1986, 58, 789–798. [Google Scholar] [CrossRef] [Green Version]
- Collins, E.J.; Robertus, J.D.; LoPresti, M.; Stone, K.L.; Williams, K.R.; Wu, P.; Hwang, K.; Piatak, M. Primary amino acid sequence of alpha-trichosanthin and molecular models for abrin A-chain and alpha-trichosanthin. J. BioL. Chem. 1990, 265, 8665–8669. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Z.; Ye, G.; Sun, X.; Wang, Q.; Jin, S. Revision of the primary structure of trichosanthin and study on the trichosanthin from different places of origin. Acta Chim. Sin. 1993, 51, 1023–1029. [Google Scholar]
- Chow, T.P.; Feldman, R.A.; Lovett, M.; Piatak, M. Isolation and DNA sequence of a gene encoding alpha-trichosanthin, a type I ribosome-inactivating protein. J Biol Chem 1990, 265, 8670–8674. [Google Scholar] [CrossRef]
- Shaw, P.C.; Yung, M.H.; Zhu, R.H.; Ho, W.K.; Ng, T.B.; Yeung, H.W. Cloning of trichosanthin cDNA and its expression in Escherichia coli. Gene 1991, 97, 267–272. [Google Scholar] [CrossRef]
- Zhu, R.H.; Ng, T.B.; Yeung, H.W.; Shaw, P.C. High level synthesis of biologically active recombinant trichosanthin in Escherichia coli. Int. J. Pept. Protein Res. 1992, 39, 77–81. [Google Scholar] [CrossRef]
- Zhang, J.S.; Liu, W.Y. The mechanism of action of trichosanthin on eukaryotic ribosomes--RNA N-glycosidase activity of the cytotoxin. Nucleic Acids Res. 1992, 20, 1271–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, Y.; Mitsui, K.; Motizuki, M.; Tsurugi, K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J. Biol. Chem. 1987, 262, 5908–5912. [Google Scholar] [CrossRef]
- Endo, Y.; Chan, Y.L.; Lin, A.; Tsurugi, K.; Wool, I.G. The cytotoxins alpha-sarcin and ricin retain their specificity when tested on a synthetic oligoribonucleotide (35-mer) that mimics a region of 28 S ribosomal ribonucleic acid. J. Biol. Chem. 1988, 263, 7917–7920. [Google Scholar] [CrossRef]
- Nilsson, L.; Nygard, O. The mechanism of the protein-synthesis elongation cycle in eukaryotes. Effect of ricin on the ribosomal interaction with elongation factors. Eur. J. Biochem. 1986, 161, 111–117. [Google Scholar] [CrossRef]
- Choi, A.K.; Wong, E.C.; Lee, K.M.; Wong, K.B. Structures of eukaryotic ribosomal stalk proteins and its complex with trichosanthin, and their implications in recruiting ribosome-inactivating proteins to the ribosomes. Toxins 2015, 7, 638–647. [Google Scholar] [CrossRef] [Green Version]
- Grela, P.; Szajwaj, M.; Horbowicz-Drożdżal, P.; Tchórzewski, M. How ricin damages the ribosome. Toxins 2019, 11, 241. [Google Scholar] [CrossRef] [Green Version]
- Voorhees, R.M.; Schmeing, T.M.; Kelley, A.C.; Ramakrishnan, V. The mechanism for activation of GTP hydrolysis on the ribosome. Science 2010, 330, 835–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Lu, X.; Zhu, Y.; Yang, J.; Dong, Y. N-glycosidase mechanism of Trichosanthin. Sci. China C Life Sci. 1998, 41, 174–180. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, S.; Tang, Y.; Jin, S.; Wang, Y. Studies on crystal structures, active-centre geometry and depurinating mechanism of two ribosome-inactivating proteins. Biochem. J. 1995, 309, 285–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, J.; Wang, Y.; Dong, Y.; Stuart, D.I. The N-glycosidase mechanism of ribosome-inactivating proteins implied by crystal structures of alpha-momorcharin. Structure 1994, 2, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Monzingo, A.F.; Robertus, J.D. X-ray analysis of substrate analogs in the ricin A-chain active site. J. Mol. Biol. 1992, 227, 1136–1145. [Google Scholar] [CrossRef]
- Xiong, J.P.; Xia, Z.X.; Wang, Y. Identification of a stable complex of trichosanthin with nicotinamide adenine dinucleotide phosphate. J. Protein Chem. 1995, 14, 139–144. [Google Scholar] [CrossRef]
- Xiong, J.P.; Xia, Z.X.; Wang, Y. Crystal structure of trichosanthin-NADPH complex at 1.7 A resolution reveals active-site architecture. Nat. Struct. Biol. 1994, 1, 695–700. [Google Scholar] [CrossRef]
- Shi, W.W.; Wong, K.B.; Shaw, P.C. Structural and functional investigation and pharmacological mechanism of Trichosanthin, a type 1 ribosome-inactivating protein. Toxins 2018, 10, 335. [Google Scholar] [CrossRef] [Green Version]
- Frankel, A.; Welsh, P.; Richardson, J.; Robertus, J.D. Role of arginine 180 and glutamic acid 177 of ricin toxin A chain in enzymatic inactivation of ribosomes. Mol. Cell Biol. 1990, 10, 6257–6263. [Google Scholar] [CrossRef] [Green Version]
- Ready, M.P.; Kim, Y.; Robertus, J.D. Site-directed mutagenesis of ricin A-chain and implications for the mechanism of action. Proteins 1991, 10, 270–278. [Google Scholar] [CrossRef]
- Wong, K.B.; Ke, Y.B.; Dong, Y.C.; Li, X.B.; Guo, Y.W.; Yeung, H.W.; Shaw, P.C. Structure/function relationship study of Gln156, Glu160 and Glu189 in the active site of trichosanthin. Eur. J. Biochem. 1994, 221, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.J.; Xia, Z.X. Crystal structures of the complexes of trichosanthin with four substrate analogs and catalytic mechanism of RNA N-glycosidase. Proteins 2000, 39, 37–46. [Google Scholar] [CrossRef]
- Shi, W.W.; Mak, A.N.; Wong, K.B.; Shaw, P.C. Structures and ribosomal interaction of ribosome-inactivating proteins. Molecules 2016, 21, 1588. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.H.; Hung, F.S.; Chan, D.S.; Shaw, P.C. Trichosanthin interacts with acidic ribosomal proteins P0 and P1 and mitotic checkpoint protein MAD2B. Eur. J. Biochem. 2001, 268, 2107–2112. [Google Scholar] [CrossRef]
- Tchorzewski, M. The acidic ribosomal P proteins. Int. J. Biochem. Cell Biol. 2002, 34, 911–915. [Google Scholar] [CrossRef]
- Ban, N.; Beckmann, R.; Cate, J.H.; Dinman, J.D.; Dragon, F.; Ellis, S.R.; Lafontaine, D.L.; Lindahl, L.; Liljas, A.; Lipton, J.M.; et al. A new system for naming ribosomal proteins. Curr. Opin. Struct. Biol. 2014, 24, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Grela, P.; Gajda, M.J.; Armache, J.P.; Beckmann, R.; Krokowski, D.; Svergun, D.I.; Grankowski, N.; Tchórzewski, M. Solution structure of the natively assembled yeast ribosomal stalk determined by small-angle X-ray scattering. Biochem. J. 2012, 444, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Chan, D.S.; Chu, L.O.; Lee, K.M.; Too, P.H.; Ma, K.W.; Sze, K.H.; Zhu, G.; Shaw, P.C.; Wong, K.B. Interaction between trichosanthin, a ribosome-inactivating protein, and the ribosomal stalk protein P2 by chemical shift perturbation and mutagenesis analyses. Nucleic Acids Res. 2007, 35, 1660–1672. [Google Scholar] [CrossRef] [Green Version]
- Szajwaj, M.; Wawiórka, L.; Molestak, E.; Michalec-Wawiórka, B.; Mołoń, M.; Wojda, I.; Tchórzewski, M. The influence of ricin-mediated rRNA depurination on the translational machinery in vivo-New insight into ricin toxicity. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 118554. [Google Scholar] [CrossRef]
- Too, P.H.; Ma, M.K.; Mak, A.N.; Wong, Y.T.; Tung, C.K.; Zhu, G.; Au, S.W.; Wong, K.B.; Shaw, P.C. The C-terminal fragment of the ribosomal P protein complexed to trichosanthin reveals the interaction between the ribosome-inactivating protein and the ribosome. Nucleic Acids Res. 2009, 37, 602–610. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.M.; Yusa, K.; Chu, L.O.; Yu, C.W.; Oono, M.; Miyoshi, T.; Ito, K.; Shaw, P.C.; Wong, K.B.; Uchiumi, T. Solution structure of human P1*P2 heterodimer provides insights into the role of eukaryotic stalk in recruiting the ribosome-inactivating protein trichosanthin to the ribosome. Nucleic Acids Res. 2013, 41, 8776–8787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.M.; Yu, C.W.; Chan, D.S.; Chiu, T.Y.; Zhu, G.; Sze, K.H.; Shaw, P.C.; Wong, K.B. Solution structure of the dimerization domain of ribosomal protein P2 provides insights for the structural organization of eukaryotic stalk. Nucleic Acids Res. 2010, 38, 5206–5216. [Google Scholar] [CrossRef]
- Lee, K.M.; Yu, C.W.; Chiu, T.Y.; Sze, K.H.; Shaw, P.C.; Wong, K.B. Solution structure of the dimerization domain of the eukaryotic stalk P1/P2 complex reveals the structural organization of eukaryotic stalk complex. Nucleic Acids Res. 2012, 40, 3172–3182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wawiórka, L.; Molestak, E.; Szajwaj, M.; Michalec-Wawiórka, B.; Mołoń, M.; Borkiewicz, L.; Grela, P.; Boguszewska, A.; Tchórzewski, M. Multiplication of ribosomal P-stalk proteins contributes to the fidelity of translation. Mol. Cell Biol. 2017, 37, e00060-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.P.; Grela, P.; Krokowski, D.; Tchórzewski, M.; Tumer, N.E. Pentameric organization of the ribosomal stalk accelerates recruitment of ricin a chain to the ribosome for depurination. J. Biol. Chem. 2010, 285, 41463–41471. [Google Scholar] [CrossRef] [Green Version]
- Grela, P.; Li, X.P.; Horbowicz, P.; Dźwierzyńska, M.; Tchórzewski, M.; Tumer, N.E. Human ribosomal P1-P2 heterodimer represents an optimal docking site for ricin A chain with a prominent role for P1 C-terminus. Sci. Rep. 2017, 7, 5608. [Google Scholar] [CrossRef] [Green Version]
- Horbowicz-Drozdzal, P.; Kamel, K.; Kmiecik, S.; Borkiewicz, L.; Tumer, N.E.; Shaw, P.C.; Tchorzewski, M.; Grela, P. Phosphorylation of the conserved C-terminal domain of ribosomal P-proteins impairs the mode of interaction with plant toxins. FEBS Lett. 2021, 595, 2221–2236. [Google Scholar] [CrossRef]
- Shi, W.W.; Tang, Y.S.; Sze, S.Y.; Zhu, Z.N.; Wong, K.B.; Shaw, P.C. Crystal structure of ribosome-inactivating protein ricin A chain in complex with the C-terminal peptide of the ribosomal stalk protein P2. Toxins 2016, 8, 296. [Google Scholar] [CrossRef] [Green Version]
- McGrath, M.S.; Santulli, S.; Gaston, I. Effects of GLQ223 on HIV replication in human monocyte/macrophages chronically infected in vitro with HIV. AIDS Res. Hum. Retrovir. 1990, 6, 1039–1043. [Google Scholar] [CrossRef]
- McGrath, M.S.; Hwang, K.M.; Caldwell, S.E.; Gaston, I.; Luk, K.C.; Wu, P.; Ng, V.L.; Crowe, S.; Daniels, J.; Marsh, J.; et al. GLQ223: An inhibitor of human immunodeficiency virus replication in acutely and chronically infected cells of lymphocyte and mononuclear phagocyte lineage. Proc. Natl. Acad. Sci. USA 1989, 86, 2844–2848. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, P.; Trabaud, M.A.; Rommain, M.; Mandine, E.; Zalisz, R.; Desgranges, C.; Smets, P. Toxicity and activity of purified trichosanthin. AIDS 1991, 5, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Ng, T.B.; Shaw, P.C.; Wong, R.N. Recent progress in medicinal investigations on trichosanthin and other ribosome inactivating proteins from the plant genus Trichosanthes. Curr. Med. Chem. 2011, 18, 4410–4417. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Q.; Zhu, Z.N.; Zheng, Y.T.; Shaw, P.C. Engineering of ribosome-inactivating proteins for improving pharmacological properties. Toxins 2020, 12, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.L.; Feng, D.; Wu, J.; Sui, S.F. Trichosanthin inhibits integration of human immunodeficiency virus type 1 through depurinating the long-terminal repeats. Mol. Biol. Rep. 2010, 37, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.L.; Zhang, F.; Feng, D.; Wu, J.; Chen, S.; Sui, S.F. A novel sorting strategy of trichosanthin for hijacking human immunodeficiency virus type 1. Biochem. Biophys. Res. Commun. 2009, 384, 347–351. [Google Scholar] [CrossRef]
- Li, M.X.; Yeung, H.W.; Pan, L.P.; Chan, S.I. Trichosanthin, a potent HIV-1 inhibitor, can cleave supercoiled DNA in vitro. Nucleic Acids Res. 1991, 19, 6309–6312. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.H.; Nie, H.L.; Huang, H.; Tam, S.C.; Zheng, Y.T. Independency of anti-HIV-1 activity from ribosome-inactivating activity of trichosanthin. Biochem. Biophys/ Res. Commun. 2003, 302, 89–94. [Google Scholar] [CrossRef]
- Huang, H.; Chan, H.; Wang, Y.Y.; Ouyang, D.Y.; Zheng, Y.T.; Tam, S.C. Trichosanthin suppresses the elevation of p38 MAPK, and Bcl-2 induced by HSV-1 infection in Vero cells. Life Sci. 2006, 79, 1287–1292. [Google Scholar] [CrossRef]
- Bodmer, D.; Gloddek, B.; Ryan, A.F.; Huverstuhl, J.; Brors, D. Inhibition of the c-Jun N-terminal kinase signaling pathway influences neurite outgrowth of spiral ganglion neurons in vitro. Laryngoscope 2002, 112, 2057–2061. [Google Scholar] [CrossRef]
- Pinching, A.J. Early trials of GLQ223/trichosanthin: What do they show? AIDS 1990, 4, 1289–1291. [Google Scholar] [CrossRef]
- Kolata, G. Trial of experimental AIDS drug to be continued, with revisions. N Y Times Web 1990, A1, A15. [Google Scholar]
- McGrath, M.S.; Lifson, J.D. Compound Q. Nature 1990, 343, 304. [Google Scholar] [CrossRef] [PubMed]
- Palca, J. Trials and tribulations of AIDS drug testing. Science 1990, 247, 1406. [Google Scholar] [CrossRef] [PubMed]
- Byers, V.S.; Baldwin, P.W. Trichosanthin treatment of HIV disease. AIDS 1991, 5, 1150–1151. [Google Scholar] [PubMed]
- An, Q.; Lei, Y.; Jia, N.; Zhang, X.; Bai, Y.; Yi, J.; Chen, R.; Xia, A.; Yang, J.; Wei, S.; et al. Effect of site-directed PEGylation of trichosanthin on its biological activity, immunogenicity, and pharmacokinetics. Biomol. Eng. 2007, 24, 643–649. [Google Scholar] [CrossRef]
- Wang, Q.C.; Ying, W.B.; Xie, H.; Zhang, Z.C.; Yang, Z.H.; Ling, L.Q. Trichosanthin-monoclonal antibody conjugate specifically cytotoxic to human hepatoma cells in vitro. Cancer. Res. 1991, 51, 3353–3355. [Google Scholar] [PubMed]
- Lu, D.Y.; Wu, H.Y.; Yarla, N.S.; Xu, B.; Ding, J.; Lu, T.R. HAART in HIV/AIDS Treatments: Future Trends. Infect. Disord. Drug Targets 2018, 18, 15–22. [Google Scholar] [CrossRef]
- Chan, W.L.; Zheng, Y.T.; Huang, H.; Tam, S.C. Relationship between trichosanthin cytotoxicity and its intracellular concentration. Toxicology 2002, 177, 245–251. [Google Scholar] [CrossRef]
- Sandvig, K.; Olsnes, S.; Pihl, A. Kinetics of binding of the toxic lectins abrin and ricin to surface receptors of human cells. J. Biol. Chem. 1976, 251, 3977–3984. [Google Scholar] [CrossRef]
- Spooner, R.A.; Lord, J.M. Ricin trafficking in cells. Toxins 2015, 7, 49–65. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.F.; Zhang, F.; Shaw, P.C.; Sui, S.F. Trichosanthin induces leakage and membrane fusion of liposome. IUBMB Life 2003, 55, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.Y.; Huang, H.; Tam, S.C. Receptor-mediated endocytosis of trichosanthin in choriocarcinoma cells. Toxicology 2003, 186, 191–203. [Google Scholar] [CrossRef]
- Chan, W.L.; Shaw, P.C.; Tam, S.C.; Jacobsen, C.; Gliemann, J.; Nielsen, M.S. Trichosanthin interacts with and enters cells via LDL receptor family members. Biochem. Biophys. Res. Commun. 2000, 270, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xiang, D.; Zhang, Y.; Jiang, S.; Tu, S.; Qiao, M.; Shen, B. Cytotoxicity of trichosanthin to gastric and colonic cancer cells in vitro. Chin. J. Dig. 1993, 13, 263–266. [Google Scholar]
- Zheng, Y.T.; Zhang, W.F.; Ben, K.L.; Wang, J.H. In vitro immunotoxicity and cytotoxicity of trichosanthin against human normal immunocytes and leukemia-lymphoma cells. Immunopharmacol. Immunotoxicol. 1995, 17, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, D.J. Effect of trichosanthin an anti-leukemia protein on normal mouse spleen cells. Anticancer. Res. 1998, 18, 357–361. [Google Scholar]
- Chen, Y.; Han, L.; Bai, L.; Tang, H.; Zheng, A. Trichosanthin inhibits the proliferation of cervical cancer cells and downregulates STAT-5/C-myc signaling pathway. Pathol. Res. Pract. 2019, 215, 632–638. [Google Scholar] [CrossRef]
- Cui, L.; Song, J.; Wu, L.; Huang, L.; Wang, Y.; Huang, Y.; Yu, H.; Huang, Y.; You, C.C.; Ye, J. Smac is another pathway in the anti-tumour activity of Trichosanthin and reverses Trichosanthin resistance in CaSki cervical cancer cells. Biomed. Pharm. 2015, 69, 119–124. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, C.; Cui, X.; Wu, J.; Cui, Z.; Shen, X. Trichosanthin inhibits cervical cancer by regulating oxidative stress-induced apoptosis. Bioengineered 2021, 12, 2779–2790. [Google Scholar] [CrossRef]
- Tang, Y.; Liang, J.; Wu, A.; Chen, Y.; Zhao, P.; Lin, T.; Zhang, M.; Xu, Q.; Wang, J.; Huang, Y. Co-delivery of Trichosanthin and Albendazole by nano-self-assembly for overcoming tumor multidrug-resistance and metastasis. ACS Appl. Mater. Interfaces 2017, 9, 26648–26664. [Google Scholar] [CrossRef]
- You, C.; Sun, Y.; Zhang, S.; Tang, G.; Zhang, N.; Li, C.; Tian, X.; Ma, S.; Luo, Y.; Sun, W.; et al. Trichosanthin enhances sensitivity of non-small cell lung cancer (NSCLC) TRAIL-resistance cells. Int. J. Biol. Sci. 2018, 14, 217–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, W.H.; Wong, C.C.; Yeung, H.W.; Yung, M.H.; Shaw, P.C.; Tam, S.C. Increasing the plasma half-life of trichosanthin by coupling to dextran. Biochem. Pharmacol. 1991, 42, 1721–1728. [Google Scholar] [CrossRef]
- He, X.H.; Shaw, P.C.; Xu, L.H.; Tam, S.C. Site-directed polyethylene glycol modification of trichosanthin: Effects on its biological activities, pharmacokinetics, and antigenicity. Life Sci. 1999, 64, 1163–1175. [Google Scholar] [CrossRef]
- Gilabert-Oriol, R.; Weng, A.; Mallinckrodt, B.; Melzig, M.F.; Fuchs, H.; Thakur, M. Immunotoxins constructed with ribosome-inactivating proteins and their enhancers: A lethal cocktail with tumor specific efficacy. Curr. Pharm. Des. 2014, 20, 6584–6643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pincus, S.H.; Fang, H.; Wilkinson, R.A.; Marcotte, T.K.; Robinson, J.E.; Olson, W.C. In vivo efficacy of anti-glycoprotein 41, but not anti-glycoprotein 120, immunotoxins in a mouse model of HIV infection. J. Immunol. 2003, 170, 2236–2241. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, S.; Koito, A.; Maeda, Y.; Hattori, T.; Takatsuki, K. Selective killing of HIV-infected cells by anti-gp120 immunotoxins. AIDS Res Hum Retrovir. 1990, 6, 193–203. [Google Scholar] [CrossRef]
- Law, S.K.; Wang, R.R.; Mak, A.N.; Wong, K.B.; Zheng, Y.T.; Shaw, P.C. A switch-on mechanism to activate maize ribosome-inactivating protein for targeting HIV-infected cells. Nucleic Acids Res. 2010, 38, 6803–6812. [Google Scholar] [CrossRef] [Green Version]
- Au, K.Y.; Wang, R.R.; Wong, Y.T.; Wong, K.B.; Zheng, Y.T.; Shaw, P.C. Engineering a switch-on peptide to ricin A chain for increasing its specificity towards HIV-infected cells. Biochim. Biophys. Acta. 2014, 1840, 958–963. [Google Scholar] [CrossRef]
- Wang, R.R.; Au, K.Y.; Zheng, H.Y.; Gao, L.M.; Zhang, X.; Luo, R.H.; Law, S.K.; Mak, A.N.; Wong, K.B.; Zhang, M.X.; et al. The recombinant maize ribosome-inactivating protein transiently reduces viral load in SHIV89.6 infected Chinese Rhesus Macaques. Toxins 2015, 7, 156–169. [Google Scholar] [CrossRef] [Green Version]
- Kreitman, R.J. Immunotoxins for targeted cancer therapy. AAPS J. 2006, 8, E532–E551. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Z.; Wang, J.; Xie, H.; Yang, Z. Trichosanthin has potent inhibiting activity of protein synthesis in a cell-free system and its antibody-conjugate exhibits potentiated cytotoxicity to tumor cells in vitro. Shi Yan Sheng Wu Xue Bao 1987, 20, 515–519. [Google Scholar] [PubMed]
- Casellas, P.; Dussossoy, D.; Falasca, A.I.; Barbieri, L.; Guillemot, J.C.; Ferrara, P.; Bolognesi, A.; Cenini, P.; Stirpe, F. Trichokirin, a ribosome-inactivating protein from the seeds of Trichosanthes kirilowii Maximowicz. Purification, partial characterization and use for preparation of immunotoxins. Eur. J. Biochem. 1988, 176, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Bourrie, B.J.; Casellas, P.; Blythman, H.E.; Jansen, F.K. Study of the plasma clearance of antibody--ricin-A-chain immunotoxins. Evidence for specific recognition sites on the A chain that mediate rapid clearance of the immunotoxin. Eur. J. Biochem. 1986, 155, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kwok, K.H.; Law, K.B.; Wong, R.N.; Yung, K.K. Immunolesioning of nerve growth factor p75 receptor-containing neurons in the rat brain by a novel immunotoxin: Anti-p75-anti-mouse IgG-trichosanthin conjugates. Brain Res. 1999, 846, 154–163. [Google Scholar] [CrossRef]
- Lu, J.; Liu, X.; Wang, M. The Preparation of McAB TCS Conjugate and Its in VItro Tests of Cytotoxicity Against Human Lung Cancer Cell. J. China Med. Univ. 1994, 4, 335–338. [Google Scholar]
- Yuan, H.; Ji, R.; Zhang, R.; Cao, H.; Zhang, Z. Purification of trichosanthin-monoclonal antibody conjugate by monoclonal antibody affinity chromatography. Zhongguo Mian Yi Xue Za Zhi 1996, 12, 246–249. [Google Scholar]
- Li, Y.; Chen, J.; Yang, H.; Luo, R. Purification of EGF-TCS recombinant fusion protein and its targeting action on human tumor cells in vitro. J. Third Mil. Med. Univ. 2007, 13, 1316–1319. [Google Scholar]
- Li, Y.; Huo, Y.; Yang, M. Antitumor Effect of Recombinant Immunotoxin EGF-TCSredlk on Tumor-bearing Mouse Model. J. Chang. Univ. Tradit. Chin. Med. 2011, 5, 9. [Google Scholar]
- Yang, H.W.; Yang, H.W.; Li, Y.M. Antitumor effect of recombinant immunotoxin EGF-TCS in nude mice bearing human hepatocellular carcinoma. Nan Fang Yi Ke Da Xue Xue Bao 2007, 27, 1535–1536. [Google Scholar]
- Wen-Jie, L.Q.J.Y.-C.L. Antitumor effect of TCS-hepama-1 immunotoxin and its combined use with ADM on human hepatoma bearing nude mice. Chin. J. Clin. Oncol. 1997, 24, 4–8. [Google Scholar]
- Gao, H.L.; Zhou, G.Y.; Lu, D.Y.; Zhang, W.Y. Trichosanthin-CEA MAb conjugate cytotoxic to human colonic carcinoma. Chin. J. Immunol. 1992, 8, 300. [Google Scholar]
- Dai, R.X.; Xu, G.J.; Lin, X.Y.; Liu, L.Y.; Shen, H.P.; Zhou, X.G.; Gao, T.; Wang, Y.H. Studies on injury-mechanism of trichosanthin on trophoblast cells and choriocarcinoma cells in culture. Shi Yan Sheng Wu Xue Bao 1993, 26, 411–427. [Google Scholar] [PubMed]
- Lu, J.; Li, Y.; Liu, X. Targeting treatment with immunotoxin Trichosanthin conjugated with monoclonal antibody on nude mice model bearing humen lung adenocarcinoma. China Med. Univ. 1995, 3, 243–246. [Google Scholar]
- Lan, P.; Zhan, W.; Wang, J. Effect of donor antigen-TCS on survival time of mouse to rat cardiac xenografts. Zhonghua Yi Xue Za Zhi 1997, 77, 847–849. [Google Scholar] [PubMed]
- Hegele, R.A.; Maltman, G.M. Insulin’s centenary: The birth of an idea. Lancet Diabetes Endocrinol. 2020, 8, 971–977. [Google Scholar] [CrossRef]
- Roth, J.; Qureshi, S.; Whitford, I.; Vranic, M.; Kahn, C.R.; Fantus, I.G.; Dirks, J.H. Insulin’s discovery: New insights on its ninetieth birthday. Diabetes Metab. Res. Rev. 2012, 28, 293–304. [Google Scholar] [CrossRef]
- Lee, S.H.; Yoon, K.H. A century of progress in diabetes care with Insulin: A history of innovations and foundation for the future. Diabetes Metab J. 2021, 45, 629–640. [Google Scholar] [CrossRef]
- Lam, Y.; Wong, Y.; Wang, B.; Wong, R.; Yeung, H.; Shaw, P.C. Use of trichosanthin to reduce infection by turnip mosaic virus. Plant Sci. 1996, 114, 111–117. [Google Scholar] [CrossRef]
- Jiang, G.Y.; Jin, D.M.; Weng, M.L.; Guo, B.T.; Wang, B. Transformation and expression of trichosanthin gene in tomato. J. Integr. Plant Biol. 1999, 41, 334–336. [Google Scholar]
- Krishnan, R.; McDonald, K.A.; Dandekar, A.M.; Jackman, A.P.; Falk, B. Expression of recombinant trichosanthin, a ribosome-inactivating protein, in transgenic tobacco. J. Biotechnol. 2002, 97, 69–88. [Google Scholar] [CrossRef]
- Huang, A.; Friesen, J.; Brunton, J.L. Characterization of a bacteriophage that carries the genes for production of Shiga-like toxin 1 in Escherichia coli. J. Bacteriol. 1987, 169, 4308–4312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbieri, L.; Valbonesi, P.; Bondioli, M.; Alvarez, M.L.; Dal Monte, P.; Landini, M.P.; Stirpe, F. Adenine glycosylase activity in mammalian tissues: An equivalent of ribosome-inactivating proteins. FEBS Lett. 2001, 505, 196–197. [Google Scholar] [CrossRef] [Green Version]
- Lam, S.K.; Ng, T.B. First simultaneous isolation of a ribosome inactivating protein and an antifungal protein from a mushroom (Lyophyllum shimeji) together with evidence for synergism of their antifungal effects. Arch. Biochem. Biophys. 2001, 393, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.Z.; Yu, M.M.; Ooi, L.S.; Ng, T.B.; Chang, S.T.; Sun, S.S.; Ooi, V.E. Isolation and Characterization of a Type 1 Ribosome-Inactivating Protein from Fruiting Bodies of the Edible Mushroom (Volvariella volvacea). J. Agric. Food Chem. 1998, 46, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.; Bao, H.; Ng, T.B.; Chan, H.H.L.; Ng, C.C.W.; Man, G.C.W.; Wang, H.; Guan, S.; Zhao, S.; Fang, E.F.; et al. New ribosome-inactivating proteins and other proteins with protein synthesis-inhibiting activities. Appl. Microbiol. Biotechnol. 2020, 104, 4211–4226. [Google Scholar] [CrossRef]
- Lu, J.Q.; Shi, W.W.; Xiao, M.J.; Tang, Y.S.; Zheng, Y.T.; Shaw, P.C. Lyophyllin, a mushroom protein from the peptidase M35 superfamily is an RNA N-glycosidase. Int. J. Mol. Sci. 2021, 22, 11598. [Google Scholar] [CrossRef]
- Baker, M.P.; Reynolds, H.M.; Lumicisi, B.; Bryson, C.J. Immunogenicity of protein therapeutics: The key causes, consequences and challenges. Self Nonself 2010, 1, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Xin, X.; Ma, J.; Tan, C.; Osna, N.; Mahato, R.I. Therapeutic targets, novel drugs, and delivery systems for diabetes associated NAFLD and liver fibrosis. Adv. Drug Deliv. Rev. 2021, 176, 113888. [Google Scholar] [CrossRef]
- Meyer, D.W.; Bou, L.B.; Shum, S.; Jonas, M.; Anderson, M.E.; Hamilton, J.Z.; Hunter, J.H.; Wo, S.W.; Wong, A.O.; Okeley, N.M. An in vitro assay using cultured kupffer cells can predict the impact of drug conjugation on in vivo antibody pharmacokinetics. Mol. Pharm. 2020, 17, 802–809. [Google Scholar] [CrossRef]
- Breous, E.; Somanathan, S.; Vandenberghe, L.H.; Wilson, J.M. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology 2009, 50, 612–621. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhang, M.; Jin, H.; Li, D.; Xu, F.; Wu, A.; Wang, J.; Huang, Y. Glioma Dual-Targeting Nanohybrid Protein Toxin Constructed by Intein-Mediated Site-Specific Ligation for Multistage Booster Delivery. Theranostics 2017, 7, 3489–3503. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Yao, S.; Chen, Y.; Huang, J.; Wu, A.; Zhang, M.; Xu, F.; Li, F.; Huang, Y. Genetically-engineered protein prodrug-like nanoconjugates for tumor-targeting biomimetic delivery via a SHEATH strategy. Nanoscale 2019, 11, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, M.; Jin, H.; Tang, Y.; Wang, H.; Xu, Q.; Li, Y.; Li, F.; Huang, Y. Intein-mediated site-specific synthesis of tumor-targeting protein delivery system: Turning PEG dilemma into prodrug-like feature. Biomaterials 2017, 116, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.; Chen, Y.; Wang, H.; Chang, Y.; Zhang, M.; Zhao, P.; Tang, Y.; Xu, Q.; Zhu, Z.; Cao, Y.; et al. Genetically-engineered "all-in-one" vaccine platform for cancer immunotherapy. Acta Pharm. Sin. B 2021, 11, 3622–3635. [Google Scholar] [CrossRef] [PubMed]



| Time | Country/City | Agent | Target Disease | Effect | ADR 1 | References |
|---|---|---|---|---|---|---|
| 1989 2 | CA, US 3 | GLQ223 4 | AIDS | - | - | [17] |
| 1990 | CA, US | GLQ223 | AIDS | No consistent or sustained changes 5 | No significant toxicity, except one with a severe neurological ADR. | [18] |
| 1990 | Nottingham, UK; CA, US; FL, US 6 | GLQ223 (Phase I/II) | AIDS | Serum p24 antigen decreased after one month in 10–18 patients; one converted to negative. | Severe fatigue and myalgias; dementia or even coma in two patients; one death | [19] |
| 1990 | Shanghai, China | TCS7 | Abortion (10–14 weeks) | High success rate, better in cervical injections | Increased body temperature; pain at the injection site; alleviated by dexamethasone | [11] |
| 1991 | CA, US; Pavia, Italy | GLQ223 | AIDS | Pharmacokinetic study; predictability of elimination and distribution among species | - | [20] |
| 1991 | Shanghai, China | TCS with dexamethasone | Midterm abortion and anti-early pregnancy | High success rate (>83%) | Flu-like syndrome; can be alleviated with dexamethasone | [16] |
| 1992 | Shanghai, China | TCS | AIDS | Reduced p24 antigen, increased CD4 cells | Flu-like syndrome; pain and erythema at the injection sites | [21] |
| 1993 | Nottingham, UK; CA, US; MD, US 8 | GLQ223 | AIDS | - | Two patients developed coma; multifocal neurological deficits after treatment. | [22] |
| 1993 | CA, US; WA, US 9 | GLQ223 | AIDS | For patients who received 36 and 50 μg/kg, an increase in CD4+ and CD8+ T cells was sustained; Beta-Microglobulin levels increased during the infusion. | Flu-like syndrome with muscle; joint aches; increase in creatinine kinase levels | [23] |
| 1994 | Shanghai, China | TCS with dexamethasone | Abortion | High success rate (100%) | Flu-like syndrome; alleviated with dexamethasone | [24] |
| 1994 | Shanghai, China | TCS | Abortion | - | TCS may have a transient effect on the myocardium | [25] |
| 1994 | Nottingham, UK | GLQ223 combined with zidovudine | AIDS | Significant increase in CD4+ cell levels | Myalgias, fevers, mild elevation in liver function tests; mild-moderate anaphylactic reactions; two with mental status changes | [26] |
| 2000 | Shanghai, China | TCS | Tubal pregnancy | 92% success rate | Flu-like syndrome | [27] |
| 2001 | Shanghai, China | TCS | Midterm abortion | High success rate (98%) | Flu-like syndrome | [28] |
| Time | Immunotoxin | Target Antigen | Disease | Effect | References |
|---|---|---|---|---|---|
| 1991 | TCS-Hepama-1 | Hepatoma-associated antigen | Hepatoma | Potent and specific antihepatoma agents; might have considerable potential in hepatoma therapy. | [89,123] |
| 1992 | B3-IgG-TCS | CEA 1-MAb antigen | Colorectal cancer; Cervical cancer | Anti-tumor activity in vitro and in vivo | [124] |
| 1993 | TCS-Hepama-1-gold | Hepatoma-associated antigen and gold | Hepatoma | TCS-Hepama-1-gold particles are bound to the microvilli of human liver carcinoma cells and can be inhibited competitively by pretreatment with Hepama-1 for an hour. | [125] |
| 1995 | CMU15—TCS | Lung cancer antigen | Lung cancer | Significant tumor-inhibited effect in vivo, inhibition rate of tumor growth reaching 76% in the group of peritoneal injection and 99.4% in the group of intra-tumor injection without apparent toxic effect to host mice | [118,126] |
| 1996 | TCS-Ng76 | Melanoma antigen | Melanoma | The affinity gel may be used to purify different TCS-composed immunotoxins. | [119] |
| 1997 | H-2 Ag-TCS | Mouse major histocompatibility complex antigens (H-2 Ag) | Rat cardiac xenografts | The proliferation of recipient immune cells pretreated with conjugate H-2Ag-TCS was inhibited. H-2 Ag-TCS significantly prolonged the cardiac survival time | [127] |
| 1999 | p75-TCS (anti-p75 anti-mouse IgG-TCS) | Rat nerve growth factor (NGF) receptor (p75 receptor) (p75NTR) | Immuno-lesioning (cholinergic basal forebrain neurons) | Potent and caused a selective and specific depletion of cholinergic neurons in the neostriatum | [117] |
| 2010 | EGF-TCS | EGFR 2 | Hepatocellular carcinoma | Inhibits the growth of solid tumors in nude mice | [120] |
| 2011 | EGF-TCSredlk | EGFR | Hepatocellular carcinoma | Anti-cancer activity in vivo and in vitro | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.-Q.; Wong, K.-B.; Shaw, P.-C. A Sixty-Year Research and Development of Trichosanthin, a Ribosome-Inactivating Protein. Toxins 2022, 14, 178. https://doi.org/10.3390/toxins14030178
Lu J-Q, Wong K-B, Shaw P-C. A Sixty-Year Research and Development of Trichosanthin, a Ribosome-Inactivating Protein. Toxins. 2022; 14(3):178. https://doi.org/10.3390/toxins14030178
Chicago/Turabian StyleLu, Jia-Qi, Kam-Bo Wong, and Pang-Chui Shaw. 2022. "A Sixty-Year Research and Development of Trichosanthin, a Ribosome-Inactivating Protein" Toxins 14, no. 3: 178. https://doi.org/10.3390/toxins14030178




