Effects of Two Toxin-Producing Harmful Algae, Alexandrium catenella and Dinophysis acuminata (Dinophyceae), on Activity and Mortality of Larval Shellfish
Abstract
:1. Introduction
2. Results
2.1. Larval Inactivity and Mortality
2.1.1. Live-Cell Bioassay
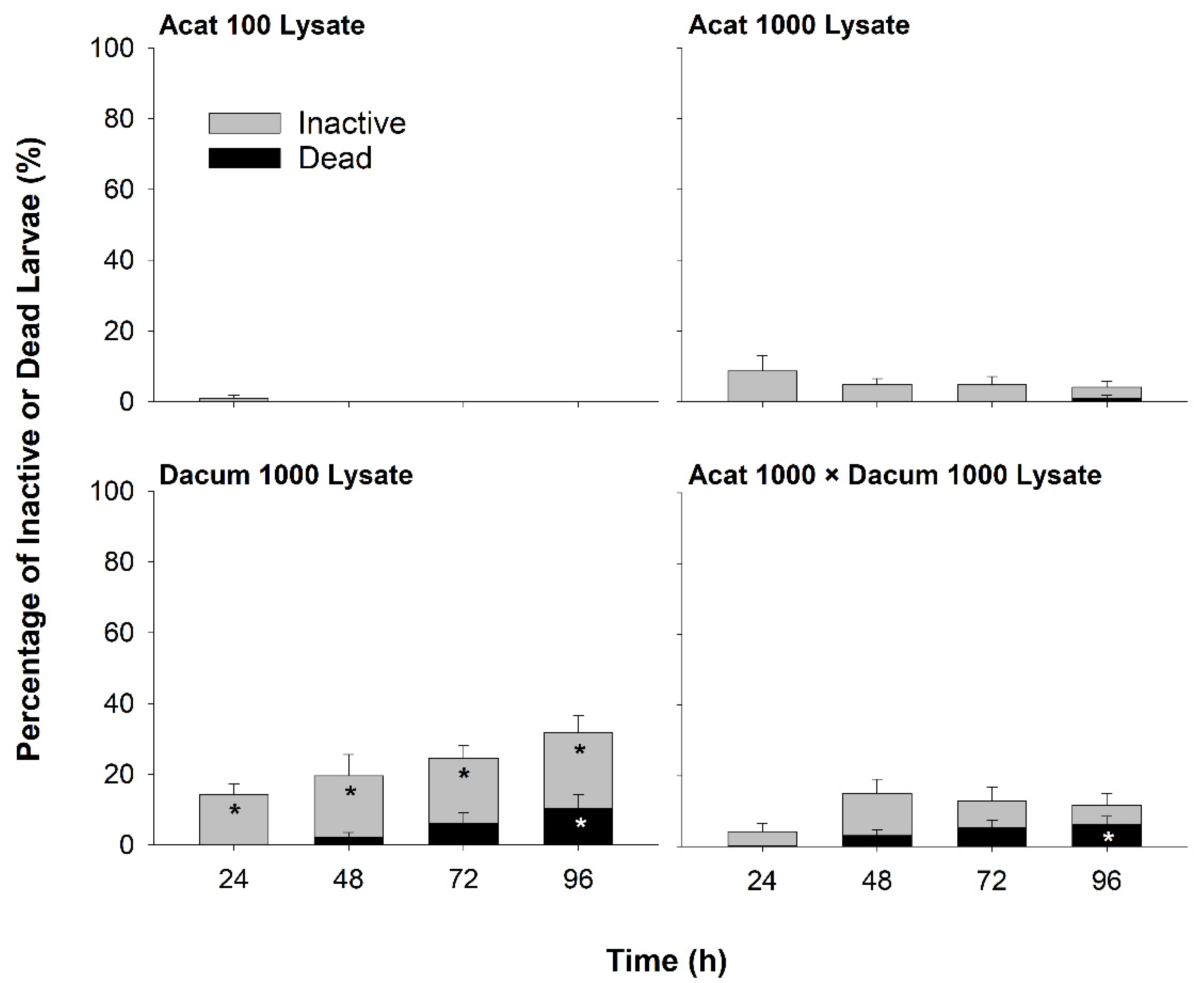
2.1.2. Lysate Bioassay
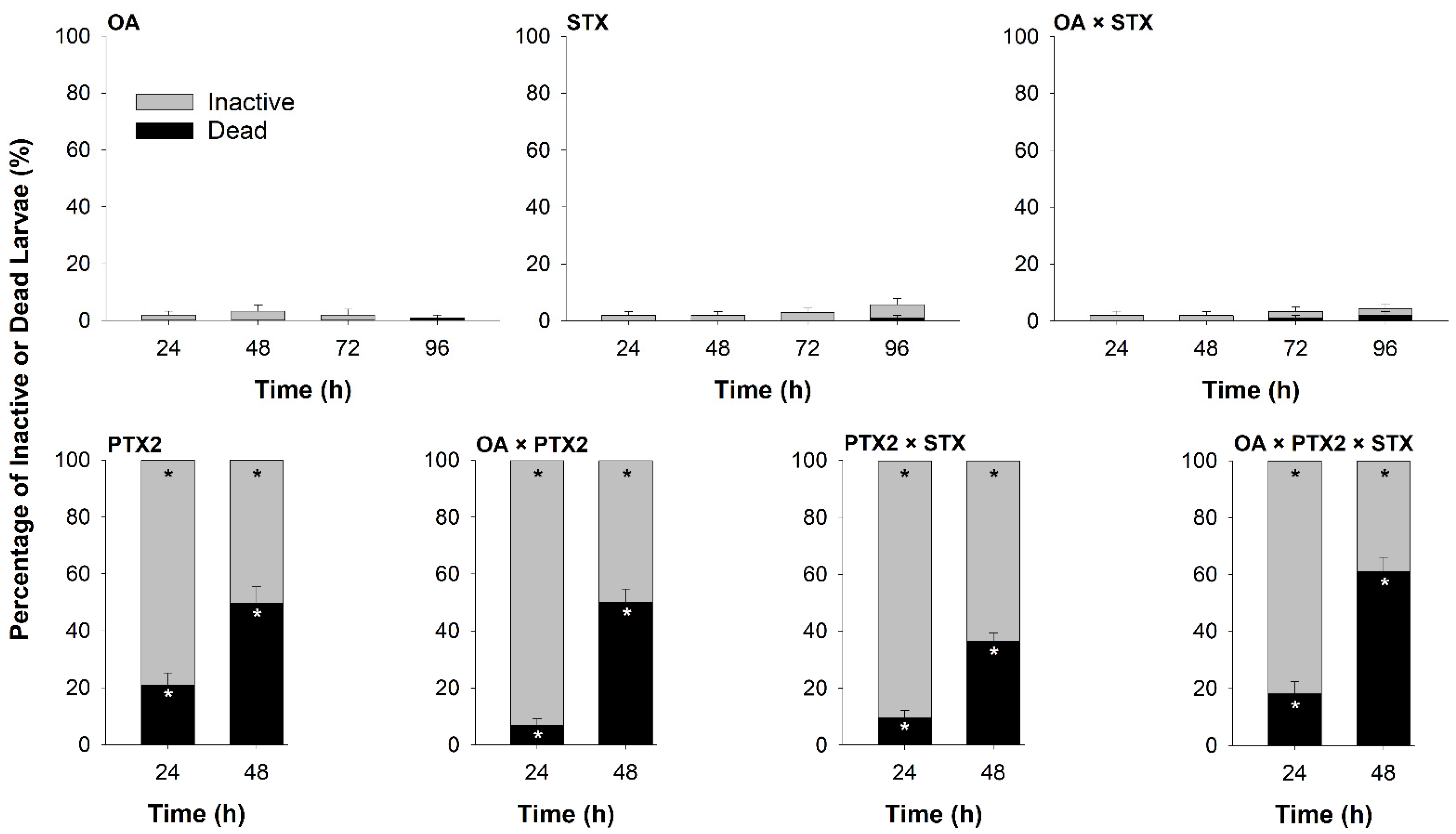
2.1.3. Pure Toxin Bioassay
2.2. Toxins in HAB Cultures, Water, and Oysters
| Bioassay | Treatments a | Time Collected (h) | Oysters per Sample b | Toxin (pg/oyster) c | ||
|---|---|---|---|---|---|---|
| OA | DTX1 | PTX2 | ||||
| N/A | Control | 0 | 70 * | <DL | <LOQ | <DL |
| Live-Cell | Dacum 10 | 96 | 80 | <DL | <LOQ | <LOQ |
| Dacum 100 | 96 | 69 | <DL | <DL | <LOQ | |
| Dacum 500 | 96 | 72 | <LOQ | <DL | 1.8 | |
| Dacum 1000 | 96 | 70 | <DL | <DL | 5.2 | |
| Lysate | Dacum 1000 | 96 | 68 | <LOQ | <LOQ | 3.7 |
| Acat 1000 × Dacum 1000 | 96 | 65 | <DL | <LOQ | 2.5 | |
| Pure Toxin | PTX2 | 48 | 74 | <DL | <DL | 40.2 |
| OA × PTX2 | 48 | 70 | <DL | <DL | 45.3 | |
| PTX2 × STX | 48 | 71 | <DL | <DL | 50.0 | |
| OA × PTX2 × STX | 48 | 70 | <DL | <DL | 47.9 | |
3. Discussion
3.1. Effects of A. catenella on Larval Oysters
3.2. Effects of D. acuminata on Larval Oysters
3.3. Potential Effects of Co-Exposure to A. catenella and D. acuminata
4. Conclusions
5. Materials and Methods
5.1. Experimental Design
5.1.1. Live-Cell Bioassay
5.1.2. Lysate Bioassay
5.1.3. Pure Toxin Bioassay
5.2. Larval Oyster Metrics and Well Water
5.3. Toxin Analyses
5.4. Culturing
5.5. Data Analysis and Statistics
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brosnahan, M.L.; Ralston, D.K.; Fischer, A.D.; Solow, A.R.; Anderson, D.M. Bloom termination of the toxic dinoflagellate Alexandrium catenella: Vertical migration behavior, sediment infiltration, and benthic cyst yield. Limnol. Oceanogr. 2017, 62, 2829–2849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crespo, B.G.; Keafer, B.A.; Ralston, D.K.; Lind, H.; Farber, D.; Anderson, D.M. Dynamics of Alexandrium fundyense blooms and shellfish toxicity in the Nauset Marsh System of Cape Cod (Massachusetts, USA). Harmful Algae 2011, 12, 26–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattenrath-Lehmann, T.K.; Marcoval, M.A.; Berry, D.L.; Fire, S.; Wang, Z.; Morton, S.L.; Gobler, C.J. The emergence of Dinophysis acuminata blooms and DSP toxins in shellfish in New York waters. Harmful Algae 2013, 26, 33–44. [Google Scholar] [CrossRef]
- Bricelj, V.M.; Connell, L.; Konoki, K.; MacQuarrie, S.P.; Scheuer, T.; Catterall, W.A.; Trainer, V.L. Sodium channel mutation leading to saxitoxin resistance in clams increases risk of PSP. Nature 2005, 434, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Mello, D.F.; da Silva, P.M.; Barracco, M.A.; Soudant, P.; Hégaret, H. Effects of the dinoflagellate Alexandrium minutum and its toxin (saxitoxin) on the functional activity and gene expression of Crassostrea gigas hemocytes. Harmful Algae 2013, 26, 45–51. [Google Scholar] [CrossRef]
- Yan, T.; Zhou, M.; Fu, M.; Wang, Y.; Yu, R.; Li, J. Inhibition of egg hatching success and larvae survival of the scallop, Chlamys farreri, associated with exposure to cells and cell fragments of the dinoflagellate Alexandrium tamarense. Toxicon 2001, 39, 1239–1244. [Google Scholar] [CrossRef]
- Yan, T.; Zhou, M.; Fu, M.; Yu, R.; Wang, Y.; Li, J. Effects of the dinoflagellate Alexandrium tamarense on early development of the scallop Argopecten irradians concentricus. Aquaculture 2003, 217, 167–178. [Google Scholar] [CrossRef]
- Supono, S.; Knowles, G.; Bolch, C. Toxicity and histopathological effects of toxic dinoflagellate, Alexandrium catenella exudates on larvae of blue mussel, Mytilus galloprovincialis, and Pacific oyster, Crassostrea gigas. J. Ilmiah Perikanan dan Kelautan 2020, 12, 188–198. [Google Scholar] [CrossRef]
- Hégaret, H.; Wikfors, G.H.; Soudant, P.; Lambert, C.; Shumway, S.E.; Bérard, J.B.; Lassus, P. Toxic dinoflagellates (Alexandrium fundyense and A. catenella) have minimal apparent effects on oyster hemocytes. Mar. Biol. 2007, 152, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Banno, K.; Oda, T.; Nagai, K.; Nagai, S.; Tanaka, Y.; Basti, L. Deleterious effects of harmful dinoflagellates and raphidophytes on egg viability and spermatozoa swimming velocity in the Japanese pearl oyster Pinctada fucata martensii. J. Shellfish Res. 2018, 37, 41–48. [Google Scholar] [CrossRef]
- Basti, L.; Nagai, S.; Go, J.; Okano, S.; Nagai, K.; Watanabe, R.; Suzuki, T.; Tanaka, Y. Differential inimical effects of Alexandrium spp. and Karenia spp. on cleavage, hatching, and two larval stages of Japanese pearl oyster Pinctada fucata martensii. Harmful Algae 2015, 43, 1–12. [Google Scholar] [CrossRef]
- Mu, C.; Li, Q. Effects of the dinoflagellate Alexandrium catenella on the early development of the Pacific oyster Crassostrea gigas. J. Shellfish. Res. 2013, 32, 689–694. [Google Scholar] [CrossRef]
- Ford, S.E.; Bricelj, V.M.; Lambert, C.; Paillard, C. Deleterious effects of a nonPST bioactive compound(s) from Alexandrium tamarense on bivalve hemocytes. Mar. Biol. 2008, 154, 241–253. [Google Scholar] [CrossRef] [Green Version]
- Castrec, J.; Hégaret, H.; Alunno-Bruscia, M.; Maïlys, P.; Soudant, P.; Petton, B.; Boulais, M.; Suquet, M.; Quéau, I.; Ratiskol, D.; et al. The dinoflagellate Alexandrium minutum affects development of the oyster Crassostrea gigas, through parental or direct exposure. Environ. Pollut. 2019, 246, 827–836. [Google Scholar] [CrossRef] [Green Version]
- Castrec, J.; Hégaret, H.; Huber, M.; Le Grand, J.; Huvet, A.; Tallec, K.; Boulais, M.; Soudant, P.; Fabioux, C. The toxic dinoflagellate Alexandrium minutum impairs the performance of oyster embryos and larvae. Harmful Algae 2020, 92, 101744. [Google Scholar] [CrossRef]
- De Rijcke, M.; Vandegehuchte, M.B.; Bussche, J.V.; Nevejan, N.; Vanhaecke, L.; De Schamphelaere, K.A.C.; Janssen, C.R. Common European harmful algal blooms affect the viability and innate immune responses of Mytilus edulis larvae. Fish Shellfish Immun. 2015, 47, 175–181. [Google Scholar] [CrossRef]
- Svensson, S.; Förlin, L. Intracellular effects of okadaic acid in the blue mussel Mytilus edulis, and rainbow trout Oncorhynchus mykiss. Mar. Environ. Res. 1998, 46, 449–452. [Google Scholar] [CrossRef]
- Gaillard, S.; Le Goïc, N.; Malo, F.; Boulais, M.; Fabioux, C.; Zaccagnini, L.; Carpentier, L.; Sibat, M.; Réveillon, D.; Séchet, V.; et al. Cultures of Dinophysis sacculus, D. acuminata and pectenotoxin 2 affect gametes and fertilization success of the Pacific oyster, Crassostrea gigas. Environ. Pollut. 2020, 265 Pt B, 114840. [Google Scholar] [CrossRef]
- Gaillard, S. Ecophysiological Studies on Dinophysis and Its Food Chain, and In Vitro Effects of the Dinoflagellate and Its Toxins on Early Life Stages of Two Models of Marine Animals (Oyster and Fish). Ph.D. Thesis, Université de Nantes, Nantes, France, 2020. Available online: https://archimer.ifremer.fr/doc/00666/77807/ (accessed on 25 March 2022).
- Loosanoff, V.L. Spawning of Ostrea virginica at low temperatures. Science 1939, 89, 177–178. [Google Scholar] [CrossRef]
- Rountos, K.J.; Kim, J.J.; Hattenrath-Lehmann, T.K.; Gobler, C.J. Effects of the harmful algae, Alexandrium catenella and Dinophysis acuminata, on the survival, growth, and swimming activity of early life stages of forage fish. Mar. Env. Res. 2019, 148, 46–56. [Google Scholar] [CrossRef]
- Fritz, L.W.; Lutz, R.A.; Foote, M.A.; van Dover, C.L.; Ewart, J.W. Selective feeding and grazing rates of oyster (Crassostrea virginica) larvae on natural phytoplankton assemblages. Estuaries 1984, 7, 513–518. [Google Scholar] [CrossRef]
- Whedon, W.F.; Kofoid, C.A. Dinoflagellata of the San Francisco region. I. On the skeletal morphology of two new species, Gonyaulax catenella and G. acatenella. Univ. Calif. Publ. Zool. 1936, 41, 25–34. [Google Scholar]
- Lefebvre, K.A.; Trainer, V.L.; Scholz, N.L. Morphological abnormalities and sensorimotor deficits in larval fish exposed to dissolved saxitoxin. Aquat. Toxicol. 2004, 66, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, K.A.; Elder, N.E.; Hershberger, P.K.; Trainer, V.L.; Stehr, C.M.; Scholz, N.L. Dissolved saxitoxin causes transient inhibition of sensorimotor function in larval Pacific herring (Clupea harengus pallasi). Mar. Biol. 2005, 147, 1393–1402. [Google Scholar] [CrossRef]
- Oberemm, A.; Becker, J.; Codd, G.A.; Steinberg, C. Effects of cyanobacterial toxins and aqueous crude extracts of cyanobacteria on the development of fish and amphibians. Environ. Toxicol. 1999, 14, 77–88. [Google Scholar] [CrossRef]
- Lassudrie, M.; Hégaret, H.; Wikfors, G.H.; da Silva, P.M. Effects of marine harmful algal blooms on bivalve cellular immunity and infectious diseases: A review. Dev. Comp. Immunol. 2020, 108, 103660. [Google Scholar] [CrossRef]
- Borcier, E.; Morvezen, R.; Boudry, P.; Miner, P.; Charrier, G.; Laroche, J.; Hégaret, H. Effects of bioactive extracellular compounds and paralytic shellfish toxins produced by Alexandrium minutum on growth and behaviour of juvenile great scallops Pecten maximus. Aquat. Toxicol. 2017, 184, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Castrec, J.; Soudant, P.; Payton, L.; Tran, D.; Miner, P.; Lambert, C.; Le Goïc, N.; Huvet, A.; Quillen, V.; Boullot, F.; et al. Bioactive extracellular compounds produced by the dinoflagellate Alexandrium minutum are highly detrimental for oysters. Aquat. Toxicol. 2018, 199, 188–198. [Google Scholar] [CrossRef] [Green Version]
- Dorantes-Aranda, J.J.; Seger, A.; Mardones, J.I.; Nichols, P.D.; Hallegraeff, G.M. Progress in understanding algal bloom-mediated fish kills: The role of superoxide radicals, phycotoxins and fatty acids. PLoS ONE 2015, 10, e0133549. [Google Scholar] [CrossRef]
- Flores, H.S.; Wikfors, G.H.; Dam, H.G. Reactive oxygen species are linked to the toxicity of the dinoflagellate Alexandrium spp. to protists. Aquat. Microb. Ecol. 2012, 66, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Krock, B.; Tillmann, U.; Cembella, A. Preliminary characterization of extracellular allelochemicals of the toxic marine dinoflagellate Alexandrium tamarense using a Rhodomonas salina bioassay. Mar. Drugs 2009, 7, 497–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mardones, J.I.; Dorantes-Aranda, J.J.; Nichols, P.D.; Hallegraeff, G.M. Fish gill damage by the dinoflagellate Alexandrium catenella from Chilean fjords: Synergistic action of ROS and PUFA. Harmful Algae 2015, 49, 40–49. [Google Scholar] [CrossRef]
- Marshall, J.; Nichols, P.D.; Hamilton, B.; Lewis, R.J.; Hallegraeff, G.M. Ichthyotoxicity of Chattonella marina (Raphidophyceae) to damselfish (Acanthochromis polycanthus): The synergistic role of reactive oxygen species and free fatty acids. Harmful Algae 2003, 2, 273–281. [Google Scholar] [CrossRef]
- Tillmann, U.; Alpermann, T.; John, U.; Cembella, A. Allelochemical interactions and short-term effects of the dinoflagellate Alexandrium on selected photoautotrophic and heterotrophic protists. Harmful Algae 2008, 7, 52–64. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, M.; Jeong, H.J.; Park, M.G. Revisiting the taxonomy of the “Dinophysis acuminata complex” (Dinophyta). Harmful Algae 2019, 88, 101657. [Google Scholar] [CrossRef]
- Miles, C.O.; Wilkins, A.L.; Munday, J.S.; Munday, R.; Hawkes, A.D.; Jensen, D.J.; Cooney, J.M.; Beuzenberg, V. Production of 7-epi-Pectenotoxin-2 seco acid and assessment of its acute toxicity to mice. J. Agric. Food Chem. 2006, 54, 1530–1534. [Google Scholar] [CrossRef]
- Figueroa, D.; Signore, A.; Araneda, O.; Contreras, H.R.; Concha, M.; García, C. Toxicity and differential oxidative stress effects on zebrafish larvae following exposure to toxins from the okadaic acid group. J. Toxicol. Env. Health A 2020, 83, 573–588. [Google Scholar] [CrossRef]
- Fux, E.; Smith, J.L.; Tong, M.; Guzmán, L.; Anderson, D.M. Toxin profiles of five geographical isolates of Dinophysis spp. from North and South America. Toxicon 2011, 57, 275–287. [Google Scholar] [CrossRef] [Green Version]
- Tong, M.; Smith, J.L.; Richlen, M.; Steidinger, K.A.; Kulis, D.M.; Fux, E.; Anderson, D.M. Characterization and comparison of toxin-producing isolates of Dinophysis acuminata from New England and Canada. J. Phycol. 2015, 51, 66–81. [Google Scholar] [CrossRef] [Green Version]
- Alarcan, J.; Biré, R.; Le Hégaret, L.; Fessard, V. Mixtures of lipophilic phycotoxins: Exposure data and toxicological assessment. Mar. Drugs 2018, 16, 46. [Google Scholar] [CrossRef] [Green Version]
- Pease, S.K.D.; Reece, K.S.; O’Brien, J.; Hobbs, P.L.M.; Smith, J.L. Oyster hatchery breakthrough of two HABs and potential effects on larval eastern oysters (Crassostrea virginica). Harmful Algae 2021, 101, 101965. [Google Scholar] [CrossRef]
- Smith, J.L.; Tong, M.; Fux, E.; Anderson, D.M. Toxin production, retention, and extracellular release by Dinophysis acuminata during extended stationary phase and culture decline. Harmful Algae 2012, 19, 125–132. [Google Scholar] [CrossRef]
- Armstrong, C.T.; Erdner, D.L.; McClelland, J.W.; Sanderson, M.P.; Anderson, D.M.; Gobler, C.J.; Smith, J.L. Impact of nitrogen chemical form on the isotope signature and toxicity of a marine dinoflagellate. Mar. Ecol. Prog. Ser. 2018, 602, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Boundy, M.J.; Selwood, A.I.; Harwood, D.T.; McNabb, P.S.; Turner, A.D. Development of a sensitive and selective liquid chromatography–mass spectrometry method for high throughput analysis of paralytic shellfish toxins using graphitised carbon solid phase extraction. J. Chromatogr. A 2015, 1387, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Onofrio, M.D.; Mallet, C.R.; Place, A.R.; Smith, J.L. A screening tool for the direct analysis of marine and freshwater phycotoxins in organic SPATT extracts from the Chesapeake Bay. Toxins 2020, 12, 322. [Google Scholar] [CrossRef] [PubMed]
- Villar-González, A.; Rodríguez-Velasco, M.L.; Ben-Gigirey, B.; Yasumoto, T.; Botana, L.M. Assessment of the hydrolysis process for the determination of okadaic acid-group toxin ester: Presence of okadaic acid 7-O-acyl-ester derivates in Spanish shellfish. Toxicon 2008, 51, 765–773. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 26–60. [Google Scholar]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Cleve. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Sehein, T.; Richlen, M.L.; Nagai, S.; Yasuike, M.; Nakamura, Y.; Anderson, D.M. Characterization of 17 new microsatellite markers for the dinoflagellate Alexandrium fundyense (Dinophyceae), a harmful algal bloom species. J. Appl. Phycol. 2016, 28, 1677–1681. [Google Scholar] [CrossRef] [Green Version]
- Park, M.G.; Kim, S.; Kim, H.S.; Myung, G.; Kang, Y.G.; Yih, W. First successful culture of the marine dinoflagellate Dinophysis acuminata. Aquat. Microb. Ecol. 2006, 45, 101–106. [Google Scholar] [CrossRef] [Green Version]

| Bioassay | Treatments a | Cells/mL or Cells/mL Equivalent | Species |
|---|---|---|---|
| Live-Cell | Fed (Pav) b | 25,000 | Pavlova pinguis |
| Unfed | 0 | None | |
| Acat 10 | 10 | Alexandrium catenella | |
| Acat 100 | 100 | Alexandrium catenella | |
| Acat 500 | 500 | Alexandrium catenella | |
| Acat 1000 | 1000 | Alexandrium catenella | |
| Dacum 10 | 10 | Dinophysis acuminata | |
| Dacum 100 | 100 | Dinophysis acuminata | |
| Dacum 500 | 500 | Dinophysis acuminata | |
| Dacum 1000 | 1000 | Dinophysis acuminata | |
| Lysate | Fed (Pav) b | 25,000 | Pavlova pinguis |
| Unfed | 0 | None | |
| Acat 100 | 100 | Alexandrium catenella | |
| Acat 1000 | 1000 | Alexandrium catenella | |
| Dacum 1000 | 1000 | Dinophysis acuminata | |
| Acat 1000 × Dacum 1000 | 1000 * | Alexandrium catenella and Dinophysis acuminata | |
| Pure Toxin | Carrier b | 0 | None |
| OA | 10,000 | None | |
| PTX2 | 10,000 | None | |
| STX | 10,000 | None | |
| OA × PTX2 | 10,000 | None | |
| OA × STX | 10,000 * | None | |
| PTX2 × STX | 10,000 * | None | |
| OA × PTX2 × STX | 10,000 * | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pease, S.K.D.; Brosnahan, M.L.; Sanderson, M.P.; Smith, J.L. Effects of Two Toxin-Producing Harmful Algae, Alexandrium catenella and Dinophysis acuminata (Dinophyceae), on Activity and Mortality of Larval Shellfish. Toxins 2022, 14, 335. https://doi.org/10.3390/toxins14050335
Pease SKD, Brosnahan ML, Sanderson MP, Smith JL. Effects of Two Toxin-Producing Harmful Algae, Alexandrium catenella and Dinophysis acuminata (Dinophyceae), on Activity and Mortality of Larval Shellfish. Toxins. 2022; 14(5):335. https://doi.org/10.3390/toxins14050335
Chicago/Turabian StylePease, Sarah K. D., Michael L. Brosnahan, Marta P. Sanderson, and Juliette L. Smith. 2022. "Effects of Two Toxin-Producing Harmful Algae, Alexandrium catenella and Dinophysis acuminata (Dinophyceae), on Activity and Mortality of Larval Shellfish" Toxins 14, no. 5: 335. https://doi.org/10.3390/toxins14050335
APA StylePease, S. K. D., Brosnahan, M. L., Sanderson, M. P., & Smith, J. L. (2022). Effects of Two Toxin-Producing Harmful Algae, Alexandrium catenella and Dinophysis acuminata (Dinophyceae), on Activity and Mortality of Larval Shellfish. Toxins, 14(5), 335. https://doi.org/10.3390/toxins14050335




