Matrix Effect of Diverse Biological Samples Extracted with Different Extraction Ratios on the Detection of β-N-Methylamino-L-Alanine by Two Common LC-MS/MS Analysis Methods
Abstract
:1. Introduction
2. Results and Discussion
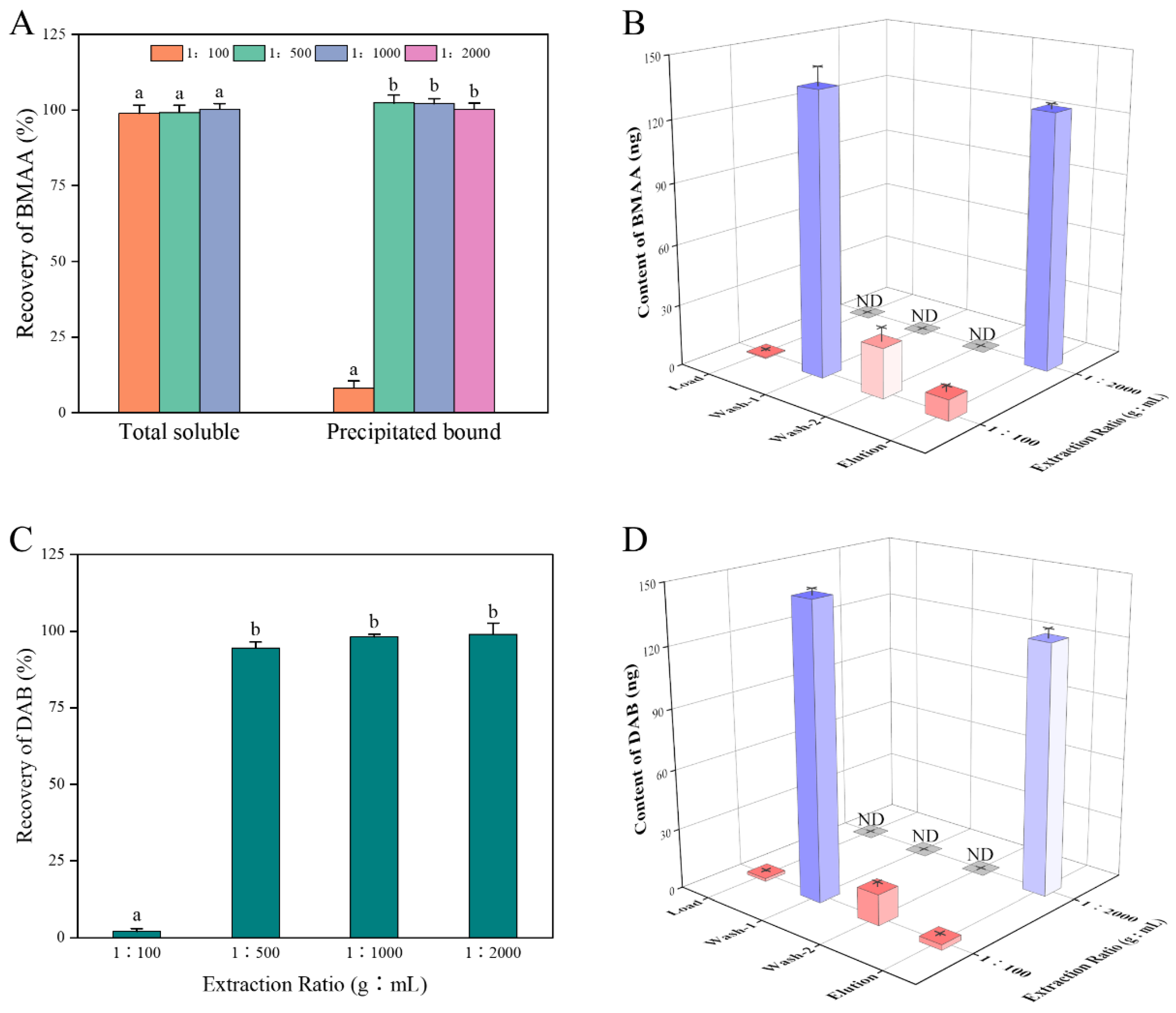
2.1. Recovery of BMAA through SPE Cartridges for Different Sample Matrices
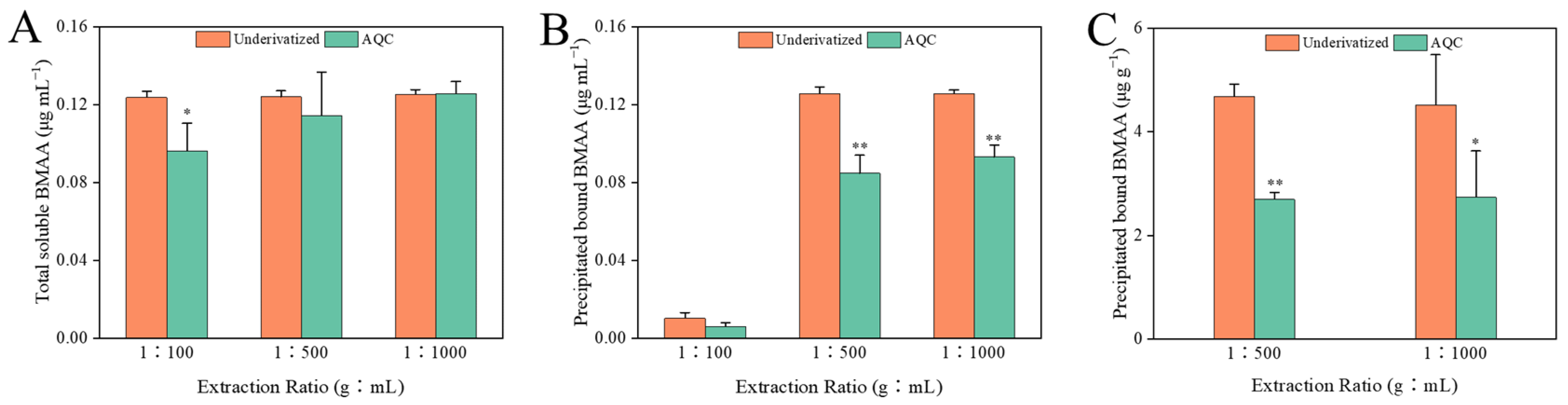
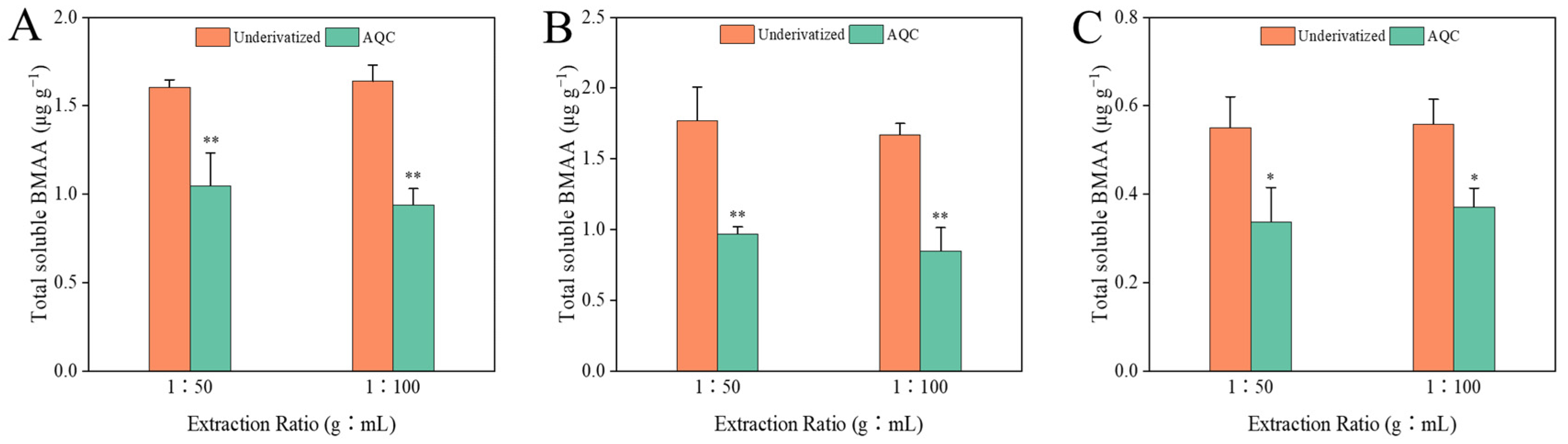
2.2. Amino Acid Analysis
2.3. Comparison between Both Analytical Methods
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Biological Samples
4.3. Extraction and Purification of Samples
4.3.1. Extraction for The Total Soluble form of BMAA
4.3.2. Extraction of Precipitated Bound BMAA
4.3.3. Purification of Extracts
4.4. Analytical Methods for BMAA
4.4.1. HILIC-MS/MS Analysis of BMAA
4.4.2. LC-MS/MS Analysis with Precolumn AQC Derivatization
4.5. LC-MS/MS Analysis of Amino Acids
4.6. Data Processing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soto, T.; Buzzi, E.D.; Rotstein, N.P.; German, O.L.; Politi, L.E. Damaging Effects of BMAA on Retina Neurons and Müller Glial Cells. Exp. Eye Res. 2020, 202, 108342. [Google Scholar] [CrossRef] [PubMed]
- Spencer, P.S.; Nunn, P.B.; Hugon, J.; Ludolph, A.C.; Ross, S.M.; Roy, D.N.; Robertson, R.C. Guam Amyotrophic Lateral Sclerosis-Parkinsonism Dementia Linked to a Plant Excitant Neurotoxin. Science 1987, 237, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Nunes-Costa, D.; Magalhães, J.D.; G-Fernandes, M.; Cardoso, S.M.; Empadinhas, N. Microbial BMAA and the Pathway for Parkinson’s Disease Neurodegeneration. Front. Aging Neurosci. 2020, 12, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glover, W.B.; Mash, D.C.; Murch, S.J. The Natural Non-Protein Amino Acid N-β-Methylamino-L-Alanine (BMAA) Is Incorporated into Protein during Synthesis. Amino Acids 2014, 46, 2553–2559. [Google Scholar] [CrossRef]
- Murch, S.J.; Cox, P.A.; Banack, S.A. A Mechanism for Slow Release of Biomagnified Cyanobacterial Neurotoxins and Neurodegenerative Disease in Guam. Proc. Natl. Acad. Sci. USA 2004, 101, 12228–12231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega, A.; Bell, E.A. α-Amino-β-Methylaminopropionic Acid, a New Amino Acid from Seeds of Cycas circinalis. Phytochemistry 1967, 6, 759–762. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse Taxa of Cyanobacteria Produce β-N-Methylamino-L-Alanine, a Neurotoxic Amino Acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Yan, C.; Qiu, J.; Liu, C.; Yan, Y.; Ji, Y.; Wang, G.; Chen, H.; Li, Y.; Li, A. Food Web Biomagnification of the Neurotoxin β-N-Methylamino-L-Alanine in a Diatom-Dominated Marine Ecosystem in China. J. Hazard. Mater. 2021, 404, 124217. [Google Scholar] [CrossRef]
- Jiang, L.; Eriksson, J.; Lage, S.; Jonasson, S.; Shams, S.; Mehine, M.; Ilag, L.L.; Rasmussen, U. Diatoms: A Novel Source for the Neurotoxin BMAA in Aquatic Environments. PLoS ONE 2014, 9, e84578. [Google Scholar] [CrossRef]
- Li, A.; Hu, Y.; Song, J.; Wang, S.; Deng, L. Ubiquity of the Neurotoxin β-N-Methylamino-L-Alanine and Its Isomers Confirmed by Two Different Mass Spectrometric Methods in Diverse Marine Mollusks. Toxicon 2018, 151, 129–136. [Google Scholar] [CrossRef]
- Main, B.J.; Bowling, L.C.; Padula, M.P.; Bishop, D.P.; Mitrovic, S.M.; Guillemin, G.J.; Rodgers, K.J. Detection of the Suspected Neurotoxin β-Methylamino-L-Alanine (BMAA) in Cyanobacterial Blooms from Multiple Water Bodies in Eastern Australia. Harmful Algae 2018, 74, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Réveillon, D.; Abadie, E.; Séchet, V.; Masseret, E.; Hess, P.; Amzil, Z. β-N-Methylamino-L-Alanine (BMAA) and Isomers: Distribution in Different Food Web Compartments of Thau Lagoon, French Mediterranean Sea. Mar. Environ. Res. 2015, 110, 8–18. [Google Scholar] [CrossRef] [Green Version]
- Jonasson, S.; Eriksson, J.; Berntzon, L.; Spáčcil, Z.; Ilag, L.L.; Ronnevi, L.O.; Rasmussen, U.; Bergman, B. Transfer of a Cyanobacterial Neurotoxin within a Temperate Aquatic Ecosystem Suggests Pathways for Human Exposure. Proc. Natl. Acad. Sci. USA 2010, 107, 9252–9257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.; Aigret, B.; De Borggraeve, W.M.; Spacil, Z.; Ilag, L.L. Selective LC-MS/MS Method for the Identification of BMAA from Its Isomers in Biological Samples. Anal. Bioanal. Chem. 2012, 403, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Faassen, E.J.; Antoniou, M.G.; Beekman-Lukassen, W.; Blahova, L.; Chernova, E.; Christophoridis, C.; Combes, A.; Edwards, C.; Fastner, J.; Harmsen, J.; et al. A Collaborative Evaluation of LC-MS/MS Based Methods for BMAA Analysis: Soluble Bound BMAA Found to Be an Important Fraction. Mar. Drugs 2016, 14, 45. [Google Scholar] [CrossRef] [Green Version]
- Faassen, E.J.; Gillissen, F.; Lürling, M. A Comparative Study on Three Analytical Methods for the Determination of the Neurotoxin BMAA in Cyanobacteria. PLoS ONE 2012, 7, e3667. [Google Scholar] [CrossRef]
- Banack, S.A.; Murch, S.J. Methods for the Chemical Analysis of β-N-Methylamino-L-A Lanine: What Is Known and What Remains to Be Determined. Neurotox. Res. 2018, 33, 184–191. [Google Scholar] [CrossRef]
- Bishop, S.L.; Murch, S.J. A Systematic Review of Analytical Methods for the Detection and Quantification of β-N-Methylamino-L-Alanine (BMAA). Analyst 2020, 145, 13–28. [Google Scholar] [CrossRef]
- Duncan, M.W.; Kopin, I.J.; Crowley, J.S.; Jones, S.M.; Markey, S.P. Quantification of the Putative Neurotoxin 2-Amino-3-(Methylamino)Propanoic Acid (Bmaa) in Cycadales: Analysis of the Seeds of Some Members of the Family Cycadaceae. J. Anal. Toxicol. 1989, 13, 169–175. [Google Scholar] [CrossRef]
- Duncan, M.W.; Markey, S.P.; Weick, B.G.; Pearson, P.G.; Ziffer, H.; Hu, Y.; Kopin, I.J. 2-Amino-3-(Methylamino)Propanoic Acid (BMAA) Bioavailability in the Primate. Neurobiol. Aging 1992, 13, 333–337. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J. Biomagnification of Cyanobacterial Neurotoxins and Neurodegenerative Disease among the Chamorro People of Guam. Proc. Natl. Acad. Sci. USA 2003, 100, 13380–13383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banack, S.A.; Cox, P.A. Biomagnification of Cycad Neurotoxins in Flying Foxes: Implications for ALS-PDC in Guam. Neurology 2003, 61, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Banack, S.A.; Johnson, H.E.; Cheng, R.; Alan Cox, P. Production of the Neurotoxin BMAA by a Marine Cyanobacterium. Mar. Drugs 2007, 5, 180–196. [Google Scholar] [CrossRef]
- Esterhuizen-Londt, M.; Downing, S.; Downing, T.G. Improved Sensitivity Using Liquid Chromatography Mass Spectrometry (LC-MS) for Detection of Propyl Chloroformate Derivatised β-N-Methylamino-L-Alanine (BMAA) in Cyanobacteria. Water SA 2011, 37, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Lampinen Salomonsson, M.; Hansson, A.; Bondesson, U. Development and In-House Validation of a Method for Quantification of BMAA in Mussels Using Dansyl Chloride Derivatization and Ultra Performance Liquid Chromatography Tandem Mass Spectrometry. Anal. Methods 2013, 5, 4865–4874. [Google Scholar] [CrossRef]
- Kubo, T.; Kato, N.; Hosoya, K.; Kaya, K. Effective Determination Method for a Cyanobacterial Neurotoxin, β-N-Methylamino-L-Alanine. Toxicon 2008, 51, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Rosén, J.; Hellenäs, K.E. Determination of the Neurotoxin BMAA (β-N-Methylamino-L-Alanine) in Cycad Seed and Cyanobacteria by LC-MS/MS (Liquid Chromatography Tandem Mass Spectrometry). Analyst 2008, 133, 1785–1789. [Google Scholar] [CrossRef]
- Faassen, E.J. Presence of the Neurotoxin BMAA in Aquatic Ecosystems: What Do We Really Know? Toxins 2014, 6, 1109–1138. [Google Scholar] [CrossRef] [Green Version]
- Tymm, F.J.M.; Bishop, S.L.; Murch, S.J. A Single Laboratory Validation for the Analysis of Underivatized β-N-Methylamino-L-Alanine (BMAA). Neurotox. Res. 2021, 39, 49–71. [Google Scholar] [CrossRef]
- Taylor, P.J. Matrix Effects: The Achilles Heel of Quantitative High-Performance Liquid Chromatography-Electrospray-Tandem Mass Spectrometry. Clin. Biochem. 2005, 38, 328–334. [Google Scholar] [CrossRef]
- Roney, B.R.; Renhui, L.; Banack, S.A.; Murch, S.; Honegger, R.; Cox, P.A. Consumption of Fa Cai Nostoc Soup: A Potential for BMAA Exposure from Nostoc Cyanobacteria in China? Amyotroph. Lateral Scler. 2009, 10, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Spáil, Z.; Eriksson, J.; Jonasson, S.; Rasmussen, U.; Ilag, L.L.; Bergman, B. Analytical Protocol for Identification of BMAA and DAB in Biological Samples. Analyst 2010, 135, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Liu, Z.; Huang, R.; Xu, Y.; Liu, D.; Lin, T.F.; Cui, F. Optimization of the Determination Method for Dissolved Cyanobacterial Toxin BMAA in Natural Water. Anal. Chem. 2017, 89, 10991–10998. [Google Scholar] [CrossRef]
- Krüger, T.; Mönch, B.; Oppenhäuser, S.; Luckas, B. LC-MS/MS Determination of the Isomeric Neurotoxins BMAA (β-N-Methylamino-L-Alanine) and DAB (2,4-Diaminobutyric Acid) in Cyanobacteria and Seeds of Cycas revoluta and Lathyrus latifolius. Toxicon 2010, 55, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Réveillon, D.; Abadie, E.; Séchet, V.; Brient, L.; Savar, V.; Bardouil, M.; Hess, P.; Amzil, Z. Beta-N-Methylamino-L-Alanine: LC-MS/MS Optimization, Screening of Cyanobacterial Strains and Occurrence in Shellfish from Thau, a French Mediterranean Lagoon. Mar. Drugs 2014, 12, 5441–5467. [Google Scholar] [CrossRef]
- Aparicio-Muriana, M.M.; Carmona-Molero, R.; Lara, F.J.; García-Campaña, A.M.; del Olmo-Iruela, M. Multiclass Cyanotoxin Analysis in Reservoir Waters: Tandem Solid-Phase Extraction Followed by Zwitterionic Hydrophilic Interaction Liquid Chromatography-Mass Spectrometry. Talanta 2022, 237, 122929. [Google Scholar] [CrossRef]
- Faassen, E.J.; Gillissen, F.; Zweers, H.A.J.; Lrling, M. Determination of the Neurotoxins BMAA (β-N-Methylamino-L-Alanine) and DAB (α-,γ-Diaminobutyric Acid) by LC-MSMS in Dutch Urban Waters with Cyanobacterial Blooms. Amyotroph. Lateral Scler. 2009, 10, 79–84. [Google Scholar] [CrossRef]
- Jiao, Y.; Chen, Q.; Chen, X.; Wang, X.; Liao, X.; Jiang, L.; Wu, J.; Yang, L. Occurrence and Transfer of a Cyanobacterial Neurotoxin β-Methylamino-L-Alanine within the Aquatic Food Webs of Gonghu Bay (Lake Taihu, China) to Evaluate the Potential Human Health Risk. Sci. Total Environ. 2014, 468–469, 457–463. [Google Scholar] [CrossRef]
- Lepoutre, A.; Faassen, E.J.; Zweers, A.J.; Lürling, M.; Ge, A.; Lance, E. How the Neurotoxin β-N-Methylamino-L-Alanine Accumulates in Bivalves: Distribution of the Different Accumulation Fractions among Organs. Toxins 2020, 12, 61. [Google Scholar] [CrossRef] [Green Version]
- Beach, D.G.; Kerrin, E.S.; Giddings, S.D.; Quilliam, M.A.; McCarron, P. Differential Mobility-Mass Spectrometry Double Spike Isotope Dilution Study of Release of β-Methylaminoalanine and Proteinogenic Amino Acids during Biological Sample Hydrolysis. Sci. Rep. 2018, 8, 117. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Johnston, E.; Åberg, K.M.; Nilsson, U.; Ilag, L.L. Strategy for Quantifying Trace Levels of BMAA in Cyanobacteria by LC/MS/MS. Anal. Bioanal. Chem. 2013, 405, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Fan, H.; Ma, F.; McCarron, P.; Thomas, K.; Tang, X.; Quilliam, M.A. Elucidation of Matrix Effects and Performance of Solid-Phase Extraction for LC-MS/MS Analysis of β-N-Methylamino-l-Alanine (BMAA) and 2,4-Diaminobutyric Acid (DAB) Neurotoxins in Cyanobacteria. Analyst 2012, 137, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Esterhuizen-Londt, M.; Downing, T.G. Solid Phase Extraction of β-N-Methylamino-L-Alanine (BMAA) from South African Water Supplies. Water SA 2011, 37, 523–528. [Google Scholar] [CrossRef] [Green Version]
- Baker, T.C.; Tymm, F.J.M.; Murch, S.J. Assessing Environmental Exposure to β-N-Methylamino-L-Alanine (BMAA) in Complex Sample Matrices: A Comparison of the Three Most Popular LC-MS/MS Methods. Neurotox. Res. 2018, 33, 43–54. [Google Scholar] [CrossRef]
- Lage, S.; Burian, A.; Rasmussen, U.; Costa, P.R.; Annadotter, H.; Godhe, A.; Rydberg, S. BMAA Extraction of Cyanobacteria Samples: Which Method to Choose? Environ. Sci. Pollut. Res. 2016, 23, 338–350. [Google Scholar] [CrossRef]

| ER # | Total Soluble Form | Precipitated Bound Form | ||||
|---|---|---|---|---|---|---|
| 1:100 | 1:200 | 1:500 | 1:100 | 1:500 | 1:1000 | |
| BMAA | LOD | ND | ND | 1.2 ± 0.24 a | 4.7 ± 0.24 b | 4.5 ± 0.97 b |
| DAB | 0.54 ± 0.03 | LOD | LOD | ND | ND | ND |
| ER # | Total Soluble Form Extracts | Precipitated Bound Form Extracts | ||
|---|---|---|---|---|
| 1:100 | 1:100 | 1:500 | 1:2000 | |
| Glycine | 35 ± 2.7 | 612 ± 52 | 122 ± 10 | 30 ± 2.5 |
| Alanine | 58 ± 2.0 | 1462 ± 199 | 282 ± 40 | 70 ± 10 |
| Serine | 20 ± 3.8 | 371 ± 38 | 74 ± 7.6 | 18 ± 1.9 |
| Proline | 32 ± 1.7 | 662 ± 50 | 132 ± 10 | 33 ± 2.5 |
| Valine | 50 ± 4.6 | 1282 ± 193 | 256 ± 10 | 64 ± 2.5 |
| Threonine | 30 ± 1.5 | 688 ± 82 | 138 ± 16 | 34 ± 4.0 |
| Isoleucine | 44 ± 1.7 | 1224 ± 98 | 245 ± 20 | 61 ± 5.0 |
| Leucine | 54 ± 2.2 | 1385 ± 70 | 277 ± 14 | 69 ± 3.5 |
| Aspartic acid | 41 ± 0.49 | 199 ± 29 | 40 ± 5.8 | 10 ± 1.4 |
| Glutamine | 85 ± 8.6 | 1704 ± 136 | 341 ± 27 | 85 ± 6.8 |
| Lysine | 79 ± 7.8 | 1605 ± 169 | 321 ± 34 | 80 ± 8.5 |
| Glutamic | 9.0 ± 0.28 | 95 ± 9.9 | 19 ± 2.0 | 4.8 ± 0.49 |
| Methionine | 0.50 ± 0.38 | 310 ± 16 | 62 ± 3.2 | 16 ± 0.79 |
| Histidine | 5.0 ± 1.2 | 108 ± 1.2 | 22 ± 0.24 | 5.5 ± 0.060 |
| Phenylalanine | 62 ± 1.2 | 1402 ± 80 | 280 ± 16 | 70 ± 4.0 |
| Arginine | 88 ± 5.8 | 830 ± 64 | 166 ± 13 | 42 ± 3.2 |
| Tyrosine | 2.6 ± 0.58 | 109 ± 16 | 22 ± 3.2 | 5.5 ± 0.81 |
| Tryptophan | 0.089 ± 0.011 | 2.3 ± 0.0076 | 0.46 ± 0.0015 | 0.12 ± 0.00038 |
| Total | 694 ± 40 | 14047 ± 1348 | 2810 ± 270 | 702 ± 62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, P.; Qiu, J.; Li, A.; Yan, G.; Li, M.; Ji, Y. Matrix Effect of Diverse Biological Samples Extracted with Different Extraction Ratios on the Detection of β-N-Methylamino-L-Alanine by Two Common LC-MS/MS Analysis Methods. Toxins 2022, 14, 387. https://doi.org/10.3390/toxins14060387
Zhao P, Qiu J, Li A, Yan G, Li M, Ji Y. Matrix Effect of Diverse Biological Samples Extracted with Different Extraction Ratios on the Detection of β-N-Methylamino-L-Alanine by Two Common LC-MS/MS Analysis Methods. Toxins. 2022; 14(6):387. https://doi.org/10.3390/toxins14060387
Chicago/Turabian StyleZhao, Peng, Jiangbing Qiu, Aifeng Li, Guowang Yan, Min Li, and Ying Ji. 2022. "Matrix Effect of Diverse Biological Samples Extracted with Different Extraction Ratios on the Detection of β-N-Methylamino-L-Alanine by Two Common LC-MS/MS Analysis Methods" Toxins 14, no. 6: 387. https://doi.org/10.3390/toxins14060387
APA StyleZhao, P., Qiu, J., Li, A., Yan, G., Li, M., & Ji, Y. (2022). Matrix Effect of Diverse Biological Samples Extracted with Different Extraction Ratios on the Detection of β-N-Methylamino-L-Alanine by Two Common LC-MS/MS Analysis Methods. Toxins, 14(6), 387. https://doi.org/10.3390/toxins14060387





