Wasp Venom Ameliorates Scopolamine-Induced Learning and Memory Impairment in Mice
Abstract
:1. Introduction
2. Results
2.1. In Vitro Antioxidant Activity of WV
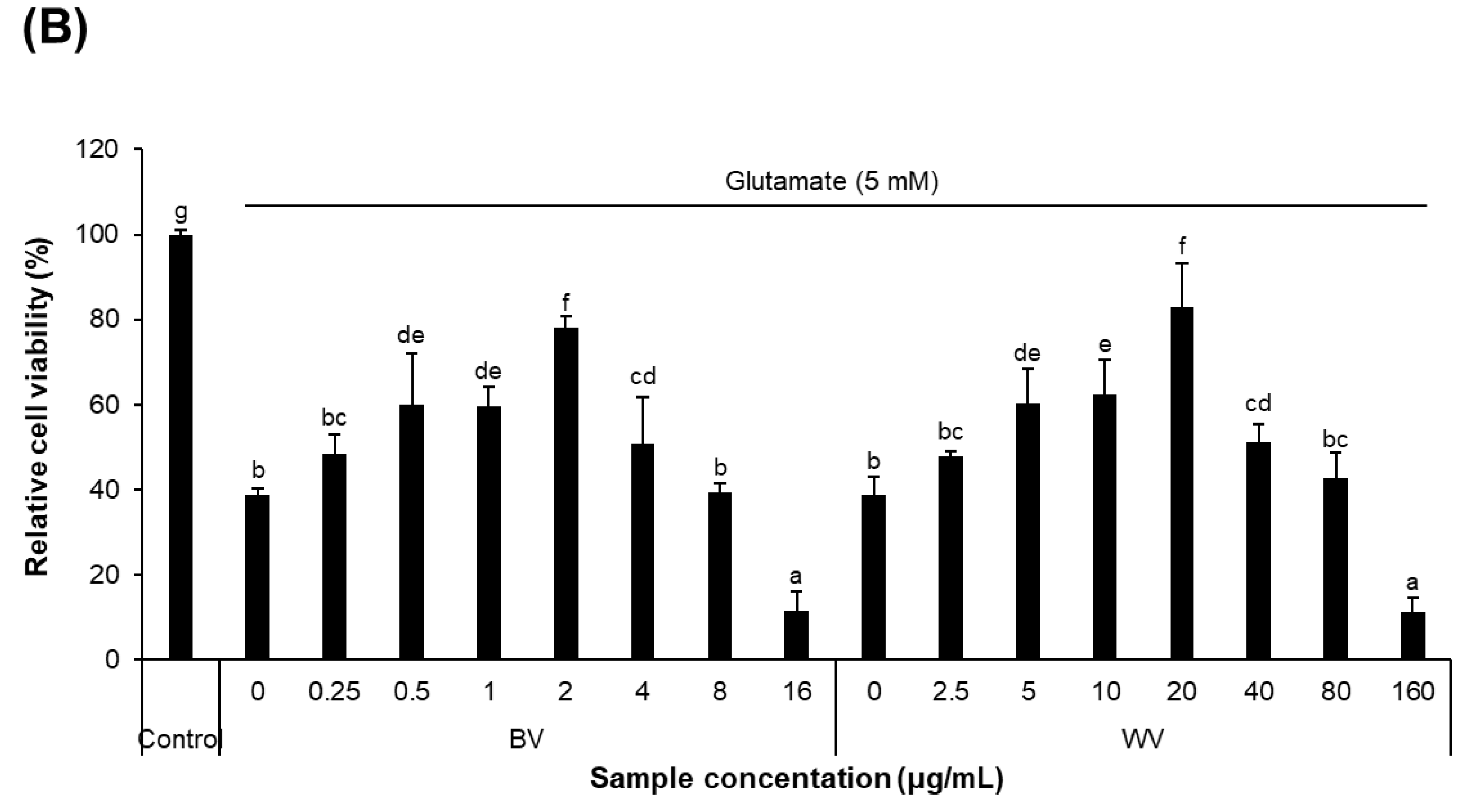
2.2. WV Protected against Glutamate-Induced Cytotoxicity
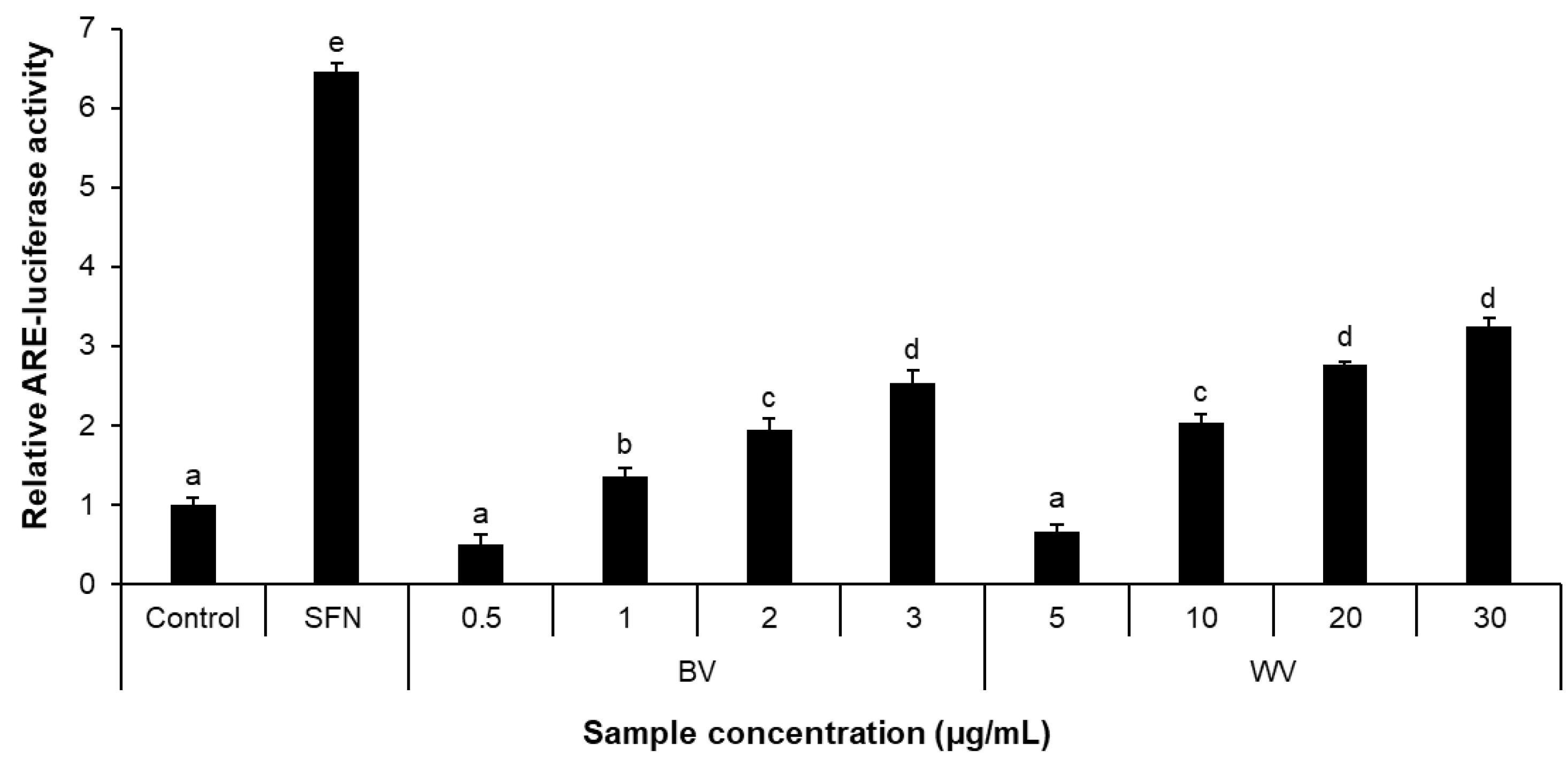
2.3. WV Increased Antioxidant Response Element (ARE)-Luciferase Activity
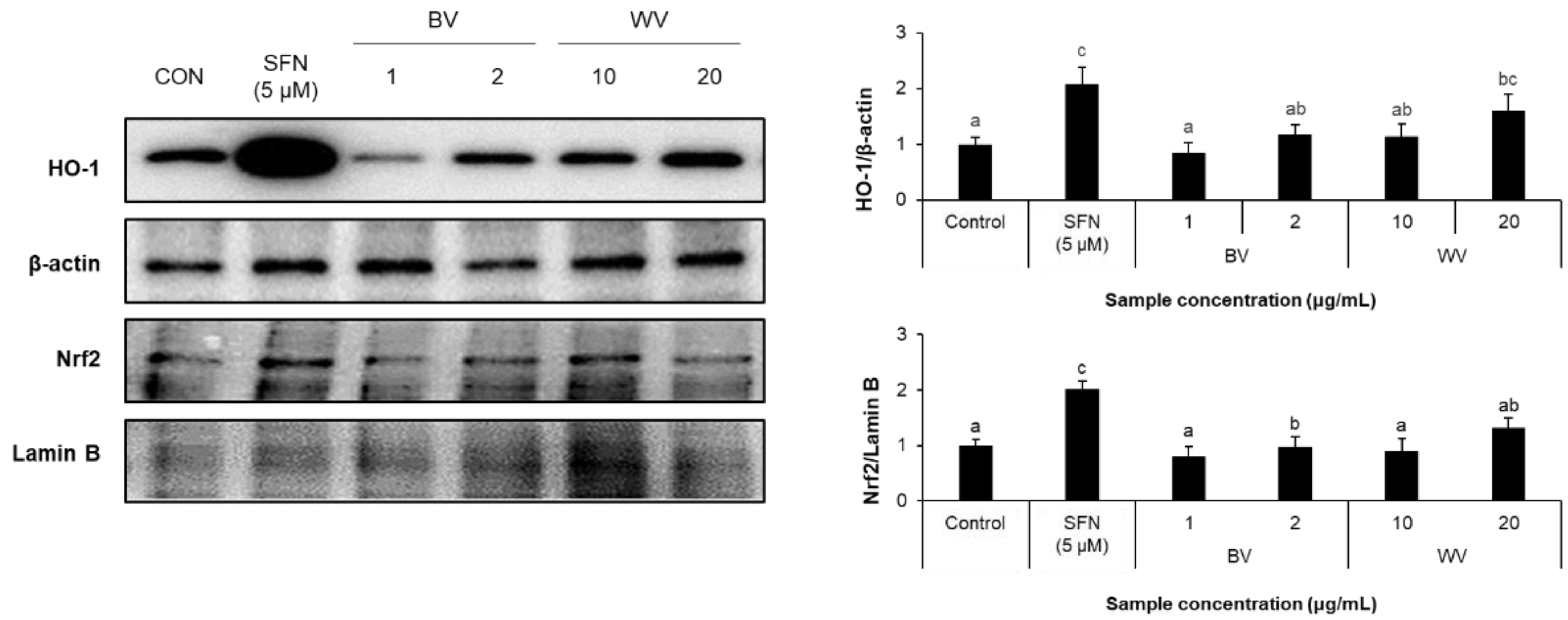
2.4. WV Upregulated Cytoplasmic HO-1 Level Downstream of Nrf2
2.5. WV Decreased Cellular ROS Level
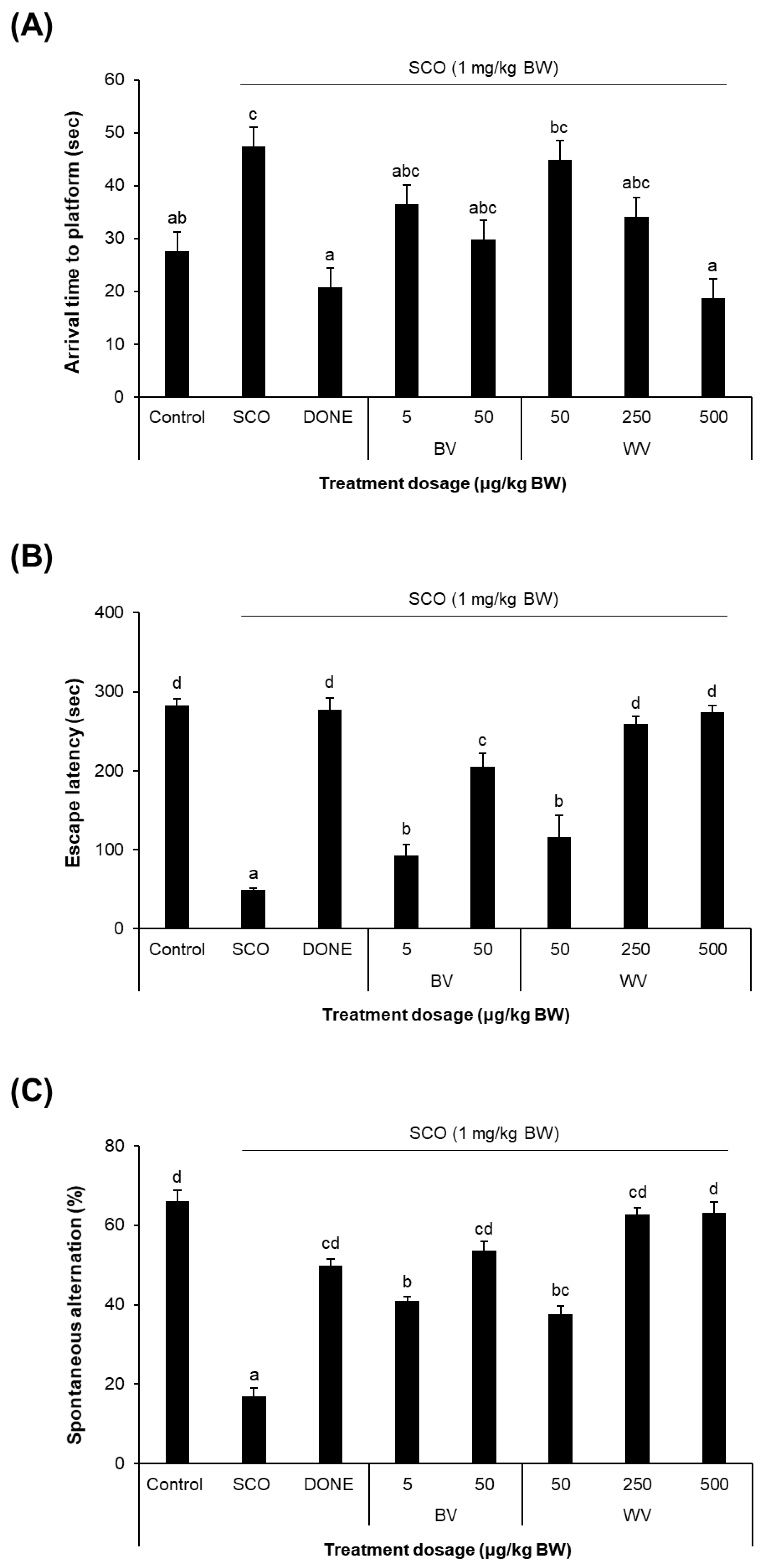
2.6. WV Improved Learning and Memory in SCO-Treated Mouse Model
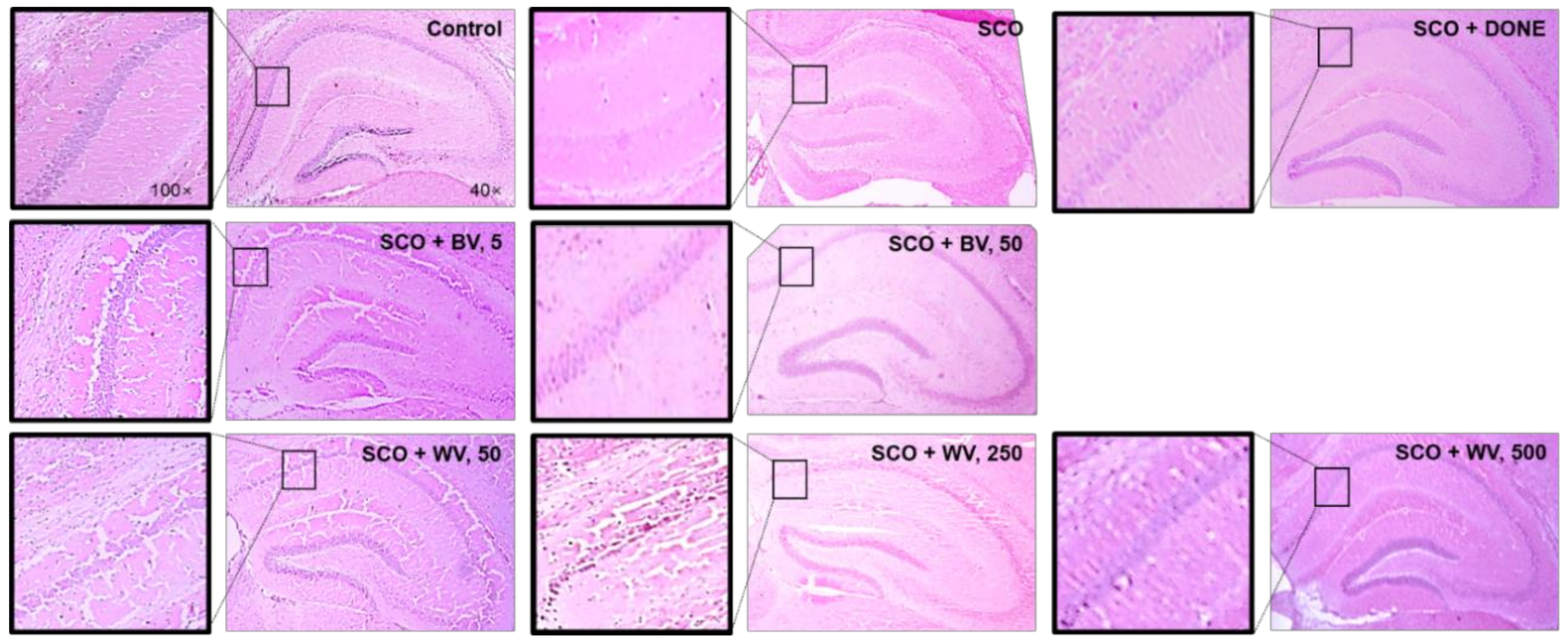
2.7. WV Protected Hippocampal Region from SCO-Induced Damage
2.8. WV Activated the Nrf2/HO-1 Axis
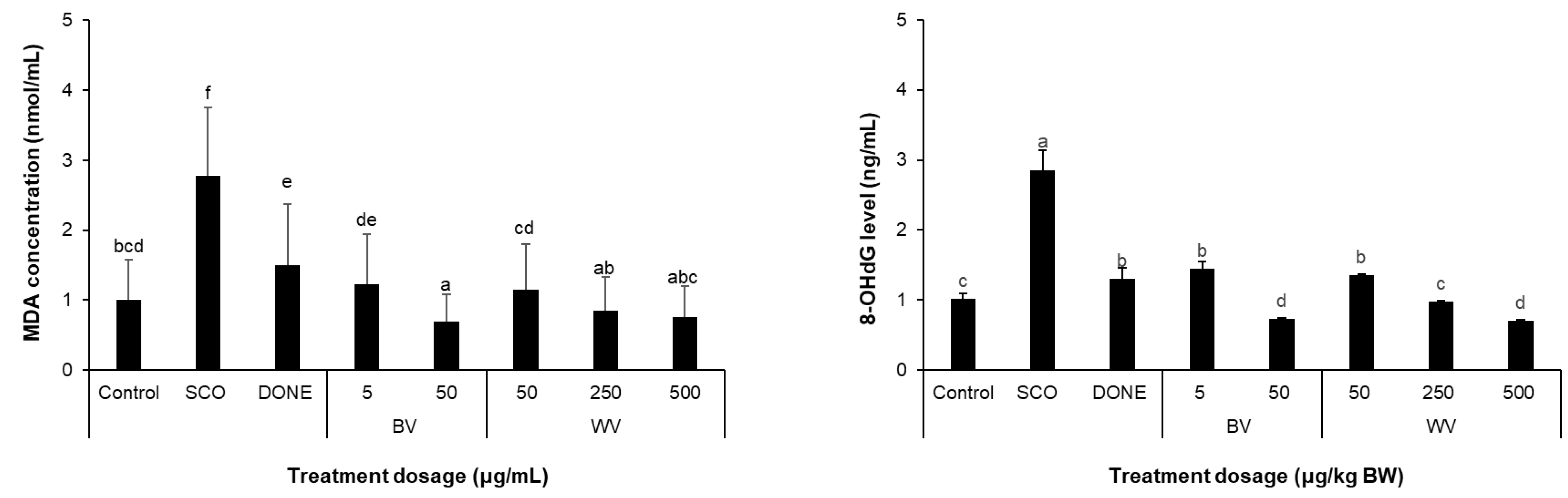
2.9. WV Decreased Scopolamine-Induced Oxidative Stress Biomarkers
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Preparation of WV and BV
5.2. Cell Culture
5.3. Free Radical Scavenging Assays
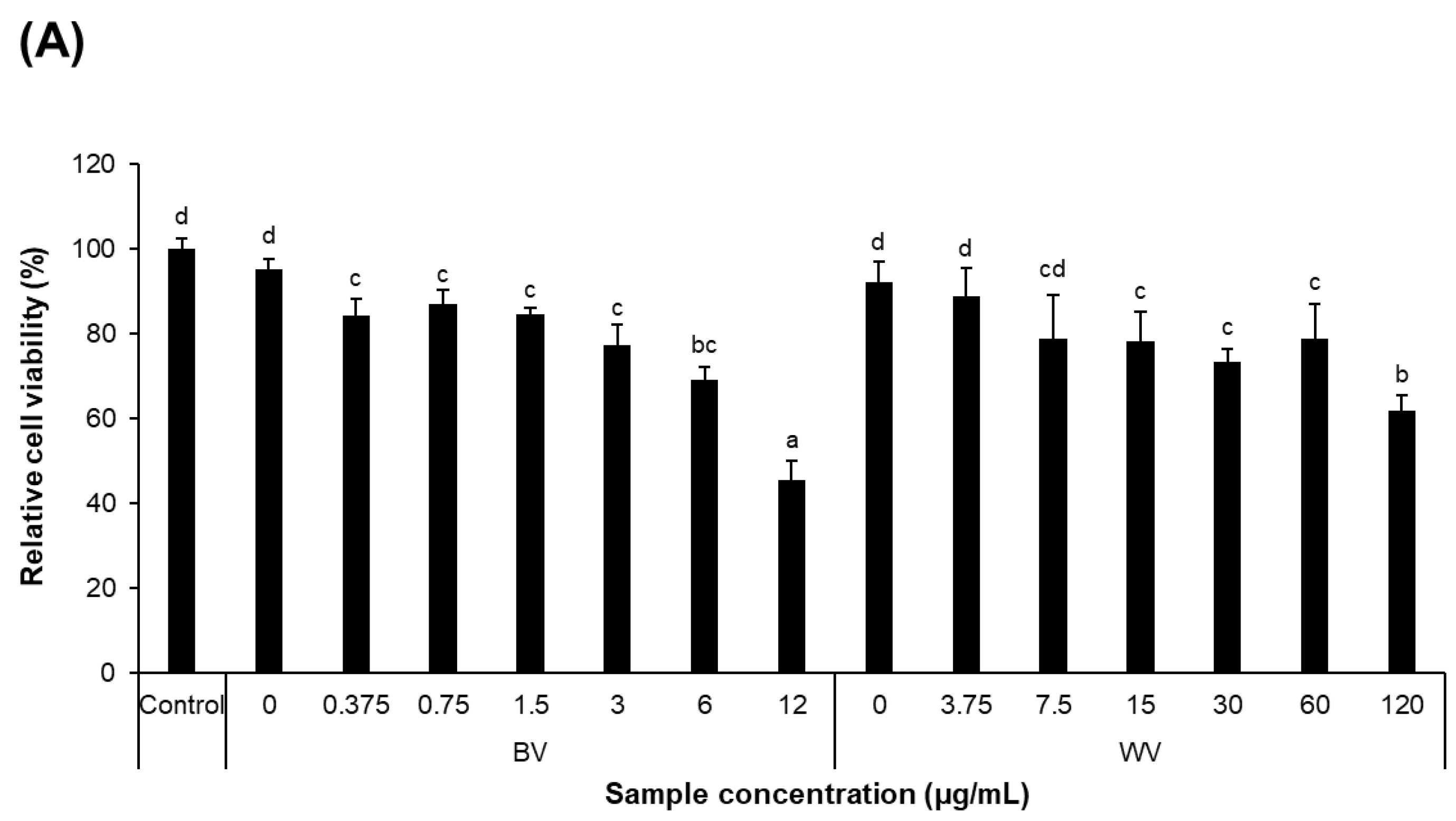
5.4. Cell Viability
5.5. ARE-Luciferase Reporter Assay
5.6. Determination of Intracellular ROS Level
5.7. Western blotting
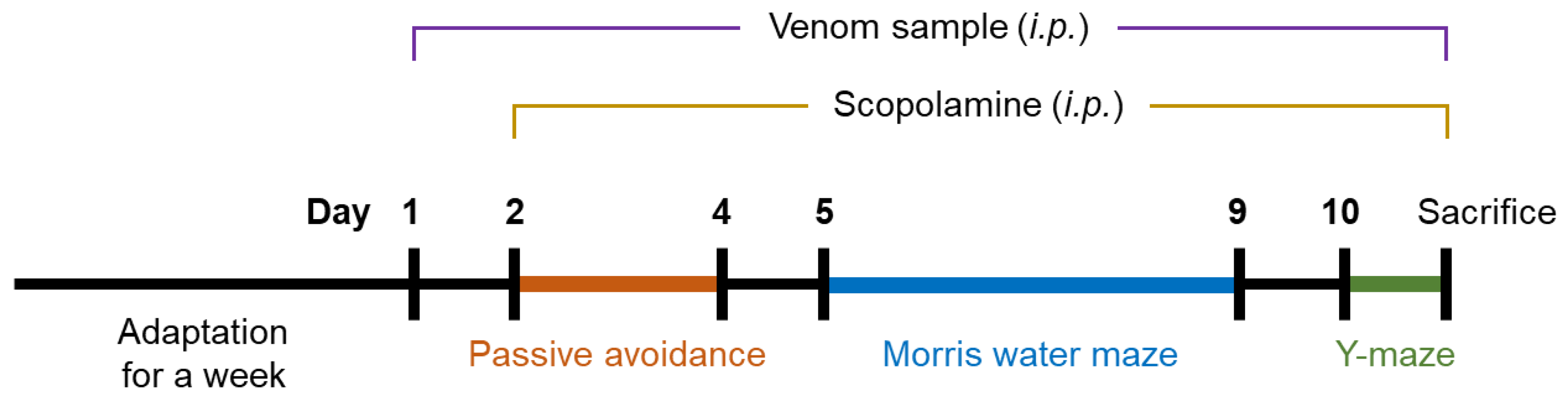
5.8. Animal Experiment
5.9. Behavioral Test
5.10. Histological Analysis by Hematoxylin and Eosin (H&E) Staining
5.11. Measurement of Plasma 8-hydroxy-2′-deoxyguanosine (8-OHdG) Level
5.12. Determination of Lipid Peroxidation in Cerebral Cortex Tissues
5.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monteiro, M.C.; Romao, P.R.; Soares, A.M. Pharmacological perspectives of wasp venom. Protein Pept. Lett. 2009, 16, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Son, M.; Noh, E.Y.; Kim, S.; Kim, C.; Yeo, J.H.; Park, C.; Lee, K.W.; Bang, W.Y. MP-V1 from the Venom of Social Wasp Vespula vulgaris Is a de Novo Type of Mastoparan that Displays Superior Antimicrobial Activities. Molecules 2016, 21, 512. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.H.; Yang, X.B.; Yang, X.L.; Zhai, L.; Lu, Z.K.; Liu, J.Z.; Yu, H.N. Antimicrobial peptides from the venoms of Vespa bicolor Fabricius. Peptides 2008, 29, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Li, D.; Tang, Q.; Wang, W.; Xie, G.; Dou, P. A Novel Peptide from Vespa ducalis Induces Apoptosis in Osteosarcoma Cells by Activating the p38 MAPK and JNK Signaling Pathways. Biol. Pharm. Bull. 2018, 41, 458–464. [Google Scholar] [CrossRef] [Green Version]
- Danneels, E.L.; Gerlo, S.; Heyninck, K.; Van Craenenbroeck, K.; De Bosscher, K.; Haegeman, G.; de Graaf, D.C. How the venom from the ectoparasitoid Wasp nasonia vitripennis exhibits anti-inflammatory properties on mammalian cell lines. PLoS ONE 2014, 9, e96825. [Google Scholar] [CrossRef] [Green Version]
- Yun, H.S.; Oh, J.; Lim, J.S.; Kim, H.J.; Kim, J.S. Anti-Inflammatory Effect of Wasp Venom in BV-2 Microglial Cells in Comparison with Bee Venom. Insects 2021, 12, 297. [Google Scholar] [CrossRef]
- Silva, J.; Monge-Fuentes, V.; Gomes, F.; Lopes, K.; dos Anjos, L.; Campos, G.; Arenas, C.; Biolchi, A.; Goncalves, J.; Galante, P.; et al. Pharmacological Alternatives for the Treatment of Neurodegenerative Disorders: Wasp and Bee Venoms and Their Components as New Neuroactive Tools. Toxins 2015, 7, 3179–3209. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.A.; Ackerman, S.L. Oxidative stress, cell cycle, and neurodegeneration. J. Clin. Investig. 2003, 111, 785–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayre, L.M.; Perry, G.; Smith, M.A. Oxidative stress and neurotoxicity. Chem. Res. Toxicol. 2008, 21, 172–188. [Google Scholar] [CrossRef] [Green Version]
- Bhat, S.A.; Sood, A.; Shukla, R.; Hanif, K. AT2R Activation Prevents Microglia Pro-inflammatory Activation in a NOX-Dependent Manner: Inhibition of PKC Activation and p47(phox) Phosphorylation by PP2A. Mol. Neurobiol. 2019, 56, 3005–3023. [Google Scholar] [CrossRef] [PubMed]
- Muche, A.; Arendt, T.; Schliebs, R. Oxidative stress affects processing of amyloid precursor protein in vascular endothelial cells. PLoS ONE 2017, 12, e0178127. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Liu, I.Y.; Bi, X.; Thompson, R.F.; Doctrow, S.R.; Malfroy, B.; Baudry, M. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc. Natl. Acad. Sci. USA 2003, 100, 8526–8531. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Hu, J.; Li, J.; Yang, Z.; Xin, X.; Wang, J.; Ding, J.; Geng, M. Effect of acidic oligosaccharide sugar chain on scopolamine-induced memory impairment in rats and its related mechanisms. Neurosci. Lett. 2005, 374, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Seo, J.Y.; Lee, S.K.; Oh, J.; Kim, J.S. Oral administration of hydrolyzed red ginseng extract improves learning and memory capability of scopolamine-treated C57BL/6J mice via upregulation of Nrf2-mediated antioxidant mechanism. J. Ginseng Res. 2021, 45, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.H.; Miyamoto, M.; Sastre, A.; Schnaar, R.L.; Coyle, J.T. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 1989, 2, 1547–1558. [Google Scholar] [CrossRef]
- Stanciu, M.; Wang, Y.; Kentor, R.; Burke, N.; Watkins, S.; Kress, G.; Reynolds, I.; Klann, E.; Angiolieri, M.R.; Johnson, J.W.; et al. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J. Biol. Chem. 2000, 275, 12200–12206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kritis, A.A.; Stamoula, E.G.; Paniskaki, K.A.; Vavilis, T.D. Researching glutamate-induced cytotoxicity in different cell lines: A comparative/collective analysis/study. Front. Cell. Neurosci. 2015, 9, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobaben, S.; Grohm, J.; Seiler, A.; Conrad, M.; Plesnila, N.; Culmsee, C. Bid-mediated mitochondrial damage is a key mechanism in glutamate-induced oxidative stress and AIF-dependent cell death in immortalized HT-22 hippocampal neurons. Cell Death Differ. 2011, 18, 282–292. [Google Scholar] [CrossRef] [Green Version]
- Lalonde, R. The neurobiological basis of spontaneous alternation. Neurosci. Biobehav. Rev. 2002, 26, 91–104. [Google Scholar] [CrossRef]
- Lee, J.D.; Park, H.J.; Chae, Y.; Lim, S. An Overview of Bee Venom Acupuncture in the Treatment of Arthritis. Evid.-Based Complement. Altern. Med. 2005, 2, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, C.; Kucuksezer, U.C.; Akdis, M.; Akdis, C.A. Mechanisms of immunotherapy to wasp and bee venom. Clin. Exp. Allergy 2011, 41, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Chung, H.S.; Lee, C.; Yoon, M.S.; Yu, A.R.; Kim, J.S.; Hwang, D.S.; Shim, I.; Bae, H. Neuroprotective effects of bee venom phospholipase A2 in the 3xTg AD mouse model of Alzheimer’s disease. J. Neuroinflamm. 2016, 13, 10. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Yu, W.X.; Duan, X.M.; Ni, L.L.; Liu, H.; Zhao, H.R.; Xiao, H.; Zhang, C.G.; Yang, Z.B. Wasp Venom Possesses Potential Therapeutic Effect in Experimental Models of Rheumatoid Arthritis. Evid.-Based Complement. Altern. Med. 2020, 2020, 6394625. [Google Scholar] [CrossRef]
- Dongol, Y.; Dhananjaya, B.L.; Shrestha, R.K.; Aryal, G. Wasp Venom Toxins as a Potential Therapeutic Agent. Protein Pept. Lett. 2016, 23, 688–698. [Google Scholar] [CrossRef]
- Bahn, G.; Jo, D.G. Therapeutic Approaches to Alzheimer’s Disease Through Modulation of NRF2. Neuromol. Med. 2019, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.T.; Braak, H.; Markesbery, W.R. Neuropathology and cognitive impairment in Alzheimer disease: A complex but coherent relationship. J. Neuropathol. Exp. Neurol. 2009, 68, 1–14. [Google Scholar] [CrossRef]
- Martins, R.N.; Villemagnen, V.; Sohrabi, H.R.; Chatterjee, P.; Shah, T.M.; Verdile, G.; Fraser, P.; Taddei, K.; Gupta, V.B.; Rainey-Smith, S.R.; et al. Alzheimer’s Disease: A Journey (f)rom Amyloid Peptides and Oxidative Stress, to Biomarker Technologies and Disease Prevention Strategies-Gains from AIBL and DIAN Cohort Studies. J. Alzheimers Dis. 2018, 62, 965–992. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Vasavda, C.; Kothari, R.; Malla, A.P.; Tokhunts, R.; Lin, A.; Ji, M.; Ricco, C.; Xu, R.; Saavedra, H.G.; Sbodio, J.I.; et al. Bilirubin Links Heme Metabolism to Neuroprotection by Scavenging Superoxide. Cell Chem. Biol. 2019, 26, 1450–1460.e7. [Google Scholar] [CrossRef]
- Abd El-Wahed, A.; Yosri, N.; Sakr, H.H.; Du, M.; Algethami, A.F.M.; Zhao, C.; Abdelazeem, A.H.; Tahir, H.E.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Wasp Venom Biochemical Components and Their Potential in Biological Applications and Nanotechnological Interventions. Toxins 2021, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Le, T.N.; Da Silva, D.; Colas, C.; Darrouzet, E.; Baril, P.; Leseurre, L.; Maunit, B. Asian hornet Vespa velutina nigrithorax venom: Evaluation and identification of the bioactive compound responsible for human keratinocyte protection against oxidative stress. Toxicon Off. J. Int. Soc. Toxinol. 2020, 176, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.A.; Sun, E.W.; Martin, A.M.; Keating, D.J. The ever-changing roles of serotonin. Int. J. Biochem. Cell B 2020, 125, 105776. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Hille, B.; Koh, D.S. Serotonin modulates melatonin synthesis as an autocrine neurotransmitter in the pineal gland. Proc. Natl. Acad. Sci. USA 2021, 118, e2113852118. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Nguyen, J.C.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Mosienko, V.; Bert, B.; Beis, D.; Matthes, S.; Fink, H.; Bader, M.; Alenina, N. Exaggerated aggression and decreased anxiety in mice deficient in brain serotonin. Transl. Psychiatry 2012, 2, e122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szoke, H.; Kovacs, Z.; Bokkon, I.; Vagedes, J.; Szabo, A.E.; Hegyi, G.; Sterner, M.G.; Kiss, A.; Kapocs, G. Gut dysbiosis and serotonin: Intestinal 5-HT as a ubiquitous membrane permeability regulator in host tissues, organs, and the brain. Rev. Neurosci. 2020, 31, 415–425. [Google Scholar] [CrossRef] [PubMed]
- El-Merahbi, R.; Loffler, M.; Mayer, A.; Sumara, G. The roles of peripheral serotonin in metabolic homeostasis. FEBS Lett. 2015, 589, 1728–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Averilla, J.N.; Oh, J.; Wu, Z.; Liu, K.H.; Jang, C.H.; Kim, H.J.; Kim, J.S.; Kim, J.S. Improved extraction of resveratrol and antioxidants from grape peel using heat and enzymatic treatments. J. Sci. Food Agric. 2019, 99, 4043–4053. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.; Lee, H.; Jeong, Y.S.; Shin, G.Y.; Oh, J.G.; Kim, J.S.; Oh, J. Antioxidant Potential of Selected Korean Edible Plant Extracts. BioMed Res. Int. 2017, 2017, 7695605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, J.Y.; Ju, S.H.; Oh, J.; Lee, S.K.; Kim, J.S. Neuroprotective and Cognition-Enhancing Effects of Compound K Isolated from Red Ginseng. J. Agric. Food Chem. 2016, 64, 2855–2864. [Google Scholar] [CrossRef]
- Kim, M.S.; Seo, J.Y.; Oh, J.; Jang, Y.K.; Lee, C.H.; Kim, J.S. Neuroprotective Effect of Halophyte Salicornia herbacea L. Is Mediated by Activation of Heme Oxygenase-1 in Mouse Hippocampal HT22 Cells. J. Med. Food 2017, 20, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Kim, B.R.; Oh, J.; Kim, J.S. Soybean-Derived Phytoalexins Improve Cognitive Function through Activation of Nrf2/HO-1 Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 268. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.Y.; Lim, S.S.; Kim, J.; Lee, K.W.; Kim, J.S. Alantolactone and Isoalantolactone Prevent Amyloid beta25-35 -induced Toxicity in Mouse Cortical Neurons and Scopolamine-induced Cognitive Impairment in Mice. Phytother. Res. PTR 2017, 31, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Oh, J.; Jang, C.H.; Kim, J.S. Improvement of cognitive function by Gochujang supplemented with tomato paste in a mouse model. Food Sci. Biotechnol. 2019, 28, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.; Lim, J.S.; Oh, J.; Lee, J.S.; Kim, J.S. Neuroprotective Effects of Euonymus alatus Extract on Scopolamine-Induced Memory Deficits in Mice. Antioxidants 2020, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lim, J.S.; Yun, H.S.; Kim, Y.; Jeong, S.; Hwang, S.D.; Kim, J.W.; Oh, J.; Kim, J.S. Dietary supplementation with Ceriporia lacerata improves learning and memory in a scopolamine-induced amnesia mouse model. Food Sci. Biotechnol. 2021, 30, 1107–1116. [Google Scholar] [CrossRef]
- Saintemarie, G. A Paraffin Embedding Technique for Studies Employing Immunofluorescence. J. Histochem. Cytochem. 1962, 10, 250–256. [Google Scholar] [CrossRef] [Green Version]



| Group No. | Treatment | |
|---|---|---|
| Sample | SCO (1 mg/kg BW) | |
| 1 | Vehicle | – |
| 2 | Vehicle | + |
| 3 | Donepezil (5 mg/kg BW) | + |
| 4 | BV at 5 μg/kg BW | + |
| 5 | BV at 50 μg/kg BW | + |
| 6 | WV at 50 μg/kg BW | + |
| 7 | WV at 250 μg/kg BW | + |
| 8 | WV at 500 μg/kg BW | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chae, J.H.; Oh, J.; Lim, J.S.; Jeong, Y.A.; Yun, H.S.; Jang, C.H.; Kim, H.J.; Kim, J.-S. Wasp Venom Ameliorates Scopolamine-Induced Learning and Memory Impairment in Mice. Toxins 2022, 14, 256. https://doi.org/10.3390/toxins14040256
Chae JH, Oh J, Lim JS, Jeong YA, Yun HS, Jang CH, Kim HJ, Kim J-S. Wasp Venom Ameliorates Scopolamine-Induced Learning and Memory Impairment in Mice. Toxins. 2022; 14(4):256. https://doi.org/10.3390/toxins14040256
Chicago/Turabian StyleChae, Ji Hyeong, Jisun Oh, Ji Sun Lim, Yoon Ah Jeong, Hyun Seok Yun, Chan Ho Jang, Hyo Jung Kim, and Jong-Sang Kim. 2022. "Wasp Venom Ameliorates Scopolamine-Induced Learning and Memory Impairment in Mice" Toxins 14, no. 4: 256. https://doi.org/10.3390/toxins14040256
APA StyleChae, J. H., Oh, J., Lim, J. S., Jeong, Y. A., Yun, H. S., Jang, C. H., Kim, H. J., & Kim, J.-S. (2022). Wasp Venom Ameliorates Scopolamine-Induced Learning and Memory Impairment in Mice. Toxins, 14(4), 256. https://doi.org/10.3390/toxins14040256





