Occurrence of BMAA Isomers in Bloom-Impacted Lakes and Reservoirs of Brazil, Canada, France, Mexico, and the United Kingdom
Abstract
:1. Introduction
2. Results and Discussion
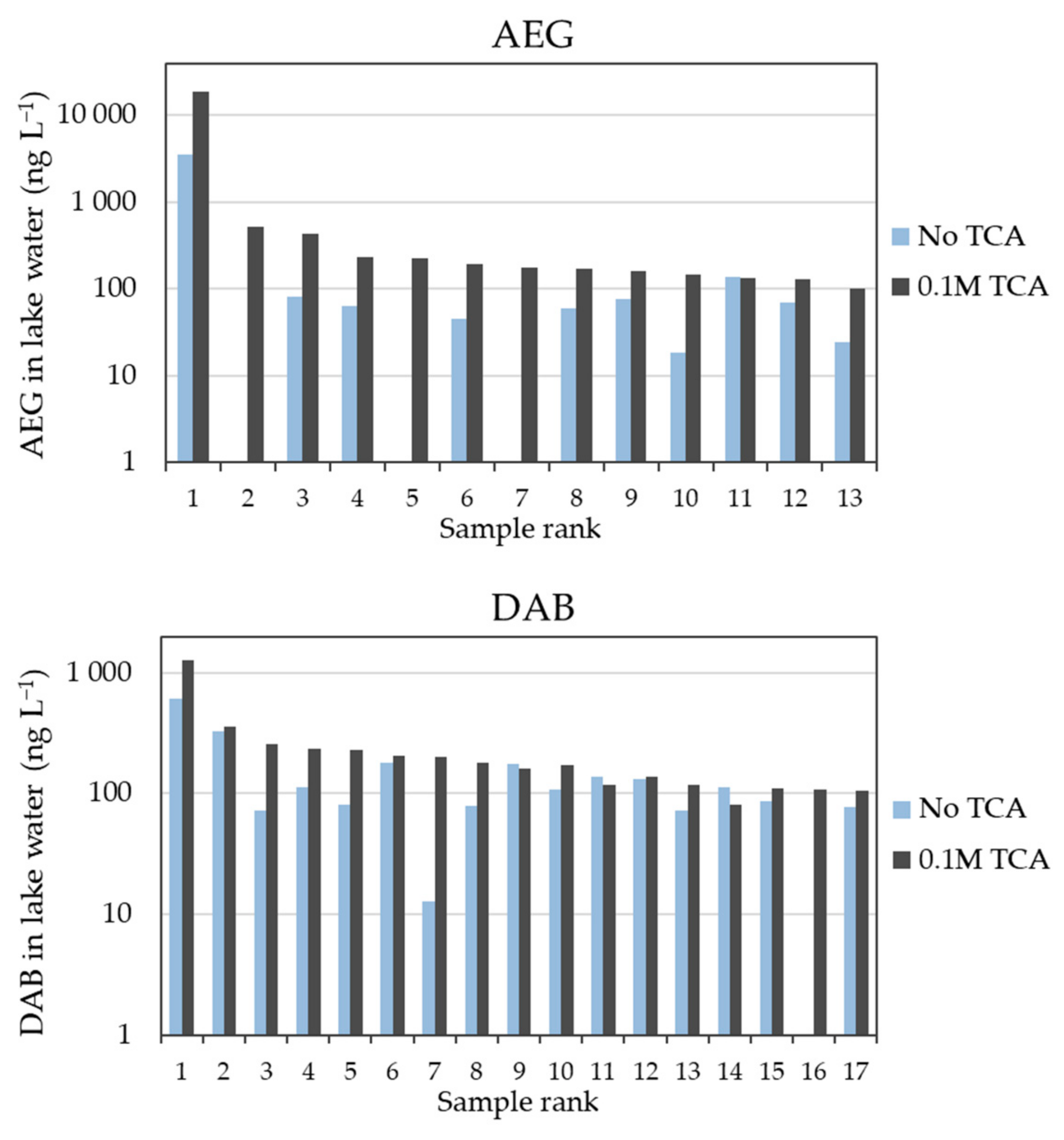
2.1. Influence of TCA Addition
2.2. Occurrence Trends of BMAA Isomers in 390 Lake Samples
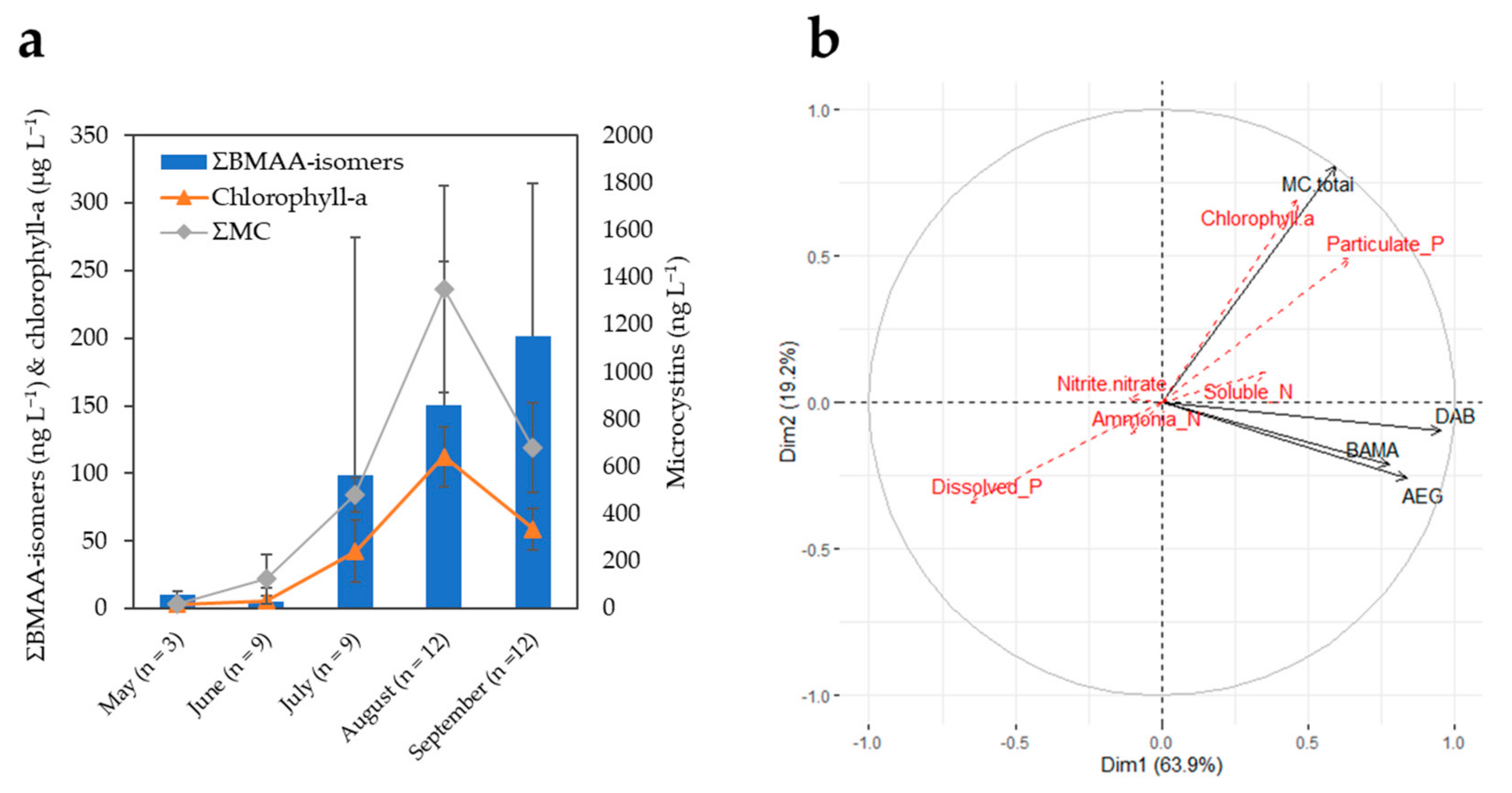
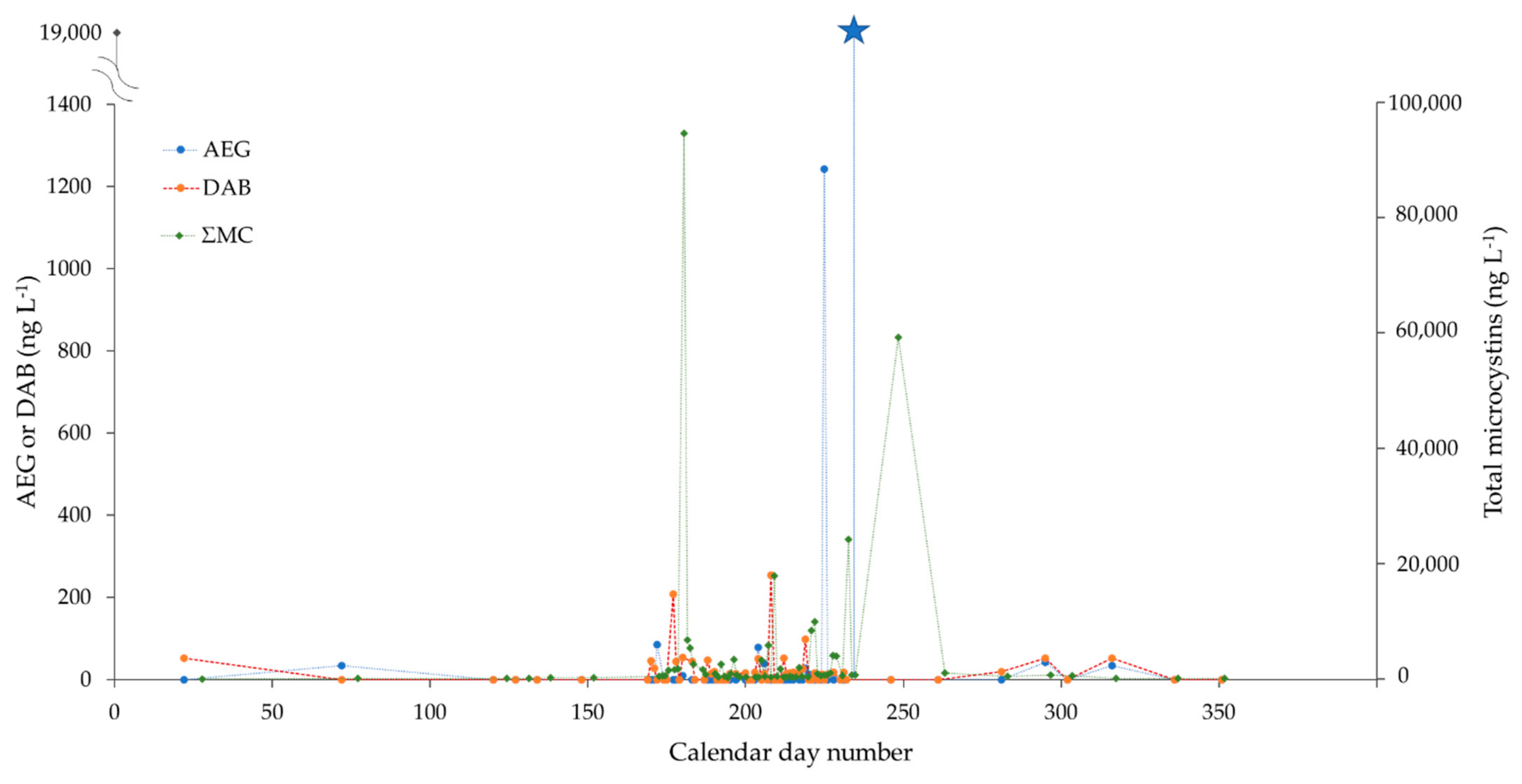
2.3. Monitoring of Two CyanoHAB Impacted Lakes during the Bloom Season
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Standards
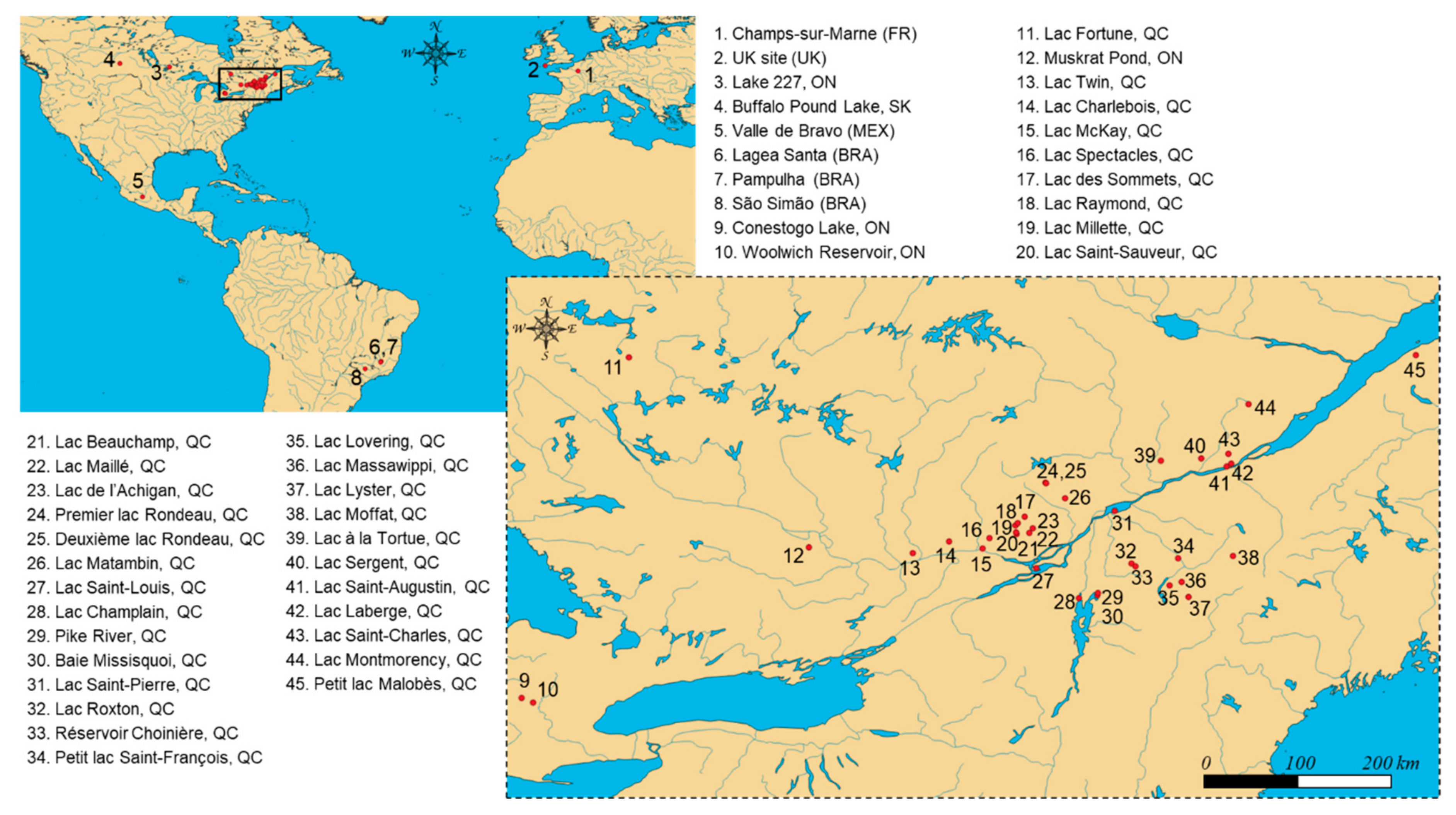
4.2. Sample Collections
4.3. Sample Preparation
4.4. Instrumental Analysis
4.5. Quality Assurance/Quality Control (QA/QC)
4.6. Data Curation and Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Costa, D.; Magalhães, J.D.; Cardoso, S.M.; Empadinhas, N. Microbial BMAA and the pathway for Parkinson’s disease neurodegeneration. Front. Aging Neurosci. 2020, 12, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, S.D.; Banack, S.A.; Cox, P.A.; Weiss, J.H. BMAA selectively injures motor neurons via AMPA/kainate receptor activation. Exp. Neurol. 2006, 201, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Lobner, D.; Piana, P.M.T.; Salous, A.K.; Peoples, R.W. β-N-methylamino-l-alanine enhances neurotoxicity through multiple mechanisms. Neurobiol. Dis. 2007, 25, 360–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esterhuizen, M.; Downing, T.G. β-N-methylamino-l-alanine (BMAA) in novel South African cyanobacterial isolates. Ecotoxicol. Environ. Saf. 2008, 71, 309–313. [Google Scholar] [CrossRef]
- Banack, S.; Johnson, H.; Cheng, R.; Cox, P. Production of the neurotoxin BMAA by a marine cyanobacterium. Mar. Drugs 2007, 5, 180–196. [Google Scholar] [CrossRef]
- Snyder, L.R.; Cruz-Aguado, R.; Sadilek, M.; Galasko, D.; Shaw, C.A.; Montine, T.J. Parkinson–dementia complex and development of a new stable isotope dilution assay for BMAA detection in tissue. Toxicol. Appl. Pharmacol. 2009, 240, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Berntzon, L.; Ronnevi, L.O.; Bergman, B.; Eriksson, J. Detection of BMAA in the human central nervous system. Neuroscience 2015, 292, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Chang, A.C.G.; Chen, T.; Li, N.; Duan, J. Perspectives on endosymbiosis in coralloid roots: Association of cycads and cyanobacteria. Front. Microbiol. 2019, 10, 1888. [Google Scholar] [CrossRef] [Green Version]
- Lance, E.; Arnich, N.; Maignien, T.; Biré, R. Occurrence of β-N-methylamino-l-alanine (BMAA) and isomers in aquatic environments and aquatic food sources for humans. Toxins 2018, 10, 83. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Chen, Q.; Chen, X.; Wang, X.; Liao, X.; Jiang, L.; Wu, J.; Yang, L. Occurrence and transfer of a cyanobacterial neurotoxin β-methylamino-l-alanine within the aquatic food webs of Gonghu Bay (Lake Taihu, China) to evaluate the potential human health risk. Sci. Total Environ. 2014, 468–469, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Manolidi, K.; Triantis, T.M.; Kaloudis, T.; Hiskia, A. Neurotoxin BMAA and its isomeric amino acids in cyanobacteria and cyanobacteria-based food supplements. J. Hazard. Mater. 2019, 365, 346–365. [Google Scholar] [CrossRef] [PubMed]
- Faassen, E. Presence of the neurotoxin BMAA in aquatic ecosystems: What do we really know? Toxins 2014, 6, 1109–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metcalf, J.S.; Banack, S.A.; Powell, J.T.; Tymm, F.J.M.; Murch, S.J.; Brand, L.E.; Cox, P.A. Public health responses to toxic cyanobacterial blooms: Perspectives from the 2016 Florida event. Water Policy 2018, 20, 919–932. [Google Scholar] [CrossRef] [Green Version]
- Chatziefthimiou, A.D.; Deitch, E.J.; Glover, W.B.; Powell, J.T.; Banack, S.A.; Richer, R.A.; Cox, P.A.; Metcalf, J.S. Analysis of neurotoxic amino acids from marine waters, microbial mats, and seafood destined for human consumption in the Arabian Gulf. Neurotox. Res. 2018, 33, 143–152. [Google Scholar] [CrossRef]
- Bishop, S.L.; Kerkovius, J.K.; Menard, F.; Murch, S.J. N-β-Methylamino-L-Alanine and its naturally occurring isomers in cyanobacterial blooms in Lake Winnipeg. Neurotox. Res. 2018, 33, 133–142. [Google Scholar] [CrossRef]
- Pip, E.; Munford, K.; Bowman, L. Seasonal nearshore occurrence of the neurotoxin β N methylamino L alanine (BMAA) in Lake Winnipeg, Canada. Environ. Pollut. 2016, 5, 110. [Google Scholar] [CrossRef]
- Al-Sammak, M.; Hoagland, K.; Cassada, D.; Snow, D. Co-occurrence of the cyanotoxins BMAA, DABA and anatoxin-a in Nebraska reservoirs, fish, and aquatic plants. Toxins 2014, 6, 488–508. [Google Scholar] [CrossRef] [Green Version]
- Roy-Lachapelle, A.; Solliec, M.; Sauvé, S. Determination of BMAA and three alkaloid cyanotoxins in lake water using dansyl chloride derivatization and high-resolution mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 5487–5501. [Google Scholar] [CrossRef]
- Bishop, S.L.; Murch, S.J. A systematic review of analytical methods for the detection and quantification of β-N-methylamino-l-alanine (BMAA). Analyst 2020, 145, 13–28. [Google Scholar] [CrossRef]
- Cohen, S.A. Analytical techniques for the detection of α-amino-β-methylaminopropionic acid. Analyst 2012, 137, 1991. [Google Scholar] [CrossRef] [PubMed]
- Faassen, E.J.; Beekman, W.; Lürling, M. Evaluation of a commercial enzyme linked immunosorbent assay (ELISA) for the determination of the neurotoxin BMAA in surface waters. PLoS ONE 2013, 8, e65260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tymm, F.J.M.; Bishop, S.L.; Murch, S.J. A single laboratory validation for the analysis of underivatized β-N-Methylamino-L-Alanine (BMAA). Neurotox. Res. 2021, 39, 49–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Husk, B.R.; Duy, S.V.; Dinh, Q.T.; Sanchez, J.S.; Sauvé, S.; Whalen, J.K. Quantitative screening for cyanotoxins in soil and groundwater of agricultural watersheds in Quebec, Canada. Chemosphere 2021, 274, 129781. [Google Scholar] [CrossRef]
- Bláhová, L.; Kohoutek, J.; Kadlecová, E.; Kozáková, L.; Bláha, L. Assessment of non-derivatized β-N-methylamino-l-alanine (BMAA) neurotoxin in free form in urine of patients with nonspecific neurological symptoms. Toxicon 2017, 133, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Faassen, E.; Antoniou, M.; Beekman-Lukassen, W.; Blahova, L.; Chernova, E.; Christophoridis, C.; Combes, A.; Edwards, C.; Fastner, J.; Harmsen, J.; et al. A collaborative evaluation of LC-MS/MS based methods for BMAA analysis: Soluble bound BMAA found to be an important fraction. Mar. Drugs 2016, 14, 45. [Google Scholar] [CrossRef] [Green Version]
- Lage, S.; Burian, A.; Rasmussen, U.; Costa, P.R.; Annadotter, H.; Godhe, A.; Rydberg, S. BMAA extraction of cyanobacteria samples: Which method to choose? Environ. Sci. Pollut. Res. 2016, 23, 338–350. [Google Scholar] [CrossRef]
- Jiang, L.; Johnston, E.; Åberg, K.M.; Nilsson, U.; Ilag, L.L. Strategy for quantifying trace levels of BMAA in cyanobacteria by LC/MS/MS. Anal. Bioanal. Chem. 2013, 405, 1283–1292. [Google Scholar] [CrossRef]
- Vo Duy, S.; Munoz, G.; Dinh, Q.T.; Do, D.T.; Simon, D.F.; Sauvé, S. Analysis of the neurotoxin β-N-methylamino-L-alanine (BMAA) and isomers in surface water by FMOC derivatization liquid chromatography high resolution mass spectrometry. PLoS ONE 2019, 14, e0220698. [Google Scholar] [CrossRef] [Green Version]
- Foss, A.J.; Chernoff, N.; Aubel, M.T. The analysis of underivatized β-Methylamino-L-alanine (BMAA), BAMA, AEG & 2, 4-DAB in Pteropus mariannus mariannus specimens using HILIC-LC-MS/MS. Toxicon 2018, 152, 150–159. [Google Scholar]
- Baptista, M.S.; Cianca, R.C.C.; Lopes, V.R.; Almeida, C.M.R.; Vasconcelos, V.M. Determination of the non protein amino acid β-N-methylamino-l-alanine in estuarine cyanobacteria by capillary electrophoresis. Toxicon 2011, 58, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, M.; Pozo, Ó.J.; Sancho, J.V.; López, F.J.; Hernández, F. Re-evaluation of glyphosate determination in water by liquid chromatography coupled to electrospray tandem mass spectrometry. J. Chromatogr. A 2006, 1134, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Yvon, M.; Chabanet, C.; Pélissier, J.-P. Solubility of peptides in trichloroacetic acid (TCA) solutions Hypothesis on the precipitation mechanism. Int. J. Pept. Protein Res. 2009, 34, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Main, B.J.; Bowling, L.C.; Padula, M.P.; Bishop, D.P.; Mitrovic, S.M.; Guillemin, G.J.; Rodgers, K.J. Detection of the suspected neurotoxin β-methylamino-l-alanine (BMAA) in cyanobacterial blooms from multiple water bodies in Eastern Australia. Harmful Algae 2018, 74, 10–18. [Google Scholar] [CrossRef]
- Kehoe, M.J.; Chun, K.P.; Baulch, H.M. Who smells? Forecasting taste and odor in a drinking water reservoir. Environ. Sci. Technol. 2015, 49, 10984–10992. [Google Scholar] [CrossRef]
- Politi, E.; Prairie, Y.T. The potential of Earth Observation in modelling nutrient loading and water quality in lakes of southern Québec, Canada. Aquat. Sci. 2018, 80, 8. [Google Scholar] [CrossRef]
- Dillenberg, H.; Dehnel, M. Toxic waterbloom in Saskatchewan, 1959. Can. Med. Assoc. J. 1960, 83, 1151. [Google Scholar]
- Painter, K.J.; Venkiterwaran, J.J.; Simon, D.F.; Vo Duy, S.; Sauvé, S.; Baulch, H.M. Early and Late Bloomers in a Shallow, Eutrophic Lake. 2022. Available online: https://www.essoar.org/doi/abs/10.1002/essoar.10510183.1 (accessed on 6 January 2022).
- Ministère de l’Environnement et de la Lutte Contre les Changements Climatiques (MELCC). Réseau de Surveillance Volontaire des lacs. Suivi de la Qualité de l’eau 2016; Technical Report; Ministère de l’Environnement et de la Lutte Contre les Changements Climatiques: Quebec City, QC, Canada, 2016.
- Available online: https://www.associationdulactomcod.org/cyanobact%C3%A9ries.php (accessed on 6 January 2022).
- Violi, J.P.; Mitrovic, S.M.; Colville, A.; Main, B.J.; Rodgers, K.J. Prevalence of β-methylamino-L-alanine (BMAA) and its isomers in freshwater cyanobacteria isolated from eastern Australia. Ecotoxicol. Environ. Saf. 2019, 172, 72–81. [Google Scholar] [CrossRef]
- Weiss, J.H.; Christine, C.W.; Choi, D.W. Bicarbonate dependence of glutamate receptor activation by β-N-methylamino-L-alanine: Channel recording and study with related compounds. Neuron 1989, 3, 321–326. [Google Scholar] [CrossRef]
- Main, B.J.; Rodgers, K.J. Assessing the combined toxicity of BMAA and its isomers 2,4-DAB and AEG in vitro using human neuroblastoma cells. Neurotox. Res. 2018, 33, 33–42. [Google Scholar] [CrossRef]
- Metcalf, J.; Banack, S.; Richer, R.; Cox, P. Neurotoxic amino acids and their isomers in desert environments. J. Arid Environ. 2015, 112, 140–144. [Google Scholar] [CrossRef]
- Larsen, M.L.; Baulch, H.M.; Schiff, S.L.; Simon, D.F.; Sauvé, S.; Venkiteswaran, J.J.; Swart, N.C. Extreme rainfall drives early onset cyanobacterial bloom. FACETS 2020, 5, 899–920. [Google Scholar] [CrossRef]
- Giani, A.; Taranu, Z.E.; von Rückert, G.; Gregory-Eaves, I. Comparing key drivers of cyanobacteria biomass in temperate and tropical systems. Harmful Algae 2020, 97, 101859. [Google Scholar] [CrossRef]
- Tran Khac, V.; Hong, Y.; Plec, D.; Lemaire, B.; Dubois, P.; Saad, M.; Vinçon-Leite, B. An automatic monitoring system for high-frequency measuring and real-time management of cyanobacterial blooms in urban water bodies. Processes 2018, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Roy-Lachapelle, A.; Duy, S.V.; Munoz, G.; Dinh, Q.T.; Bahl, E.; Simon, D.F.; Sauvé, S. Analysis of multiclass cyanotoxins (microcystins, anabaenopeptins, cylindrospermopsin and anatoxins) in lake waters using on-line SPE liquid chromatography high-resolution Orbitrap mass spectrometry. Anal. Methods 2019, 11, 5289–5300. [Google Scholar] [CrossRef] [Green Version]
- Dinh, Q.T.; Munoz, G.; Simon, D.F.; Duy, S.V.; Husk, B.; Sauvé, S. Stability issues of microcystins, anabaenopeptins, anatoxins, and cylindrospermopsin during short-term and long-term storage of surface water and drinking water samples. Harmful Algae 2021, 101, 101955. [Google Scholar] [CrossRef] [PubMed]
- Munoz, G.; Duy, S.V.; Roy-Lachapelle, A.; Husk, B.; Sauvé, S. Analysis of individual and total microcystins in surface water by on-line preconcentration and desalting coupled to liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2017, 1516, 9–20. [Google Scholar] [CrossRef]
- Meriluoto, J.; Spoof, L.; Codd, G.A. Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 6 January 2022).
| AEG | BAMA | BMAA | DAB | |
|---|---|---|---|---|
| LOD (ng L−1) | 5 | 10 | 10 | 10 |
| Detection rate % | 30 | 7.4 | 0 | 43 |
| Min (ng L−1) | 5 | 15 | - | 10 |
| Max (ng L−1) | 19,000 | 56 | - | 1100 |
| Average * (ng L−1) | 220 | NC *** | NC *** | 58 |
| Average ** (ng L−1) | 67 | NC *** | NC *** | 25 |
| Sites >100 ng L−1 (max, ng L−1) | PLSF (19,000) BPL (518) Conestogo (225) Lac Millette (178) UK site (125) | - | - | PLSF (1100) Lac Saint-Augustin (359) Lac Fortune (316) Lac Millette (230) Lac Saint-Pierre (224) Lac Beauchamp (136) BPL (119) Missisquoi Bay (109) |
| Abbes et al. (This Study) | Al Samaak et al. [18] | Chatziefthimiou et al. [15] | Roy-Lachapelle et al. [19] | Vo Duy et al. [29] | |
|---|---|---|---|---|---|
| Instrumental method | LC-HRMS | LC-FLD | LC-MS | LC-HRMS | LC-HRMS |
| LOD range ng L−1 | 5–10 | 5000–7000 | N/A | 7–9 | 2–5 |
| Water matrix | Lakes | Lakes | Seawater | Lakes | Lakes & rivers |
| Site location(s) | 5 countries * | USA | Qatar | Canada | Canada |
| Year of collections | 2016–2021 | 2009–2010 | 2012–2013 | 2009, 2013 | 2016–2018 |
| Number of samples | n = 390 | n = 24 | n = 18 | n = 12 | n = 82 |
| AEG (min-max) ng L−1 | 5–19,000 | Not analyzed | 35/38 ** | 9–80 | 2–4900 |
| BAMA (min-max) ng L−1 | 15–56 | Not analyzed | Not analyzed | Not analyzed | 41–130 |
| BMAA (min-max) ng L−1 | Not detected | 1800–25,300 | 6.5/7 ** | 10–300 | 110 *** |
| DAB (min-max) ng L−1 | 10–1100 | 1780–21,100 | 430/610 ** | 8–40 | 13–1900 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbes, S.; Vo Duy, S.; Munoz, G.; Dinh, Q.T.; Simon, D.F.; Husk, B.; Baulch, H.M.; Vinçon-Leite, B.; Fortin, N.; Greer, C.W.; et al. Occurrence of BMAA Isomers in Bloom-Impacted Lakes and Reservoirs of Brazil, Canada, France, Mexico, and the United Kingdom. Toxins 2022, 14, 251. https://doi.org/10.3390/toxins14040251
Abbes S, Vo Duy S, Munoz G, Dinh QT, Simon DF, Husk B, Baulch HM, Vinçon-Leite B, Fortin N, Greer CW, et al. Occurrence of BMAA Isomers in Bloom-Impacted Lakes and Reservoirs of Brazil, Canada, France, Mexico, and the United Kingdom. Toxins. 2022; 14(4):251. https://doi.org/10.3390/toxins14040251
Chicago/Turabian StyleAbbes, Safa, Sung Vo Duy, Gabriel Munoz, Quoc Tuc Dinh, Dana F. Simon, Barry Husk, Helen M. Baulch, Brigitte Vinçon-Leite, Nathalie Fortin, Charles W. Greer, and et al. 2022. "Occurrence of BMAA Isomers in Bloom-Impacted Lakes and Reservoirs of Brazil, Canada, France, Mexico, and the United Kingdom" Toxins 14, no. 4: 251. https://doi.org/10.3390/toxins14040251
APA StyleAbbes, S., Vo Duy, S., Munoz, G., Dinh, Q. T., Simon, D. F., Husk, B., Baulch, H. M., Vinçon-Leite, B., Fortin, N., Greer, C. W., Larsen, M. L., Venkiteswaran, J. J., Martínez Jerónimo, F. F., Giani, A., Lowe, C. D., Tromas, N., & Sauvé, S. (2022). Occurrence of BMAA Isomers in Bloom-Impacted Lakes and Reservoirs of Brazil, Canada, France, Mexico, and the United Kingdom. Toxins, 14(4), 251. https://doi.org/10.3390/toxins14040251








