Biocontrol of Fusarium graminearum, a Causal Agent of Fusarium Head Blight of Wheat, and Deoxynivalenol Accumulation: From In Vitro to In Planta
Abstract
:1. Introduction
2. Results
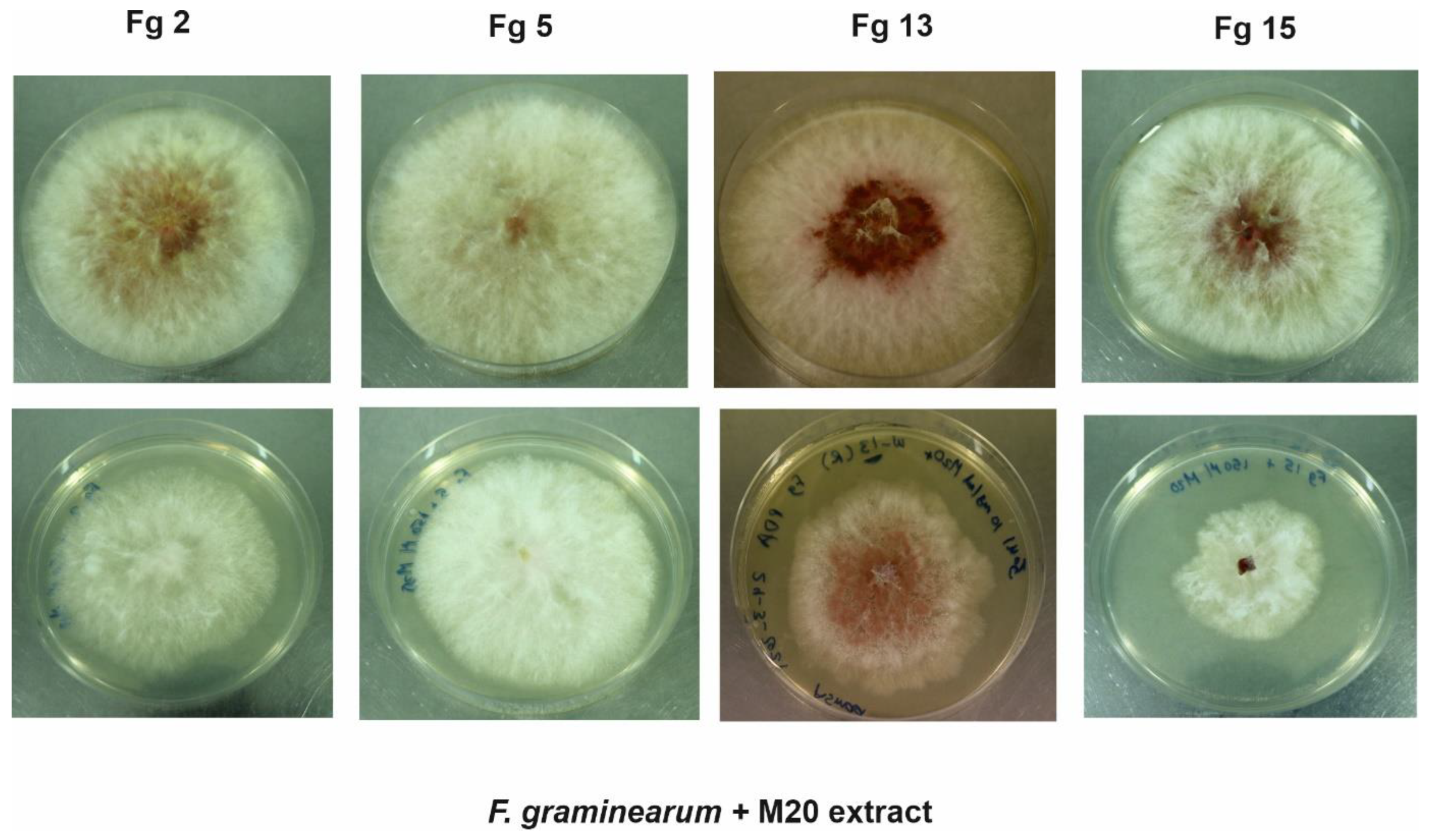
2.1. In Vitro Bioassay—Effect of M20 Extract on Mycelium Growth
2.2. Quantification of F. graminearum DNA in Wheat Using qPCR
2.3. M20 Extract Inhibited DON Production by F. graminearum
2.4. Correlation between Fungal DNA and DON Content
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals, Reagents, and Zanthoxylum bungeanum Plant
5.2. Pathogen Inoculum Production
5.3. In Vitro Bioassay—Effect of M20 Extract on Mycelium Growth
5.4. Treatments of Wheat Grains by Methanolic Extract of Zanthoxylum bungeanum in the Field
5.5. DNA Extraction and qPCR Analysis
5.6. Extraction and Evaluation of Deoxynivalenol Accumulated in Wheat Heads
5.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drakopoulos, D.; Meca, G.; Torrijos, R.; Marty, A.; Kägi, A.; Jenny, E.; Forrer, H.R.; Six, J.; Vogelgsang, S. Control of Fusarium graminearum in wheat with mustard-based botanicals: From in vitro to in planta. Front. Microbiol. 2020, 11, 1595. [Google Scholar] [CrossRef]
- Yang, F.; Jacobsen, S.; Jørgensen, H.J.L.; Collinge, D.B.; Svensson, B.; Finnie, C. Fusarium graminearum and its interactions with cereal heads: Studies in the proteomics era. Front. Plant Sci. 2013, 4, 37. [Google Scholar] [CrossRef] [Green Version]
- Morimura, H.; Ito, M.; Yoshida, S.; Koitabashi, M.; Tsushima, S.; Camagna, M.; Chiba, S.; Takemoto, D.; Kawakita, K.; Sato, I. In vitro assessment of biocontrol effects on Fusarium Head Blight and deoxynivalenol (DON) accumulation by DON-degrading bacteria. Toxins 2020, 12, 399. [Google Scholar] [CrossRef]
- Rotter, B.A.; Prelusky, D.B.; Pestka, J.J. Invited Review: Toxicology of deoxynivalenol (Vomitoxin). J. Toxicol. Environ. Health 1996, 48, 1–34. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef]
- Vogelgsang, S.; Beyer, M.; Pasquali, M.; Jenny, E.; Musa, T.; Bucheli, T.D.; Wettstein, F.E.; Forrer, H.R. An eight-year survey of wheat shows distinctive effects of cropping factors on different Fusarium species and associated mycotoxins. Eur. J. Agron. 2019, 105, 62–77. [Google Scholar] [CrossRef]
- Goswami, R.S.; Kistler, H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004, 5, 515–525. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [Green Version]
- Gimeno, A.; Sohlberg, E.; Pakula, T.; Limnell, J.; Keller, B.; Laitila, A.; Vogelgsang, S. TaqMan QPCR for quantification of Clonostachys rosea used as a biological control agent against Fusarium graminearum. Front. Microbiol. 2019, 10, 1627. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.; Zhou, M. A major gene for resistance to carbendazim, in field isolates of Gibberella zeae. Can. J. Plant Pathol. 2005, 27, 58–63. [Google Scholar] [CrossRef]
- Miedaner, T.; Gwiazdowska, D.; Waśkiewicz, A. Editorial: Management of Fusarium species and their mycotoxins in cereal food and feed. Front. Microbiol. 2017, 8, 1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, Y.; Zhang, X.; Ge, C.; Wang, Y.; Cao, J.; Jia, X.; Wang, J.; Zhou, M. Development and application of loop-mediated isothermal amplification for detection of the f167y mutation of carbendazim-resistant isolates in Fusarium graminearum. Sci. Rep. 2014, 4, 7094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia, M.; Rodrigues, M.; Paíga, P.; Delerue-Matos, C. Fungicides. In Encyclopedia of Food and Health; Academic Press: Cambridge, MA, USA, 2015; pp. 169–176. [Google Scholar] [CrossRef]
- Schöneberg, A.; Musa, T.; Voegele, R.T.; Vogelgsang, S. The potential of antagonistic fungi for control of Fusarium graminearum and Fusarium crookwellense varies depending on the experimental approach. J. Appl. Microbiol. 2015, 118, 1165–1179. [Google Scholar] [CrossRef] [PubMed]
- Xue, A.G.; Chen, Y.; Voldeng, H.D.; Fedak, G.; Savard, M.E.; Längle, T.; Zhang, J.; Harman, G.E. Concentration and cultivar effects on efficacy of clo-1 biofungicide in controlling fusarium head blight of wheat. Biol. Control 2014, 73, 2–7. [Google Scholar] [CrossRef]
- Li, J.; Duan, Y.; Bian, C.; Pan, X.; Yao, C.; Wang, J.; Zhou, M. Effects of validamycin in controlling Fusarium Head Blight caused by Fusarium graminearum: Inhibition of DON biosynthesis and induction of host resistance. Pestic. Biochem. Physiol. 2019, 153, 152–160. [Google Scholar] [CrossRef]
- Colombo, E.M.; Kunova, A.; Gardana, C.; Pizzatti, C.; Simonetti, P.; Cortesi, P.; Saracchi, M.; Pasquali, M. Investigating useful properties of four Streptomyces strains active against Fusarium graminearum growth and deoxynivalenol production on wheat grains by QPCR. Toxins 2020, 12, 560. [Google Scholar] [CrossRef]
- Palazzini, J.M.; Alberione, E.; Torres, A.; Donat, C.; Köhl, J.; Chulze, S. Biological control of Fusarium graminearum sensu stricto, causal agent of Fusarium Head Blight of wheat, using formulated antagonists under field conditions in Argentina. Biol. Control 2016, 94, 56–61. [Google Scholar] [CrossRef]
- He, J.; Boland, G.J.; Zhou, T. Concurrent selection for microbial suppression of Fusarium graminearum, Fusarium Head Blight and deoxynivalenol in wheat. J. Appl. Microbiol. 2009, 106, 1805–1817. [Google Scholar] [CrossRef]
- Masika, P.J.; Afolayan, A.J. Antimicrobial activity of some plants used for the treatment of livestock disease in the Eastern Cape, South Africa. J. Ethnopharmacol. 2002, 83, 129–134. [Google Scholar] [CrossRef]
- Sultana, S.; Akhtar, N.; Asif, H.M. Phytochemical screening and antipyretic effects of hydro-methanol extract of Melia azedarach leaves in rabbits. Bangladesh J. Pharmacol. 2013, 8, 214–217. [Google Scholar] [CrossRef] [Green Version]
- Fandohan, P.; Gbenou, J.D.; Gnonlonfin, B.; Hell, K.; Marasas, W.F.; Wingfield, M.J. Effect of essential oils on the growth of Fusarium verticillioides and fumonisin contamination in corn. J. Agric. Food Chem. 2004, 52, 6824–6829. [Google Scholar] [CrossRef] [PubMed]
- MartÍnez, J.A. Natural fungicides obtained from plants, fungicides for plant and animal diseases. In Fungicides for Plant and Animal Diseases; Dhanasekaran, D., Ed.; InTech Open: Shanghai, China, 2012. [Google Scholar]
- Chen, C.; Long, L.; Zhang, F.; Chen, Q.; Chen, C.; Yu, X.; Liu, Q.; Bao, J.; Long, Z. Antifungal activity, main active components and mechanism of Curcuma longa extract against Fusarium graminearum. PLoS ONE 2018, 13, e0194284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.-C.; Li, R.; Tan, J.; Jiang, Z.-T. Polyphenolics composition of the leaves of Zanthoxylum bungeanum maxim. grown in Hebei, China, and their radical scavenging activities. J. Agric. Food Chem. 2013, 61, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia. Science and Technology Press of Shanghai; Chinese Pharmacopoeia Commission: Shanghai, China, 2015; pp. 159–160. (In Chinese)
- Xiong, Q.; Shi, D.; Yamamoto, H.; Mizuno, M. Alkylamides from pericarps of Zanthoxylum bungeanum. Phytochemistry 1997, 46, 1123–1126. [Google Scholar] [CrossRef]
- Gong, Y.; Huang, Y.; Zhou, L.; Shi, X.; Guo, Z.; Wang, M.; Jiang, W. Chemical composition and antifungal activity of the fruit oil of Zanthoxylum bungeanum maxim. (Rutaceae) from China. J. Essent. Oil Res. 2009, 21, 174–178. [Google Scholar] [CrossRef]
- Xing-dong, L.; Hua-li, X. Antifungal activity of the essential oil of Zanthoxylum bungeanum and its major constituent on Fusarium sulphureum and dry rot of potato tubers. Phytoparasitica 2014, 42, 509–517. [Google Scholar] [CrossRef]
- Tellenbach, C.; Grünig, C.R.; Sieber, T.N. Suitability of quantitative real-time pcr to estimate the biomass of fungal root endophytes. Appl. Environ. Microbiol. 2010, 76, 5764–5772. [Google Scholar] [CrossRef] [Green Version]
- Yli-Mattila, T.; Hussien, T.; Abbas, A. Comparison of biomass and deoxynivalenol production of northern european and southern european Fusarium graminearum isolates in the infection of wheat and oat grains. J. Plant Pathol. 2022; submitted for publication. [Google Scholar]
- Abbas, A.; Wright, C.W.; El-Sawi, N.; Malinen, A.M. A methanolic extract of Zanthoxylum bungeanum modulates secondary metabolism regulator genes in Aspergillus flavus and shuts down aflatoxin production. Sci. Rep. 2022, 12, 5995. [Google Scholar] [CrossRef]
- da Cruz Cabral, L.; Fernández Pinto, V.; Patriarca, A. Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int. J. Food Microbiol. 2013, 166, 1–14. [Google Scholar] [CrossRef]
- Shah, L.; Ali, A.; Yahya, M.; Zhu, Y.; Wang, S.; Si, H.; Rahman, H.; Ma, C. Integrated control of Fusarium Head Blight and deoxynivalenol mycotoxin in wheat. Plant Pathol. 2018, 67, 532–548. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J.; Zhu, L.; Li, T.; Jiang, W.; Zhou, J.; Peng, W.; Wu, C. Zanthoxylum Bungeanum maxim. (Rutaceae): A systematic review of its traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics, and toxicology. Int. J. Mol. Sci. 2017, 18, 2172. [Google Scholar] [CrossRef] [PubMed]
- Dykes, L.; Rooney, L.W. Phenolic compounds in cereal grains and their health benefits. Cereal Foods World 2007, 52, 105–111. [Google Scholar] [CrossRef]
- Pagnussatt, F.A.; Del Ponte, E.M.; Garda-Buffon, J.; Badiale-Furlong, E. Inhibition of Fusarium graminearum growth and mycotoxin production by phenolic extract from Spirulina sp. Pestic. Biochem. Physiol. 2014, 108, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Schöneberg, T.; Kibler, K.; Sulyok, M.; Musa, T.; Bucheli, T.D.; Mascher, F.; Bertossa, M.; Voegele, R.T.; Vogelgsang, S. Can plant phenolic compounds reduce Fusarium growth and mycotoxin production in cereals? Food Addit. Contam. Part A 2018, 35, 2455–2470. [Google Scholar] [CrossRef] [Green Version]
- Skadhauge, B.; Thomsen, K.K.; Von Wettstein, D. The role of the barley testa layer and its flavonoid content in resistance to Fusarium infections. Hereditas 1997, 126, 147–160. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Najjar, A.A.; Alzahrani, S.O.; Alkhatib, F.M.; Shafi, M.E.; Selem, E.; Desoky, E.S.M.; Fouda, S.E.E.; El-Tahan, A.M.; et al. The use of biological selenium nanoparticles to suppress Triticum aestivum l. crown and root rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J. Biol. Sci. 2021, 28, 4461–4471. [Google Scholar] [CrossRef]
- Seepe, H.A.; Ramakadi, T.G.; Lebepe, C.M.; Amoo, S.O.; Nxumalo, W. Antifungal activity of isolated compounds from the leaves of Combretum erythrophyllum (Burch.) Sond. and Withania Somnifera (L.) Dunal against Fusarium pathogens. Molecules 2021, 26, 4732. [Google Scholar] [CrossRef]
- Yli-Mattila, T.; Rämö, S.; Hietaniemi, V.; Hussien, T.; Carlobos-Lopez, A.L.; Cumagun, C.J.R. Molecular quantification and genetic diversity of toxigenic Fusarium species in northern Europe as compared to those in southern Europe. Microorganisms 2013, 1, 162–174. [Google Scholar] [CrossRef]
- Alisaac, E.; Rathgeb, A.; Karlovsky, P.; Mahlein, A.-K. Fusarium Head Blight: Effect of infection timing on spread of Fusarium graminearum and spatial distribution of deoxynivalenol within wheat spikes. Microorganisms 2021, 9, 79. [Google Scholar] [CrossRef]
- Jayashree, T.; Subramanyam, C. Antiaflatoxigenic activity of eugenol is due to inhibition of lipid peroxidation. Lett. Appl. Microbiol. 1999, 28, 179–183. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, L.; Bonnin-Verdal, M.N.; Marchegay, G.; Pinson-Gadais, L.; Ducos, C.; Richard-Forget, F.; Atanasova-Penichon, V. Fungal biotransformation of chlorogenic and caffeic acids by Fusarium graminearum: New insights in the contribution of phenolic acids to resistance to deoxynivalenol accumulation in cereals. Int. J. Food Microbiol. 2016, 221, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Marín, S.; Velluti, A.; Ramos, A.J.; Sanchis, V. Effect of essential oils on zearalenone and deoxynivalenol production by Fusarium graminearum in non-sterilized maize grain. Food Microbiol. 2004, 21, 313–318. [Google Scholar] [CrossRef]
- Garda-Buffon, J.; Baraj, E.; Badiale-Furlong, E. Effect of deoxynivalenol and T-2 toxin in malt amylase activity. Brazilian Arch. Biol. Technol. 2010, 53, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Kolawole, O.; Meneely, J.; Petchkongkaew, A.; Elliott, C. A Review of mycotoxin biosynthetic pathways: Associated genes and their expressions under the influence of climatic factors. Fungal Biol. Rev. 2021, 37, 8–26. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, Y.; Li, X.; Peng, C.; Long, Z. Antifungal activity and mechanism of major compound isolated from hexane extract of Curcuma Zedoaria. Asian J. Chem. 2013, 25, 6597–6600. [Google Scholar] [CrossRef]
- Boshoff, W.H.P.; Prins, R.; De Klerk, C.; Krattinger, S.G.; Bender, C.M.; Maree, G.J.; Rothmann, L.; Pretorius, Z.A. Point inoculation method for measuring adult plant response of wheat to stripe rust infection. Plant Dis. 2019, 103, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Mitina, G.V.; Tokarev, Y.S.; Movila, A.A.; Yli-Mattila, T. Polymorphism of Beauveria Bassiana (Deuteromycota: Hyphomycetes) strains isolated from Ixodes Ricinus (Acari: Ixodidae) in Moldova. Ticks Tick-Borne Dis. 2011, 2, 50–54. [Google Scholar] [CrossRef]
- Yli-Mattila, T.; Paavanen-Huhtala, S.; Jestoi, M.; Parikka, P.; Hietaniemi, V.; Gagkaeva, T.; Sarlin, T.; Haikara, A.; Laaksonen, S.; Rizzo, A. Real-Time PCR detection and quantification of Fusarium poae, F. Graminearum, F. Sporotrichioides and F. Langsethiae in cereal grains in Finland and Russia. Arch. Phytopathol. Plant Prot. 2008, 41, 243–260. [Google Scholar] [CrossRef]




| Strain Number | Strain ID | Isolation Source | Plant | Year | Genotype |
|---|---|---|---|---|---|
| 2 | MFG 59065 | Southern Western (Finland) | wheat | 2017 | 3ADON |
| 5 | MFG 59068 | Southern Western (Finland) | wheat | 2017 | 3ADON |
| 13 | MFG 58703 | Krasnodar krai (Russia) | wheat | 2014 | 15ADON |
| 15 | MFG 58772 | Stavropol krai (Russia) | wheat | 2015 | 15ADON |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, A.; Yli-Mattila, T. Biocontrol of Fusarium graminearum, a Causal Agent of Fusarium Head Blight of Wheat, and Deoxynivalenol Accumulation: From In Vitro to In Planta. Toxins 2022, 14, 299. https://doi.org/10.3390/toxins14050299
Abbas A, Yli-Mattila T. Biocontrol of Fusarium graminearum, a Causal Agent of Fusarium Head Blight of Wheat, and Deoxynivalenol Accumulation: From In Vitro to In Planta. Toxins. 2022; 14(5):299. https://doi.org/10.3390/toxins14050299
Chicago/Turabian StyleAbbas, Asmaa, and Tapani Yli-Mattila. 2022. "Biocontrol of Fusarium graminearum, a Causal Agent of Fusarium Head Blight of Wheat, and Deoxynivalenol Accumulation: From In Vitro to In Planta" Toxins 14, no. 5: 299. https://doi.org/10.3390/toxins14050299
APA StyleAbbas, A., & Yli-Mattila, T. (2022). Biocontrol of Fusarium graminearum, a Causal Agent of Fusarium Head Blight of Wheat, and Deoxynivalenol Accumulation: From In Vitro to In Planta. Toxins, 14(5), 299. https://doi.org/10.3390/toxins14050299





