The Occurrence, Distribution, and Toxicity of High-Risk Ciguatera Fish Species (Grouper and Snapper) in Kiritimati Island and Marakei Island of the Republic of Kiribati
Abstract
:1. Introduction
2. Results
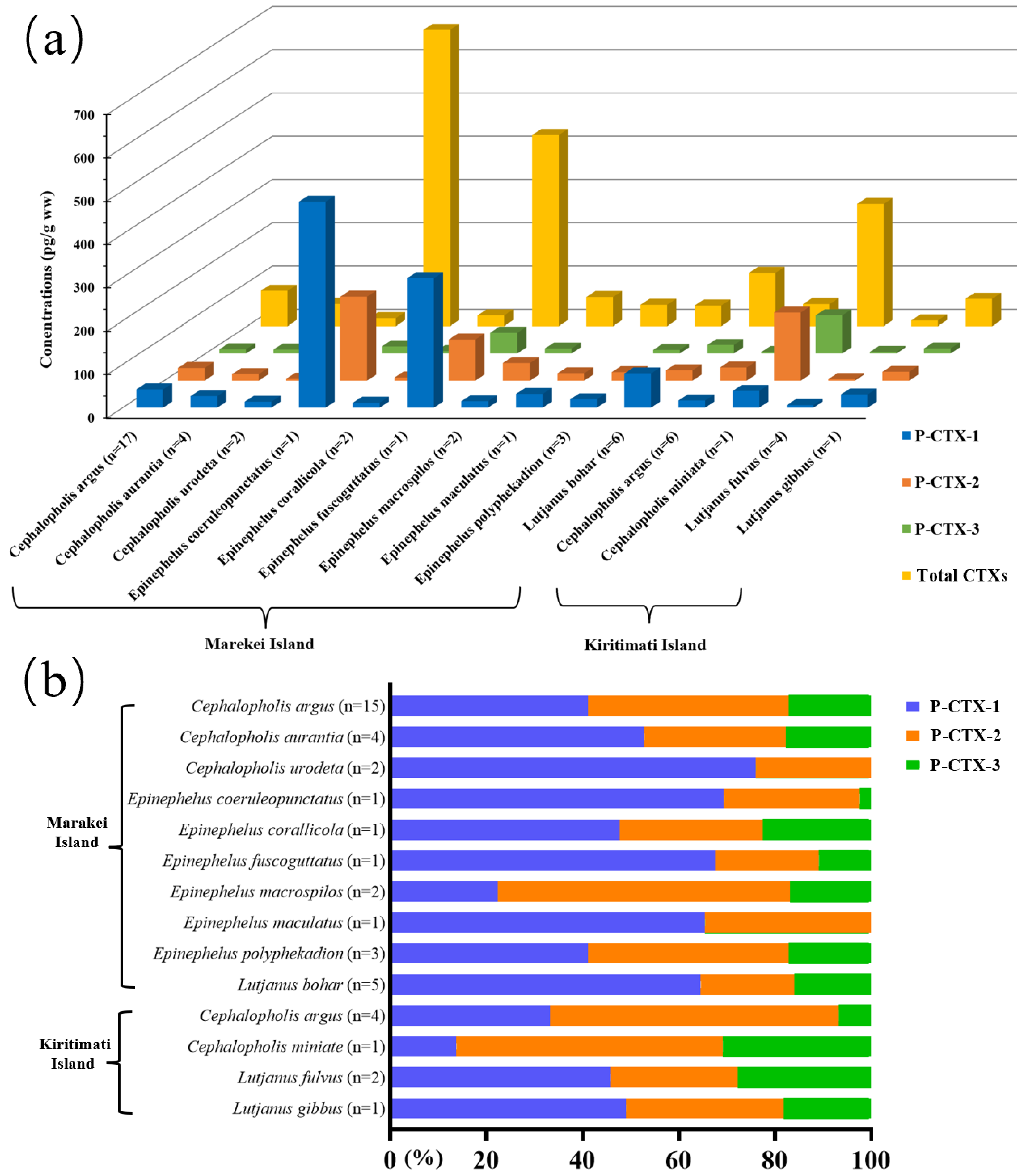
2.1. Concentrations and Composition Profiles of CTXs
2.1.1. CTX concentrations and Composition Profiles in Marakei Island
2.1.2. CTX Concentrations and Composition Profiles in Kiritimati Island
2.1.3. Relationship between Body Size (Total Length and Body Weight) and CTX Levels of Grouper and Snapper from Marakei Island and Kiritimati Island
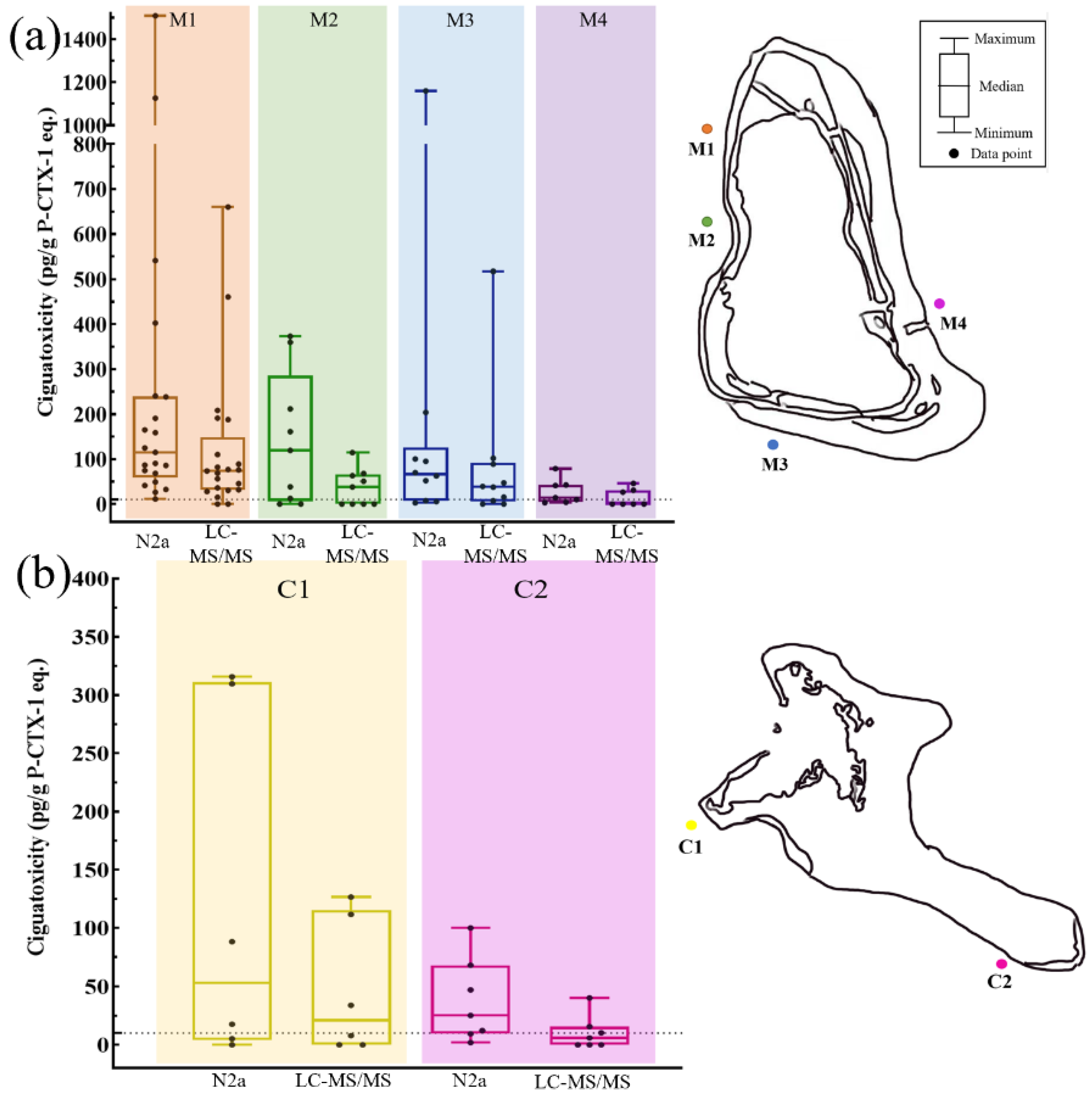
2.2. Ciguatoxicity Determined by N2a and LC-MS/MS from Marakei Island and Kiritimati Island
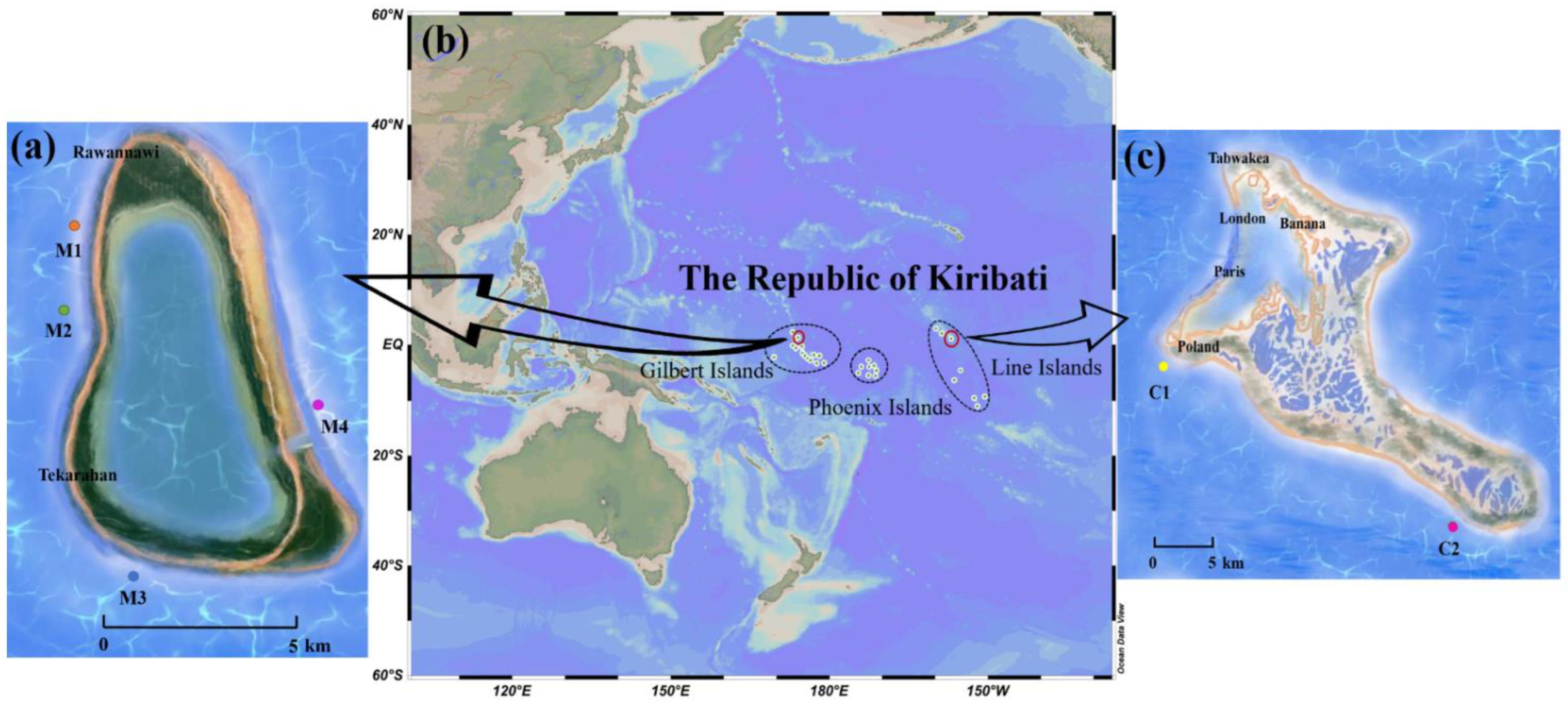
2.3. Spatial Distribution of Ciguatoxic Grouper and Snapper from Marakei Island and Kiritimati Island
2.3.1. Ciguatoxicities of Fish Samples Determined by N2a and LC-MS/MS in Marakei Island
2.3.2. Ciguatoxicities of Fish Samples Determined by N2a and LC-MS/MS in Kiritimati Islands
3. Discussion
3.1. CTX Levels in Ciguateric Groupers and Snappers of Marakei Island and Kiritimati Island
3.2. Ciguatoxicity of Grouper and Snapper Determined by N2a and LC-MS/MS
3.3. Relationship between Body Size and CTX Levels of Groupers and Snappers from Marakei Island and Kiritimati Island
3.4. Composition Profiles of Ciguatoxic Groupers and Snappers from Marakei Island and Kiritimati Island
3.5. Spatial Distribution of Ciguatoxic Groupers and Snappers from Marakei Island and Kiritimati Island
4. Conclusions
5. Materials and Methods
5.1. Sample Collection
5.2. CTXs Extraction Method
5.3. CTXs Clean-Up Procedure
5.4. Determination of CTX Concentrations
5.4.1. Mouse Neuroblastoma (N2a) Assay
5.4.2. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
5.4.3. Quality Assurance and Control
5.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chung, K.; Oehler, E.; Pierce, T.J.; Gribble, M.O.; Chinain, M. Screening for Predictors of Chronic Ciguatera Poisoning: An Exploratory Analysis among Hospitalized Cases from French Polynesia. Toxins 2021, 13, 646. [Google Scholar] [CrossRef]
- Kusche, H.; Hanel, R. Consumers of mislabeled tropical fish exhibit increased risks of ciguatera intoxication: A report on substitution patterns in fish imported at Frankfurt Airport, Germany. Food Control. 2021, 121, 107647. [Google Scholar] [CrossRef]
- Mak, Y.L.; Wai, T.C.; Murphy, M.B.; Chan, W.H.; Wu, J.J.; Lam, J.C.W.; Chan, L.L.; Lam, P.K.S. Pacific ciguatoxins in food web components of coral reef systems in the Republic of Kiribati. Environ. Sci. Technol. 2013, 47, 14070–14079. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.A.; Fernandez, M.; Backer, L.C.; Dickey, R.W.; Bernstein, J.; Schrank, K.; Kibler, S.; Stephan, W.; Gribble, M.O.; Bienfang, P.; et al. An Updated Review of Ciguatera Fish Poisoning: Clinical, Epidemiological, Environmental, and Public Health Management. Mar. Drugs 2017, 15, 72. [Google Scholar] [CrossRef]
- Hamilton, B.; Whittle, N.; Shaw, G.; Eaglesham, G.; Moore, M.R.; Lewis, R.J. Human fatality associated with Pacific ciguatoxin contaminated fish. Toxicon 2010, 56, 668–673. [Google Scholar] [CrossRef]
- Chan, W.H.; Mak, Y.L.; Wu, J.J.; Jin, L.; Sit, W.H.; Lam, J.C.W.; Sadovy de Mitcheson, Y.; Chan, L.L.; Lam, P.K.S.; Murphy, M.B. Spatial distribution of ciguateric fish in the Republic of Kiribati. Chemosphere 2011, 84, 117–123. [Google Scholar] [CrossRef]
- Wu, N.; Huan, Q.; Du, K.; Hu, R.; Jiang, T. Ciguatera toxins in wild coral reef fish along the southern coast of China. Mar. Freshw. Res. 2015, 66, 1168–1175. [Google Scholar] [CrossRef]
- Darius, T.; Revel, T.; Cruchet, P. Deep-Water Fish Are Potential Vectors of Ciguatera Poisoning in the Gambier Islands, French Polynesia. Mar. Drugs 2021, 19, 644. [Google Scholar] [CrossRef]
- Rongo, T.; van Woesik, R. Ciguatera poisoning in Rarotonga, southern Cook Islands. Harmful Algae 2011, 10, 345–355. [Google Scholar] [CrossRef]
- Chinain, M.; Gatti, C.M.; Ung, A.; Cruchet, P.; Revel, T.; Viallon, J.; Sibat, M.; Varney, P.; Laurent, V.; Hess, P.; et al. Evidence for the Range Expansion of Ciguatera in French Polynesia: A Revisit of the 2009 Mass-Poisoning Outbreak in Rapa Island (Australes Archipelago). Toxins 2020, 12, 759. [Google Scholar] [CrossRef]
- L’Herondelle, K.; Talagas, M.; Mignen, O.; Misery, L.; Le Garrec, R. Neurological Disturbances of Ciguatera Poisoning: Clinical Features and Pathophysiological Basis. Cells 2020, 9, 2291. [Google Scholar] [CrossRef]
- Mangubhai, S.; Lovell, E.; Abeta, R.; Donner, S.; Redfern, F.M.; O’Brien, M.; Aram, K.T.; Gillett, R.; Rotjan, R.; Eria, T.; et al. Chapter 37—Kiribati: Atolls and Marine Ecosystems. In World seas: An Environmental Evaluation, 2nd ed.; Sheppard, C.B.T.-W.S., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 807–826. ISBN 978-0-08-100853-9. [Google Scholar]
- Cauchi, J.P.; Bambrick, H.; Moncada, S.; Correa-Velez, I. Nutritional diversity and community perceptions of health and importance of foods in Kiribati: A case study. Food Secur. 2021, 13, 351–367. [Google Scholar] [CrossRef]
- Xu, Y.; Richlen, M.L.; Morton, S.L.; Mak, Y.L.; Chan, L.L.; Tekiau, A.; Anderson, D.M. Distribution, abundance and diversity of Gambierdiscus spp. from a ciguatera-endemic area in Marakei, Republic of Kiribati. Harmful Algae 2014, 34, 56–68. [Google Scholar] [CrossRef] [Green Version]
- Watson, M.S.; Claar, D.C.; Baum, J.K. Subsistence in isolation: Fishing dependence and perceptions of change on Kiritimati, the world’s largest atoll. Ocean Coast. Manag. 2016, 123, 1–8. [Google Scholar] [CrossRef]
- Dimoff, S.A.; Halliday, W.D.; Pine, M.K.; Tietjen, K.L.; Juanes, F.; Baum, J.K. The utility of different acoustic indicators to describe biological sounds of a coral reef soundscape. Ecol. Indic. 2021, 124, 107435. [Google Scholar] [CrossRef]
- Amorim, P.; Sousa, P.; Westmeyer, M.; Menezes, G.M. Generic Knowledge Indicator (GKI): A tool to evaluate the state of knowledge of fisheries applied to snapper and grouper. Mar. Policy 2018, 89, 40–49. [Google Scholar] [CrossRef]
- Schoelinck, C.; Hinsinger, D.D.; Dettaï, A.; Cruaud, C.; Justine, J.-L. A Phylogenetic Re-Analysis of Groupers with Applications for Ciguatera Fish Poisoning. PLoS ONE 2014, 9, e98198. [Google Scholar] [CrossRef] [Green Version]
- Oyafuso, Z.S.; Drazen, J.C.; Moore, C.H.; Franklin, E.C. Habitat-based species distribution modelling of the Hawaiian deepwater snapper-grouper complex. Fish. Res. 2017, 195, 19–27. [Google Scholar] [CrossRef]
- Chain, E.P. on C. in the F. Scientific Opinion on marine biotoxins in shellfish—Emerging toxins: Ciguatoxin group. EFSA J. 2010, 8, 1627. [Google Scholar] [CrossRef]
- Li, J.; Mak, Y.L.; Chang, Y.H.; Xiao, C.; Chen, Y.M.; Shen, J.; Wang, Q.; Ruan, Y.; Lam, P.K.S. Uptake and Depuration Kinetics of Pacific Ciguatoxins in Orange-Spotted Grouper (Epinephelus coioides). Environ. Sci. Technol. 2020, 54, 4475–4483. [Google Scholar] [CrossRef]
- Yogi, K.; Oshiro, N.; Inafuku, Y.; Hirama, M.; Yasumoto, T. Regional and Species Characteristics in Fish and Causative Alga from. Anal. Chem. 2011, 83, 8886–8891. [Google Scholar] [CrossRef] [PubMed]
- Soliño, L.; Costa, P.R. Differential toxin profiles of ciguatoxins in marine organisms: Chemistry, fate and global distribution. Toxicon 2018, 150, 124–143. [Google Scholar] [CrossRef]
- Gaboriau, M.; Ponton, D.; Darius, H.T.; Chinain, M. Ciguatera fish toxicity in French Polynesia: Size does not always matter. Toxicon 2014, 84, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.J.; Williams, A.J.; Wakefield, C.B.; Nicol, S.J.; Taylor, B.M.; O’Malley, J.M. Review of the life history characteristics, ecology and fisheries for deep-water tropical demersal fish in the Indo-Pacific region. Rev. Fish Biol. Fish. 2016, 26, 537–562. [Google Scholar] [CrossRef]
- Oshiro, N.; Yogi, K.; Asato, S.; Sasaki, T.; Tamanaha, K.; Hirama, M.; Yasumoto, T.; Inafuku, Y. Ciguatera incidence and fish toxicity in Okinawa, Japan. Toxicon 2010, 56, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Ikehara, T.; Kuniyoshi, K.; Oshiro, N.; Yasumoto, T. Biooxidation of Ciguatoxins Leads to Species-Specific Toxin Profiles. Toxins 2017, 9, 205. [Google Scholar] [CrossRef] [Green Version]
- Loeffler, C.R.; Abraham, A.; Stopa, J.E.; Flores Quintana, H.A.; Jester, E.L.E.; La Pinta, J.; Deeds, J.; Benner, R.A.; Adolf, J. Ciguatoxin in Hawai’i: Fisheries forecasting using geospatial and environmental analyses for the invasive Cephalopholis argus (Epinephelidae). Environ. Res. 2021, 207, 112164. [Google Scholar] [CrossRef]
- Soliño, L.; Costa, P.R. Global impact of ciguatoxins and ciguatera fish poisoning on fish, fisheries and consumers. Environ. Res. 2020, 182, 109111. [Google Scholar] [CrossRef]
- Costa, P.R.; Estévez, P.; Soliño, L.; Castro, D.; Rodrigues, S.M.; Timoteo, V.; Leao-Martins, J.M.; Santos, C.; Gouveia, N.; Diogène, J.; et al. An Update on Ciguatoxins and CTX-like Toxicity in Fish from Different Trophic Levels of the Selvagens Islands (NE Atlantic, Madeira, Portugal). Toxins 2021, 13, 580. [Google Scholar] [CrossRef]
- Boydron-Le Garrec, R.; Benoit, E.; Sauviat, M.-P.; Lewis, R.J.; Molgó, J.; Laurent, D. Ability of some plant extracts, traditionally used to treat ciguatera fish poisoning, to prevent the in vitro neurotoxicity produced by sodium channel activators. Toxicon 2005, 46, 625–634. [Google Scholar] [CrossRef]
- Bienfang, P.; DeFelice, S.; Dowling, A. Quantitative Evaluation of Commercially Available Test Kit for Ciguatera in Fish. Food Nutr. Sci. 2011, 2, 594–598. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Henao, J.A.; García-Álvarez, N.; Fernández, A.; Saavedra, P.; Silva Sergent, F.; Padilla, D.; Acosta-Hernández, B.; Martel Suárez, M.; Diogène, J.; Real, F. Predictive score and probability of CTX-like toxicity in fish samples from the official control of ciguatera in the Canary Islands. Sci. Total Environ. 2019, 673, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Bentur, Y.; Spanier, E. Ciguatoxin-like substances in edible fish on the eastern Mediterranean. Clin. Toxicol. 2007, 45, 695–700. [Google Scholar] [CrossRef]
- Yan, M.; Mak, M.Y.L.; Cheng, J.; Li, J.; Gu, J.R.; Leung, P.T.Y.; Lam, P.K.S. Effects of dietary exposure to ciguatoxin P-CTX-1 on the reproductive performance in marine medaka (Oryzias melastigma). Mar. Pollut. Bull. 2020, 152, 110837. [Google Scholar] [CrossRef]
- Yogi, K.; Sakugawa, S.; Oshiro, N.; Ikehara, T.; Sugiyama, K.; Yasumoto, T. Determination of Toxins Involved in Ciguatera Fish Poisoning in the Pacific by LC/MS. J. AOAC Int. 2014, 97, 398–402. [Google Scholar] [CrossRef]
- Lewis, R.J. The changing face of ciguatera. Toxicon 2001, 39, 97–106. [Google Scholar] [CrossRef]
- Cooper, M.J. Ciguatera and other marine poisoning in the Gilbert Islands. Pac. Sci. 1964, 18, 411–440. [Google Scholar]
- Anderson, A.; Wallin, P.; Martinsson-Wallin, H.; Fankhauser, B.; Hope, G. Towards a first prehistory of kiritimati (christmas) island, republic of kiribati. J. Polyn. Soc. 2000, 109, 273–293. [Google Scholar]
- Roué, M.; Smith, K.F.; Sibat, M.; Viallon, J.; Henry, K.; Ung, A.; Biessy, L.; Hess, P.; Darius, H.T.; Chinain, M. Assessment of Ciguatera and Other Phycotoxin-Related Risks in Anaho Bay (Nuku Hiva Island, French Polynesia): Molecular, Toxicological, and Chemical Analyses of Passive Samplers. Toxins 2020, 12, 321. [Google Scholar] [CrossRef]
- Wu, J.J.; Mak, Y.L.; Murphy, M.B.; Lam, J.C.W.; Chan, W.H.; Wang, M.; Chan, L.L.; Lam, P.K.S. Validation of an accelerated solvent extraction liquid chromatography-tandem mass spectrometry method for Pacific ciguatoxin-1 in fish flesh and comparison with the mouse neuroblastoma assay. Anal. Bioanal. Chem. 2011, 400, 3165–3175. [Google Scholar] [CrossRef] [PubMed]
- Catania, D.; Richlen, M.L.; Mak, Y.L.; Morton, S.L.; Laban, E.H.; Xu, Y.; Anderson, D.M.; Chan, L.L.; Berumen, M.L. The prevalence of benthic dinoflagellates associated with ciguatera fish poisoning in the central Red Sea. Harmful Algae 2017, 68, 206–216. [Google Scholar] [CrossRef]
| Fish Species | Number of Individuals (n) | Sample Site | N2a Results | LC-MS/MS Results | ||
|---|---|---|---|---|---|---|
| Occurrence | Mean Toxicity ± SD (pg/g P-CTX-1 eq.) | Occurrence | Mean Toxicity ± SD (pg/g P-CTX-1 eq.) | |||
| Marakei Island | ||||||
| Cephalopholis argus | 17 | M1, M2, M4 | 100% | 158 ± 137 | 88.2% | 54.2 ± 42.0 |
| Cephalopholis aurantia | 4 | M3, M4 | 100% | 87.4 ± 78.1 | 100% | 33.9 ± 31.5 |
| Cephalopholis urodeta | 2 | M1, M3 | 100% | 19.7 ± 17.6 | 100% | 15.0 ± 11.7 |
| Epinephelus areolatus | 1 | M4 | 50% | 3.00 | 0% | ND |
| Epinephelus coeruleopunctatus | 1 | M1 | 100% | 1508 | 100% | 537 |
| Epinephelus corallicola | 2 | M4 | 75% | 41.3 ± 52.8 | 50% | 15.4 ± 21.7 |
| Epinephelus fuscoguttatus | 1 | M1 | 100% | 1126 | 100% | 341 |
| Epinephelus hexagonatus | 1 | M2 | 0% | ND | 0% | ND |
| Epinephelus macrospilos | 2 | M3 | 100% | 76.1 ± 34.3 | 100% | 30.4 ± 12.7 |
| Epinephelus maculatus | 1 | M3 | 100% | 95.1 | 100% | 36.9 |
| Epinephelus merra | 2 | M1, M3 | 75% | 14.4 ± 16.8 | 0% | ND |
| Epinehelus polyphekadion | 3 | M1, M2 | 100% | 92.3 ± 62.0 | 100% | 27.6 ± 16.3 |
| Epinephelus tauvina | 2 | M2 | 50% | 19.1 ± 27.0 | 0% | ND |
| Lutjanus bohar | 6 | M2, M3 | 91.7% | 364 ± 416 | 75% | 92.1 ± 155 |
| Lutjanus fulvus | 2 | M1, M3 | 100% | 8.30 ± 4.20 | 0% | ND |
| Total | 95.7% | 74.5% | ||||
| Kiritimati Island | ||||||
| Cephalopholis argus | 6 | C1, C2 | 75% | 74.1 ± 118 | 66.7% | 26.7 ± 49.3 |
| Cephalopholis miniata | 1 | C1 | 100% | 315 | 100% | 111 |
| Lutjanus bohar | 1 | C2 | 100% | 9.50 | 0% | ND |
| Lutjanus fulvus | 4 | C1, C2 | 100% | 32.7 ± 37.9 | 50% | 9.90 ± 16.2 |
| Lutjanus gibbus | 1 | C2 | 100% | 100 | 100% | 40.2 |
| Total | 92.3% | 61.5% | ||||
| Species | Abbreviation | Common Name | Number of Individuals (n) | Sampling Site | Trophic Level |
|---|---|---|---|---|---|
| Cephalopholis argus | C. argus | Blue-spotted grouper | 23 | M1, M2, M4, C1, C2 | 4.5 |
| Cephalopholis aurantia | C. aurantia | Golden hind | 4 | M4, M3 | 4 |
| Cephalopholis miniata | C. miniata | Coral hind | 1 | C1 | 4.3 |
| Cephalopholis urodeta | C. urodeta | Darkfin hind | 2 | M1, M3 | 4 |
| Epinephelus areolatus | E. areolatus | Areolate grouper | 1 | M4 | 3.7 |
| Epinephelus coeruleopunctatus | E. coeruleopunctatus | White-spotted grouper | 1 | M1 | 3.7 |
| Epinephelus corallicola | E. corallicola | Coral grouper | 2 | M4 | 3.8 |
| Epinephelus fuscoguttatus | E. fuscoguttatus | Brown-marbled grouper | 1 | M1 | 4.1 |
| Epinephelus hexagonatus | E. hexagonatus | Starspotted grouper | 1 | M2 | 4.1 |
| Epinephelus macrospilos | E. macrospilos | Snubnose grouper | 2 | M3 | 3.8 |
| Epinephelus maculatus | E. maculatus | Highfin grouper | 1 | M3 | 4 |
| Epinephelus merra | E. merra | Honeycomb grouper | 2 | M1, M3 | 3.8 |
| Epinephelus polyphekadion | E. polyphekadion | Camouflage grouper | 3 | M1, M2 | 4 |
| Epinephelus tauvina | E. tauvina | Greasy grouper | 2 | M2 | 4.1 |
| Lutjanus bohar | L. bohar | Two-spot red snapper | 7 | M2, M3, C2 | 4.3 |
| Lutjanus fulvus | L. fulvus | Blacktail snapper | 6 | M1, M3, C1, C2 | 3.6 |
| Lutjanus gibbus | L. gibbus | Humpback red snapper | 1 | C1 | 4.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Lee, W.-H.; Wu, J.; Zhou, S.; Yip, K.-C.; Liu, X.; Kirata, T.; Chan, L.-L. The Occurrence, Distribution, and Toxicity of High-Risk Ciguatera Fish Species (Grouper and Snapper) in Kiritimati Island and Marakei Island of the Republic of Kiribati. Toxins 2022, 14, 208. https://doi.org/10.3390/toxins14030208
Zhu J, Lee W-H, Wu J, Zhou S, Yip K-C, Liu X, Kirata T, Chan L-L. The Occurrence, Distribution, and Toxicity of High-Risk Ciguatera Fish Species (Grouper and Snapper) in Kiritimati Island and Marakei Island of the Republic of Kiribati. Toxins. 2022; 14(3):208. https://doi.org/10.3390/toxins14030208
Chicago/Turabian StyleZhu, Jingyi, Wai-Hin Lee, Jiajun Wu, Shiwen Zhou, Ki-Chun Yip, Xiaowan Liu, Taratau Kirata, and Leo-Lai Chan. 2022. "The Occurrence, Distribution, and Toxicity of High-Risk Ciguatera Fish Species (Grouper and Snapper) in Kiritimati Island and Marakei Island of the Republic of Kiribati" Toxins 14, no. 3: 208. https://doi.org/10.3390/toxins14030208
APA StyleZhu, J., Lee, W.-H., Wu, J., Zhou, S., Yip, K.-C., Liu, X., Kirata, T., & Chan, L.-L. (2022). The Occurrence, Distribution, and Toxicity of High-Risk Ciguatera Fish Species (Grouper and Snapper) in Kiritimati Island and Marakei Island of the Republic of Kiribati. Toxins, 14(3), 208. https://doi.org/10.3390/toxins14030208





