Toxic Effects and Tumor Promotion Activity of Marine Phytoplankton Toxins: A Review
Abstract
:1. Introduction
2. Marine Phytoplankton: The Most Important Source of Toxins
3. Shellfish Poisoning Toxins: The Most Hazardous Impact to Human Health
3.1. Marine Cyanobacterial Toxins Association with Clinical Symptoms
3.2. Marine Diatoms Toxins and Their Toxic Effects and Clinical Symptoms
3.3. Marine Dinoflagellates Toxins and Their Toxic Effects and Clinical Symptoms
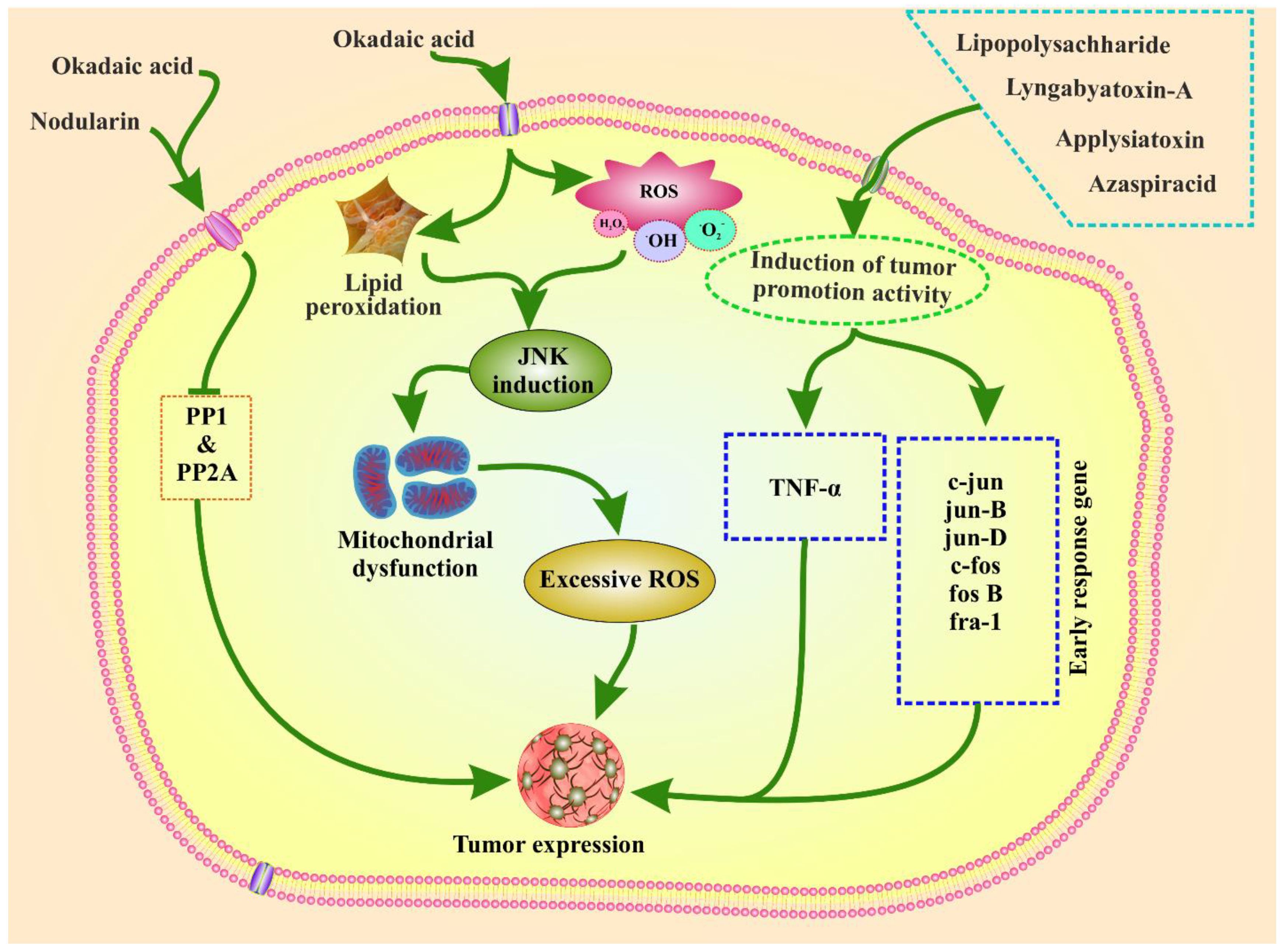
4. Tumor Promotion Activity by Marine Phytoplankton Toxins
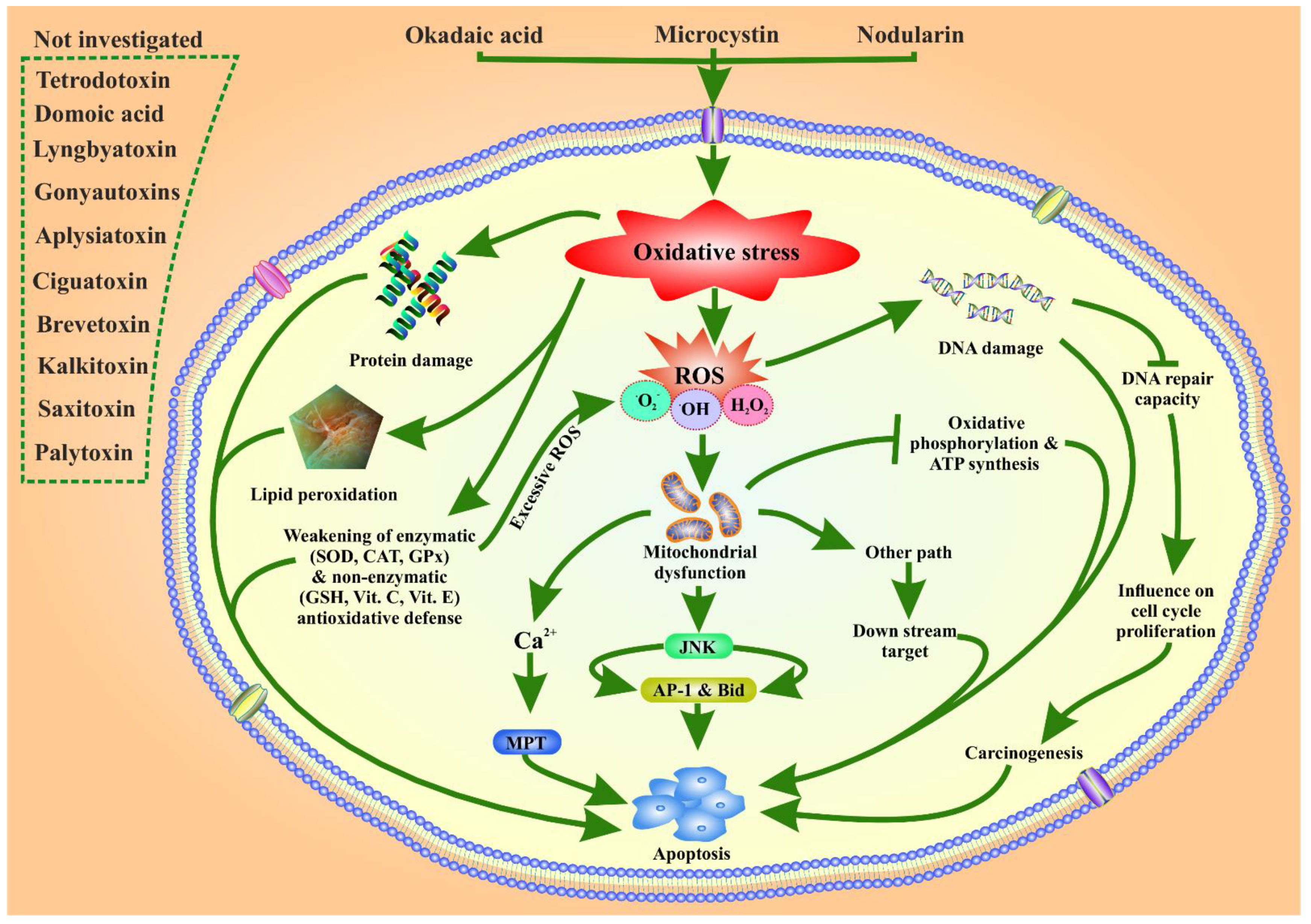
5. Possible Role of Marine Phytoplankton Toxins in Oxidative Stress Related ROS Toxicity
6. Phytoplankton Toxin and Their Disease Preventing Activities
7. Conclusions and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pradhan, B.; Maharana, S.; Bhakta, S.; Jena, M. Marine phytoplankton diversity of Odisha coast, India with special reference to new record of diatoms and dinoflagellates. Vegetos 2021. [Google Scholar] [CrossRef]
- Behera, C.; Pradhan, B.; Panda, R.; Nayak, R.; Nayak, S.; Jena, M. Algal Diversity of Saltpans, Huma (Ganjam), India. J. Indian Bot. Soc. 2021, 101, 107–120. [Google Scholar] [CrossRef]
- Dash, S.; Pradhan, B.; Behera, C.; Nayak, R.; Jena, M. Algal Flora of Tampara Lake, Chhatrapur, Odisha, India. J. Indian Bot. Soc. 2021, 101, 1–15. [Google Scholar] [CrossRef]
- Dash, S.; Pradhan, B.; Behera, C.; Jena, M. Algal Diversity of Kanjiahata Lake, Nandankanan, Odisha, India. J. Indian Bot. Soc. 2020, 99, 11–24. [Google Scholar] [CrossRef]
- Behera, C.; Dash, S.R.; Pradhan, B.; Jena, M.; Adhikary, S.P. Algal Diversity of Ansupa lake, Odisha, India. Nelumbo 2020, 62, 207–220. [Google Scholar] [CrossRef]
- Maharana, S.; Pradhan, B.; Jena, M.; Misra, M.K. Diversity of Phytoplankton in Chilika Lagoon, Odisha, India. Environ. Ecol. 2019, 37, 737–746. [Google Scholar]
- Kim, H.; Park, H.; Wang, H.; Yoo, H.Y.; Park, J.; Ki, J.-S. Low Temperature and Cold Stress Significantly Increase Saxitoxins (STXs) and Expression of STX Biosynthesis Genes sxtA4 and sxtG in the Dinoflagellate Alexandrium catenella. Mar. Drugs 2021, 19, 291. [Google Scholar] [CrossRef]
- Bui, Q.T.N.; Kim, H.; Park, H.; Ki, J.-S. Salinity Affects Saxitoxins (STXs) Toxicity in the Dinoflagellate Alexandrium pacificum, with Low Transcription of SXT-Biosynthesis Genes sxtA4 and sxtG. Toxins 2021, 13, 733. [Google Scholar] [CrossRef]
- Wang, H.; Kim, H.; Park, H.; Ki, J.-S. Temperature influences the content and biosynthesis gene expression of saxitoxins (STXs) in the toxigenic dinoflagellate Alexandrium pacificum. Sci. Total Environ. 2022, 802, 149801. [Google Scholar] [CrossRef]
- Grattan, L.M.; Holobaugh, S.; Morris, J.G., Jr. Harmful algal blooms and public health. Harmful Algae 2016, 57, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.Z. Neurotoxins from marine dinoflagellates: A brief review. Mar. Drugs 2008, 6, 349–371. [Google Scholar] [CrossRef] [PubMed]
- Muñiz, O.; Revilla, M.; Rodríguez, J.G.; Laza-Martínez, A.; Seoane, S.; Franco, J.; Orive, E. Evaluation of phytoplankton quality and toxicity risk based on a long-term time series previous to the implementation of a bivalve farm (Basque coast as a case study). Reg. Stud. Mar. Sci. 2017, 10, 10–19. [Google Scholar] [CrossRef]
- Smayda, T.J. Harmful algal blooms: Their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol. Oceanogr. 1997, 42, 1137–1153. [Google Scholar] [CrossRef]
- Ettoumi, A.; El Khalloufi, F.; El Ghazali, I.; Oudra, B.; Amrani, A.; Nasri, H.; Bouaïcha, N. Bioaccumulation of cyanobacterial toxins in aquatic organisms and its consequences for public health. In Zooplankton and Phytoplankton: Types, Characteristics and Ecology; Nova Science: Hauppauge, NY, USA, 2011; Volume 201, pp. 1–34. [Google Scholar]
- Camacho, F.G.; Rodríguez, J.G.; Mirón, A.S.; García, M.C.; Belarbi, E.; Chisti, Y.; Grima, E.M. Biotechnological significance of toxic marine dinoflagellates. Biotechnol. Adv. 2007, 25, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Maatouk, I.; Bouaïcha, N.; Plessis, M.J.; Périn, F. Detection by 32P-postlabelling of 8-oxo-7,8-dihydro-2′-deoxyguanosine in DNA as biomarker of microcystin-LR-and nodularin-induced DNA damage in vitro in primary cultured rat hepatocytes and in vivo in rat liver. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2004, 564, 9–20. [Google Scholar] [CrossRef]
- Nishiwaki-Matsushima, R.; Ohta, T.; Nishiwaki, S.; Suganuma, M.; Kohyama, K.; Ishikawa, T.; Carmichael, W.W.; Fujiki, H. Liver tumor promotion by the cyanobacterial cyclic peptide toxin microcystin-LR. Cancer Res. Clin. Oncol. 1992, 118, 420–424. [Google Scholar] [CrossRef]
- Sueoka, E.; Sueoka, N.; Okabe, S.; Kozu, T.; Komori, A.; Ohta, T.; Suganuma, M.; Kim, S.; Lim, I.; Fujiki, H. Expression of the tumor necrosis factor α gene and early response genes by nodularin, a liver tumor promoter, in primary cultured rat hepatocytes. J. Cancer Res. Clin. Oncol. 1997, 123, 413–419. [Google Scholar]
- Ohta, T.; Sueoka, E.; Iida, N.; Komori, A.; Suganuma, M.; Nishiwaki, R.; Tatematsu, M.; Kim, S.-J.; Carmichael, W.W.; Fujiki, H. Nodularin, a potent inhibitor of protein phosphatases 1 and 2A, is a new environmental carcinogen in male F344 rat liver. Cancer Res. 1994, 54, 6402–6406. [Google Scholar]
- Blossom, H.E.; Andersen, N.G.; Rasmussen, S.A.; Hansen, P.J. Stability of the intra-and extracellular toxins of Prymnesium parvum using a microalgal bioassay. Harmful Algae 2014, 32, 11–21. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Dash, S.R.; Satapathy, Y.; Nayak, S.; Mandal, A.K.; Jena, M. In vitro antidiabetic, anti-inflammatory and antibacterial activity of marine alga Enteromorpha compressa collected from Chilika lagoon, Odisha, India. Vegetos 2022, 1–8. [Google Scholar] [CrossRef]
- Pradhan, B.; Nayak, R.; Patra, S.; Bhuyan, P.P.; Dash, S.R.; Ki, J.-S.; Adhikary, S.P.; Ragusa, A.; Jena, M. Cyanobacteria and Algae-Derived Bioactive Metabolites as Antiviral Agents: Evidence, Mode of Action, and Scope for Further Expansion; A Comprehensive Review in Light of the SARS-CoV-2 Outbreak. Antioxidants 2022, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Pradhan, B.; Patra, S.; Behera, C.; Nayak, R.; Jena, M. Screening for nutritive bioactive compounds in some algal strains isolated from coastal Odisha. J. Adv. Plant Sci. 2020, 10, 1–8. [Google Scholar]
- Tester, P.A. Harmful marine phytoplankton and shellfish toxicity. Ann. N. Y. Acad. Sci. 1994, 740, 69–76. [Google Scholar] [CrossRef]
- Pradhan, B.; Nayak, R.; Patra, S.; Bhuyan, P.P.; Behera, P.K.; Mandal, A.K.; Behera, C.; Ki, J.-S.; Adhikary, S.P.; MubarakAli, D.; et al. A state-of-the-art review on fucoidan as an antiviral agent to combat viral infections. Carbohydr. Polym. 2022, 291, 119551. [Google Scholar] [CrossRef]
- Anderson, P.D. Bioterrorism: Toxins as weapons. J. Pharm. Pract. 2012, 25, 121–129. [Google Scholar] [CrossRef]
- Anderson, D.; Kulis, D.; Sullivan, J.; Hall, S.; Lee, C. Dynamics and physiology of saxitoxin production by the dinoflagellates Alexandrium spp. Mar. Biol. 1990, 104, 511–524. [Google Scholar] [CrossRef]
- Daneshian, M.; Botana, L.M.; Dechraoui Bottein, M.-Y.; Buckland, G.; Campàs, M.; Dennison, N.; Dickey, R.W.; Diogène, J.; Fessard, V.; Hartung, T. A roadmap for hazard monitoring and risk assessment of marine biotoxins on the basis of chemical and biological test systems. Altern. Anim. Exp. ALTEX 2013, 30, 487–545. [Google Scholar]
- Backer, L.; Fleming, L.; Rowan, A.; Baden, D. Epidemiology, public health and human diseases associated with harmful marine algae. Man. Mar. Microalgae Monogr. Ocean. Methodol. 2003, 11, 725–750. [Google Scholar]
- Edmunds, J.; McCarthy, R.; Ramsdell, J. Ciguatoxin reduces larval survivability in finfish. Toxicon 1999, 37, 1827–1832. [Google Scholar] [CrossRef]
- Landsberg, J.H.; Hall, S.; Johannessen, J.N.; White, K.D.; Conrad, S.M.; Abbott, J.P.; Flewelling, L.J.; Richardson, R.W.; Dickey, R.W.; Jester, E.L. Saxitoxin puffer fish poisoning in the United States, with the first report of Pyrodinium bahamense as the putative toxin source. Environ. Health Perspect. 2006, 114, 1502–1507. [Google Scholar] [CrossRef] [Green Version]
- Etheridge, S.M. Paralytic shellfish poisoning: Seafood safety and human health perspectives. Toxicon 2010, 56, 108–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewitus, A.J.; Horner, R.A.; Caron, D.A.; Garcia-Mendoza, E.; Hickey, B.M.; Hunter, M.; Huppert, D.D.; Kudela, R.M.; Langlois, G.W.; Largier, J.L. Harmful algal blooms along the North American west coast region: History, trends, causes, and impacts. Harmful Algae 2012, 19, 133–159. [Google Scholar] [CrossRef] [Green Version]
- Cusick, K.D.; Sayler, G.S. An overview on the marine neurotoxin, saxitoxin: Genetics, molecular targets, methods of detection and ecological functions. Mar. Drugs 2013, 11, 991–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallegraeff, G. Harmful algal blooms: A global overview. Man. Harmful Mar. Microalgae 2003, 33, 1–22. [Google Scholar]
- Visciano, P.; Schirone, M.; Berti, M.; Milandri, A.; Tofalo, R.; Suzzi, G. Marine biotoxins: Occurrence, toxicity, regulatory limits and reference methods. Front. Microbiol. 2016, 7, 1051. [Google Scholar] [CrossRef] [Green Version]
- Bricelj, V.M.; Connell, L.; Konoki, K.; MacQuarrie, S.P.; Scheuer, T.; Catterall, W.A.; Trainer, V.L. Sodium channel mutation leading to saxitoxin resistance in clams increases risk of PSP. Nature 2005, 434, 763–767. [Google Scholar] [CrossRef]
- Snyder, R.; Gibbs, P.; Palacios, A.; Abiy, L.; Dickey, R.; Lopez, J.V.; Rein, K. Polyketide synthase genes from marine dinoflagellates. Mar. Biotechnol. 2003, 5, 1–12. [Google Scholar]
- Rein, K.S.; Snyder, R.V. The biosynthesis of polyketide metabolites by dinoflagellates. Adv. Appl. Microbiol. 2006, 59, 93–125. [Google Scholar]
- Anderson, D. Red tides. Sci. Am. 1994, 27, 62–68. [Google Scholar] [CrossRef]
- Hawser, S.; Codd, G. The toxicity of Trichodesmium blooms from Caribbean waters. In Marine Pelagic Cyanobacteria: Trichodesmium and Other Diazotrophs; Springer: Berlin/Heidelberg, Germany, 1992; pp. 319–329. [Google Scholar]
- Jiang, L.; Eriksson, J.; Lage, S.; Jonasson, S.; Shams, S.; Mehine, M.; Ilag, L.L.; Rasmussen, U. Diatoms: A novel source for the neurotoxin BMAA in aquatic environments. PLoS ONE 2014, 9, e84578. [Google Scholar] [CrossRef]
- Xu, Q.-H.; Zhao, X.-N.; Wei, C.-H.; Rong, K.-T. Immunologic protection of anti-tetrodotoxin vaccines against lethal activities of oral tetrodotoxin challenge in mice. Int. Immunopharmacol. 2005, 5, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.-H.; Wei, C.-H.; Huang, K.; Rong, K.-T. Toxin-neutralizing effect and activity-quality relationship for mice tetrodotoxin-specific polyclonal antibodies. Toxicology 2005, 206, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Burja, A.M.; Banaigs, B.; Abou-Mansour, E.; Burgessd, J.G.; Wrighta, P.C. Marine cyanobacteriaÐa prolific source of natural products. Tetrahedron 2001, 57, 9347–9377. [Google Scholar] [CrossRef]
- Elleman, T.C.; Falconer, I.; Jackson, A.; Runnegar, M. Isolation, characterization and pathology of the toxin from a Microcystis aeruginosa (=Anacystis cyanea) bloom. Aust. J. Biol. Sci. 1978, 31, 209–218. [Google Scholar] [CrossRef]
- Falconer, I.; Jackson, R.; Langley, B.; Runnegar, M. Liver pathology in mice in poisoning by the blue-green alga Microcystis aeruginosa. Aust. J. Biol. Sci. 1981, 34, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Mynderse, J.S.; Moore, R.E. Toxins from blue-green algae: Structures of oscillatoxin A and three related bromine-containing toxins. J. Org. Chem. 1978, 43, 2301–2303. [Google Scholar] [CrossRef]
- Fujiki, H.; Suganuma, M.; Yatsunami, J.; Komori, A.; Okabe, S.; Nishiwaki-Matsushima, R.; Ohta, T. Significant marine natural products in cancer research. ChemInform 1993, 123, 309–316. [Google Scholar] [CrossRef]
- Fujiki, H.; Suganuma, M.; Suguri, H.; Yoshizawa, S.; Takagi, K.; Nakayasu, M.; Ojika, M.; Yamada, K.; Yasumoto, T.; Moore, R.E.; et al. New Tumor Promoters from Marine Natural Products. In Marine Toxins; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1990; Volume 418, pp. 232–240. [Google Scholar]
- Ito, E.; Nagai, H. Bleeding from the small intestine caused by aplysiatoxin, the causative agent of the red alga Gracilaria coronopifolia poisoning. Toxicon 2000, 38, 123–132. [Google Scholar] [CrossRef]
- Tang, Y.-H.; Wu, J.; Fan, T.-T.; Zhang, H.-H.; Gong, X.-X.; Cao, Z.-Y.; Zhang, J.; Lin, H.-W.; Han, B.-N. Chemical and biological study of aplysiatoxin derivatives showing inhibition of potassium channel Kv1.5. RSC Adv. 2019, 9, 7594–7600. [Google Scholar] [CrossRef] [Green Version]
- Berman, F.; Gerwick, W.; Murray, T. Antillatoxin and kalkitoxin, ichthyotoxins from the tropical cyanobacterium Lyngbya majuscula, induce distinct temporal patterns of NMDA receptor-mediated neurotoxicity. Toxicon 1999, 37, 1645–1648. [Google Scholar] [CrossRef]
- Li, W.; Berman, F.; Okino, T.; Yokokawa, F.; Shioiri, T.; Gerwick, W.; Murray, T. Antillatoxin is a marine cyanobacterial toxin that potently activates voltage-gated sodium channels. Proc. Natl. Acad. Sci. USA 2001, 98, 7599–7604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, A.M.; Hall, M.; Fay, M.J.; Lamar, P.; Pearson, C.; Prozialeck, W.C.; Lehmann, V.K.; Jacobson, P.B.; Romanic, A.M.; Uz, T. Effect of a short-term in vitro exposure to the marine toxin domoic acid on viability, tumor necrosis factor-alpha, matrix metalloproteinase-9 and superoxide anion release by rat neonatal microglia. BMC Pharmacol. 2001, 1, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kao, C.Y. Structure-Activity Relations of Tetrodotoxin, Saxitoxin, and Analogues. Ann. N. Y. Acad. Sci. 1986, 479, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Tamplin, M.L. A Bacterial Source of Tetrodotoxins and Saxitoxins. In Marine Toxins; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1990; Volume 418, pp. 78–86. [Google Scholar]
- Sims, J.; Zandee Van Rilland, R. Escharotic stomatitis caused by the “stinging seaweed” Microcoleus lyngbyaceus (formerly Lyngbya majuscula): Case report and literature review. Hawaii Med. J. 1981, 40, 243–248. [Google Scholar] [PubMed]
- Cardellina, J.H.; Marner, F.-J.; Moore, R.E. Seaweed dermatitis: Structure of lyngbyatoxin A. Science 1979, 204, 193–195. [Google Scholar] [CrossRef]
- Weckesser, J.; Drews, G.; Mayer, H. Lipopolysaccharides of photosynthetic prokaryotes. Annu. Rev. Microbiol. 1979, 33, 215–239. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, S.G. Lipopolysaccharide inhibition of rat hepatic microsomal epoxide hydrolase and glutathione S-transferase gene expression irrespective of nuclear factor-κB activation. Biochem. Pharmacol. 1998, 56, 1427–1436. [Google Scholar] [CrossRef]
- Devasagayam, T.; Tilak, J.; Boloor, K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R. Free radicals and antioxidants in human health: Current status and future prospects. JAPI 2004, 52, 4. [Google Scholar]
- Prieto, A.I.; Jos, A.; Pichardo, S.; Moreno, I.; Cameán, A.M. Protective role of vitamin E on the microcystin-induced oxidative stress in tilapia fish (Oreochromis niloticus). Environ. Toxicol. Chem. Int. J. 2008, 27, 1152–1159. [Google Scholar] [CrossRef]
- Ufelmann, H.; Krüger, T.; Luckas, B.; Schrenk, D. Human and rat hepatocyte toxicity and protein phosphatase 1 and 2A inhibitory activity of naturally occurring desmethyl-microcystins and nodularins. Toxicology 2012, 293, 59–67. [Google Scholar] [CrossRef]
- Endean, R.; Monks, S.; Griffith, J.; Llewellyn, L. Apparent relationships between toxins elaborated by the cyanobacterium Trichodesmium erythraeum and those present in the flesh of the narrow-barred Spanish mackerel Scomberomorus commersoni. Toxicon 1993, 31, 1155–1165. [Google Scholar] [CrossRef]
- Wu, M.; Okino, T.; Nogle, L.M.; Marquez, B.L.; Williamson, R.T.; Sitachitta, N.; Berman, F.W.; Murray, T.F.; McGough, K.; Jacobs, R. Structure, Synthesis, and Biological Properties of Kalkitoxin, a Novel Neurotoxin from the Marine Cyanobacterium Lyngbya m ajuscula. J. Am. Chem. Soc. 2000, 122, 12041–12042. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, D.; Fang, D. Nodularins in poisoning. Clin. Chim. Acta 2013, 425, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Zabaglo, K.; Chrapusta, E.; Bober, B.; Kaminski, A.; Adamski, M.; Bialczyk, J. Environmental roles and biological activity of domoic acid: A review. Algal Res. 2016, 13, 94–101. [Google Scholar] [CrossRef]
- Bates, S.S. Domoic-acid-producing diatoms: Another genus added! J. Phycol. 2000, 36, 978–983. [Google Scholar] [CrossRef]
- Olesen, A.J.; Leithoff, A.; Altenburger, A.; Krock, B.; Beszteri, B.; Eggers, S.L.; Lundholm, N. First Evidence of the Toxin Domoic Acid in Antarctic Diatom Species. Toxins 2021, 13, 93. [Google Scholar] [CrossRef]
- Lelong, A.; Hégaret, H.; Soudant, P.; Bates, S.S. Pseudo-nitzschia (Bacillariophyceae) species, domoic acid and amnesic shellfish poisoning: Revisiting previous paradigms. Phycologia 2012, 51, 168–216. [Google Scholar] [CrossRef] [Green Version]
- Landsberg, J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002, 10, 113–390. [Google Scholar] [CrossRef]
- Pulido, O.M. Domoic acid toxicologic pathology: A review. Mar. Drugs 2008, 6, 180–219. [Google Scholar] [CrossRef]
- Lefebvre, K.A.; Robertson, A. Domoic acid and human exposure risks: A review. Toxicon 2010, 56, 218–230. [Google Scholar] [CrossRef]
- Goldstein, T.; Mazet, J.; Zabka, T.; Langlois, G.; Colegrove, K.; Silver, M.; Bargu, S.; Van Dolah, F.; Leighfield, T.; Conrad, P.A. Novel symptomatology and changing epidemiology of domoic acid toxicosis in California sea lions (Zalophus californianus): An increasing risk to marine mammal health. Proc. R. Soc. B Biol. Sci. 2008, 275, 267–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdiglesias, V.; Prego-Faraldo, M.V.; Pásaro, E.; Méndez, J.; Laffon, B. Okadaic acid: More than a diarrheic toxin. Mar. Drugs 2013, 11, 4328–4349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdiglesias, V.; Laffon, B.; Pásaro, E.; Méndez, J. Evaluation of okadaic acid-induced genotoxicity in human cells using the micronucleus test and γH2AX analysis. J. Toxicol. Environ. Health Part A 2011, 74, 980–992. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, J.; Gupta, N.; Agrawal, M.; Bala Bhaskar, A.S.; Lakshmana Rao, P.V. Modulation of ROS/MAPK signaling pathways by okadaic acid leads to cell death via, mitochondrial mediated caspase-dependent mechanism. Apoptosis Int. J. Program. Cell Death 2011, 16, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Reguera, B.; Riobó, P.; Rodríguez, F.; Díaz, P.A.; Pizarro, G.; Paz, B.; Franco, J.M.; Blanco, J. Dinophysis toxins: Causative organisms, distribution and fate in shellfish. Mar. Drugs 2014, 12, 394–461. [Google Scholar] [CrossRef]
- Tergau, F.; Weichert, J.; Quentin, I.; Opitz, R.; von Zezschwitz, C.; Marwitz, J.; Ritz, V.; Steinfelder, H.J. Inhibitors of ser/thr phosphatases 1 and 2A induce apoptosis in pituitary GH3 cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1997, 356, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, A.; Román, Y.; Vieytes, M.R.; Ofuji, K.; Satake, M.; Yasumoto, T.; Botana, L.M. Azaspiracid-4 inhibits Ca2+ entry by stored operated channels in human T lymphocytes. Biochem. Pharmacol. 2005, 69, 1627–1636. [Google Scholar] [CrossRef]
- Friedman, M.A.; Fernandez, M.; Backer, L.C.; Dickey, R.W.; Bernstein, J.; Schrank, K.; Kibler, S.; Stephan, W.; Gribble, M.O.; Bienfang, P. An updated review of ciguatera fish poisoning: Clinical, epidemiological, environmental, and public health management. Mar. Drugs 2017, 15, 72. [Google Scholar] [CrossRef]
- Lehane, L.; Lewis, R.J. Ciguatera: Recent advances but the risk remains. Int. J. Food Microbiol. 2000, 61, 91–125. [Google Scholar] [CrossRef]
- Hamilton, B.; Hurbungs, M.; Jones, A.; Lewis, R.J. Multiple ciguatoxins present in Indian Ocean reef fish. Toxicon 2002, 40, 1347–1353. [Google Scholar] [CrossRef]
- Trainer, V.L.; Thomsen, W.J.; Catterall, W.A.; Baden, D.G. Photoaffinity labeling of the brevetoxin receptor on sodium channels in rat brain synaptosomes. Mol. Pharmacol. 1991, 40, 988–994. [Google Scholar] [PubMed]
- Franchini, A.; Marchesini, E.; Poletti, R.; Ottaviani, E. Acute toxic effect of the algal yessotoxin on Purkinje cells from the cerebellum of Swiss CD1 mice. Toxicon 2004, 43, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Ramos, V.; Vasconcelos, V. Palytoxin and analogs: Biological and ecological effects. Mar. Drugs 2010, 8, 2021–2037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patocka, J.; Gupta, R.C.; Wu, Q.-h.; Kuca, K. Toxic potential of palytoxin. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 2015, 35, 773–780. [Google Scholar] [CrossRef]
- Gill, S.; Murphy, M.; Clausen, J.; Richard, D.; Quilliam, M.; MacKinnon, S.; LaBlanc, P.; Mueller, R.; Pulido, O. Neural injury biomarkers of novel shellfish toxins, spirolides: A pilot study using immunochemical and transcriptional analysis. Neurotoxicology 2003, 24, 593–604. [Google Scholar] [CrossRef]
- Faber, S. Saxitoxin and the induction of paralytic shellfish poisoning. J. Young Investig. 2012, 23, 1–7. [Google Scholar]
- Baden, D.G.; Adams, D.J. Brevetoxins: Chemistry, mechanism of action, and methods of detection. Food Sci. Technol. N. Y. Marcel Dekker 2000, 505–532. [Google Scholar] [CrossRef]
- Holmes, M. The origin of ciguatera—An update. Ciguatera Inf. Bull. Noumea 1992, 8–9. [Google Scholar]
- Cameron, J.; Flowers, A.; Capra, M. Effects of ciguatoxin on nerve excitability in rats (Part I). J. Neurol. Sci. 1991, 101, 87–92. [Google Scholar] [CrossRef]
- Cameron, J.; Flowers, A.; Capra, M. Electrophysiological studies on ciguatera poisoning in man (Part II). J. Neurol. Sci. 1991, 101, 93–97. [Google Scholar] [CrossRef]
- Ito, E.; Satake, M.; Ofuji, K.; Higashi, M.; Harigaya, K.; McMahon, T.; Yasumoto, T. Chronic effects in mice caused by oral administration of sublethal doses of azaspiracid, a new marine toxin isolated from mussels. Toxicon 2002, 40, 193–203. [Google Scholar] [CrossRef]
- Rhodes, L.; McNabb, P.; De Salas, M.; Briggs, L.; Beuzenberg, V.; Gladstone, M. Yessotoxin production by Gonyaulax spinifera. Harmful Algae 2006, 5, 148–155. [Google Scholar] [CrossRef]
- Pérez-Gómez, A.; Ferrero-Gutierrez, A.; Novelli, A.; Franco, J.M.; Paz, B.; Fernández-Sánchez, M.T. Potent neurotoxic action of the shellfish biotoxin yessotoxin on cultured cerebellar neurons. Toxicol. Sci. 2006, 90, 168–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.H. Pharmacological actions of palytoxin. Toxins Biol. Act. Compd. Microalgae 2014, 2, 663. [Google Scholar]
- Deeds, J.R.; Schwartz, M.D. Human risk associated with palytoxin exposure. Toxicon 2010, 56, 150–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cembella, A.D. Chemical ecology of eukaryotic microalgae in marine ecosystems. Phycologia 2003, 42, 420–447. [Google Scholar] [CrossRef]
- Cembella, A.; Lewis, N.; Quilliam, M. The marine dinoflagellate Alexandrium ostenfeldii (Dinophyceae) as the causative organism of spirolide shellfish toxins. Phycologia 2000, 39, 67–74. [Google Scholar] [CrossRef]
- Touzet, N.; Franco, J.M.; Raine, R. Morphogenetic diversity and biotoxin composition of Alexandrium (Dinophyceae) in Irish coastal waters. Harmful Algae 2008, 7, 782–797. [Google Scholar] [CrossRef]
- Miles, C.O.; Wilkins, A.L.; Stirling, D.J.; MacKenzie, A.L. Gymnodimine C, an isomer of gymnodimine B, from Karenia selliformis. J. Agric. Food Chem. 2003, 51, 4838–4840. [Google Scholar] [CrossRef]
- Davidson, K.; Baker, C.; Higgins, C.; Higman, W.; Swan, S.; Veszelovszki, A.; Turner, A.D. Potential threats posed by new or emerging marine biotoxins in UK waters and examination of detection methodologies used for their control: Cyclic imines. Mar. Drugs 2015, 13, 7087–7112. [Google Scholar] [CrossRef]
- Ding, W.-X.; Shen, H.-M.; Ong, C.-N. Pivotal role of mitochondrial Ca2+ in microcystin-induced mitochondrial permeability transition in rat hepatocytes. Biochem. Biophys. Res. Commun. 2001, 285, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Weng, D.; Li, F.; Zou, X.; Young, D.O.; Ji, J.; Shen, P. Involvement of JNK regulation in oxidative stress-mediated murine liver injury by microcystin-LR. Apoptosis Int. J. Program. Cell Death 2008, 13, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Nayak, R.; Patra, S.; Jit, B.P.; Ragusa, A. Bioactive Metabolites from Marine Algae as Potent Pharmacophores against Oxidative Stress-Associated Human Diseases: A Comprehensive Review. Molecules 2020, 26, 37. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Behera, C.; Nayak, R.; Patil, S.; Bhutia, S.K.; Jena, M. Enteromorpha compressa extract induces anticancer activity through apoptosis and autophagy in oral cancer. Mol. Biol. Rep. 2020, 47, 9567–9578. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Nayak, R.; Behera, C.; Dash, S.R.; Nayak, S.; Sahu, B.B.; Bhutia, S.K.; Jena, M. Multifunctional role of fucoidan, sulfated polysaccharides in human health and disease: A journey under the sea in pursuit of potent therapeutic agents. Int. J. Biol. Macromol. 2020, 164, 4263–4278. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Patra, S.; Behera, C.; Nayak, R.; Jit, B.P.; Ragusa, A. Preliminary Investigation of the Antioxidant, Anti-Diabetic, and Anti-Inflammatory Activity of Enteromorpha intestinalis Extracts. Molecules 2021, 26, 1171. [Google Scholar] [CrossRef]
- Campos, A.; Vasconcelos, V. Molecular mechanisms of microcystin toxicity in animal cells. Int. J. Mol. Sci. 2010, 11, 268–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, X.-S.; Li, X.-Y.; Duan, H.-Y.; Chung, I.-K.; Lee, J.-A. Toxic effects of Microcystis cell extracts on the reproductive system of male mice. Toxicon 2006, 48, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Sueoka, E.; Watanabe, T.; Suganuma, M. The concept of the okadaic acid class of tumor promoters is revived in endogenous protein inhibitors of protein phosphatase 2A, SET and CIP2A, in human cancers. J. Cancer Res. Clin. Oncol. 2018, 144, 2339–2349. [Google Scholar] [CrossRef] [Green Version]
- Patra, S.; Nayak, R.; Patro, S.; Pradhan, B.; Sahu, B.; Behera, C.; Bhutia, S.K.; Jena, M. Chemical diversity of dietary phytochemicals and their mode of chemoprevention. Biotechnol. Rep. 2021, 30, e00633. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomed. Int. J. Phytother. Phytopharm. 2021, 90, 153554. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Panda, K.C.; Das, S.; Jena, M. Apoptosis and autophagy modulating dietary phytochemicals in cancer therapeutics: Current evidences and future perspectives. Phytother. Res. 2021, 35, 4194–4214. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Rout, L.; Jena, M.; Efferth, T.; Bhutia, S.K. Chemotherapeutic efficacy of curcumin and resveratrol against cancer: Chemoprevention, chemoprotection, drug synergism and clinical pharmacokinetics. Semin. Cancer Biol. 2021, 73, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Bhuyan, P.P.; Patra, S.; Nayak, R.; Behera, P.K.; Behera, C.; Behera, A.K.; Ki, J.S.; Jena, M. Beneficial effects of seaweeds and seaweed-derived bioactive compounds: Current evidence and future prospective. Biocatal. Agric. Biotechnol. 2022, 39, 102242. [Google Scholar] [CrossRef]
- Jit, B.P.; Pradhan, B.; Dash, R.; Bhuyan, P.P.; Behera, C.; Behera, R.K.; Sharma, A.; Alcaraz, M.; Jena, M. Phytochemicals: Potential Therapeutic Modulators of Radiation Induced Signaling Pathways. Antioxidants 2022, 11, 49. [Google Scholar] [CrossRef]
- Jit, B.P.; Pattnaik, S.; Arya, R.; Dash, R.; Sahoo, S.S.; Pradhan, B.; Bhuyan, P.P.; Behera, P.K.; Jena, M.; Sharma, A.; et al. Phytochemicals: A potential next generation agent for radioprotection. Phytomed. Int. J. Phytother. Phytopharm. 2022, 154188. [Google Scholar] [CrossRef]
- Quarta, A.; Gaballo, A.; Pradhan, B.; Patra, S.; Jena, M.; Ragusa, A. Beneficial Oxidative Stress-Related trans-Resveratrol Effects in the Treatment and Prevention of Breast Cancer. Appl. Sci. 2021, 11, 11041. [Google Scholar] [CrossRef]
- Pradhan, B.; Patra, S.; Dash, S.R.; Nayak, R.; Behera, C.; Jena, M. Evaluation of the anti-bacterial activity of methanolic extract of Chlorella vulgaris Beyerinck [Beijerinck] with special reference to antioxidant modulation. Futur. J. Pharm. Sci 2021, 7, 17. [Google Scholar] [CrossRef]
- Pradhan, B.; Ki, J.-S. Phytoplankton Toxins and Their Potential Therapeutic Applications: A Journey toward the Quest for Potent Pharmaceuticals. Mar. Drugs 2022, 20, 271. [Google Scholar] [CrossRef]
- White, J.D.; Xu, Q.; Lee, C.-S.; Valeriote, F.A. Total synthesis and biological evaluation of (+)-kalkitoxin, a cytotoxic metabolite of the cyanobacterium Lyngbya majuscula. Org. Biomol. Chem. 2004, 2, 2092–2102. [Google Scholar] [CrossRef]
- LePage, K.; Goeger, D.; Yokokawa, F.; Asano, T.; Shioiri, T.; Gerwick, W.; Murray, T. The neurotoxic lipopeptide kalkitoxin interacts with voltage-sensitive sodium channels in cerebellar granule neurons. Toxicol. Lett. 2005, 158, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Ayed, Y.; Kouidhi, B.; Kassim, S.; Bacha, H. Proliferative effect of the phycotoxin domoic acid on cancer cell lines: A preliminary evaluation. J. Taibah Univ. Sci. 2018, 12, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Borowitzka, M.A. Microalgae as sources of pharmaceuticals and other biologically active compounds. J. Appl. Phycol. 1995, 7, 3–15. [Google Scholar] [CrossRef]
- Ronzitti, G.; Callegari, F.; Malaguti, C.; Rossini, G.P. Selective disruption of the E-cadherin-catenin system by an algal toxin. Br. J. Cancer 2004, 90, 1100–1107. [Google Scholar] [CrossRef]
- Paz, B.; Daranas, A.H.; Norte, M.; Riobó, P.; Franco, J.M.; Fernández, J.J. Yessotoxins, a group of marine polyether toxins: An overview. Mar. Drugs 2008, 6, 73–102. [Google Scholar] [CrossRef]
- Alfonso, A.; Vieytes, M.R.; Botana, L.M. Yessotoxin, a promising therapeutic tool. Mar. Drugs 2016, 14, 30. [Google Scholar] [CrossRef] [Green Version]
- Korsnes, M.S.; Korsnes, R. Mitotic catastrophe in BC3H1 cells following yessotoxin exposure. Front. Cell Dev. Biol. 2017, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Orsi, C.F.; Colombari, B.; Callegari, F.; Todaro, A.M.; Ardizzoni, A.; Rossini, G.P.; Blasi, E.; Peppoloni, S. Yessotoxin inhibits phagocytic activity of macrophages. Toxicon 2010, 55, 265–273. [Google Scholar] [CrossRef]
- López, A.M.; Rodríguez, J.J.G.; Mirón, A.S.; Camacho, F.G.; Grima, E.M. Immunoregulatory potential of marine algal toxins yessotoxin and okadaic acid in mouse T lymphocyte cell line EL-4. Toxicol. Lett. 2011, 207, 167–172. [Google Scholar] [CrossRef]
- Bloom, G.S. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [Green Version]
- Rubiolo, J.; López-Alonso, H.; Martínez, P.; Millán, A.; Cagide, E.; Vieytes, M.; Vega, F.; Botana, L. Yessotoxin induces ER-stress followed by autophagic cell death in glioma cells mediated by mTOR and BNIP3. Cell. Signal. 2014, 26, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Tobío, A.; Alfonso, A.; Madera-Salcedo, I.; Botana, L.M.; Blank, U. Yessotoxin, a marine toxin, exhibits anti-allergic and anti-tumoural activities inhibiting melanoma tumour growth in a preclinical model. PLoS ONE 2016, 11, e0167572. [Google Scholar] [CrossRef] [PubMed]
- Mattei, C.; Legros, C. The voltage-gated sodium channel: A major target of marine neurotoxins. Toxicon 2014, 91, 84–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eijkelkamp, N.; Linley, J.E.; Baker, M.D.; Minett, M.S.; Cregg, R.; Werdehausen, R.; Rugiero, F.; Wood, J.N. Neurological perspectives on voltage-gated sodium channels. Brain 2012, 135, 2585–2612. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.M.; Bourdelais, A.J.; Sabater, J.R.; Ahmed, A.; Lee, T.A.; Serebriakov, I.; Baden, D.G. Airway responses to aerosolized brevetoxins in an animal model of asthma. Am. J. Respir. Crit. Care Med. 2005, 171, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, C.J.; Leggett, S.R.; Strohbehn, K.; Pierce, R.H.; Sleasman, J.W. Effects of in vitro brevetoxin exposure on apoptosis and cellular metabolism in a leukemic T cell line (Jurkat). Mar. Drugs 2008, 6, 291–307. [Google Scholar] [CrossRef]
- Hilderbrand, S.C.; Murrell, R.N.; Gibson, J.E.; Brown, J.M. Marine brevetoxin induces IgE-independent mast cell activation. Arch. Toxicol. 2011, 85, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Sas, K.M.; Baatz, J.E. Brevetoxin-2 induces an inflammatory response in an alveolar macrophage cell line. Int. J. Hydrog. Environ. Health 2010, 213, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Murrell, R.N.; Gibson, J.E. Brevetoxins 2, 3, 6, and 9 show variability in potency and cause significant induction of DNA damage and apoptosis in Jurkat E6-1 cells. Arch. Toxicol. 2009, 83, 1009–1019. [Google Scholar] [CrossRef]
- George, J.; Baden, D.G.; Gerwick, W.H.; Murray, T.F. Bidirectional influence of sodium channel activation on NMDA receptor–dependent cerebrocortical neuron structural plasticity. Proc. Natl. Acad. Sci. USA 2012, 109, 19840–19845. [Google Scholar] [CrossRef] [Green Version]
- Baden, D.G.; Abraham, W.M.; Bourdelais, A.J. Polyether Brevetoxin Derivatives as a Treatment for Cystic Fibrosis, Mucociliary Dysfunction, and Pulmonary Diseases. U.S. Patent US7399782B2, 15 July 2008. [Google Scholar]
- Louzao, M.C.; Fraga, M.; Vilariño, N. Pharmacology of palytoxins and ostreocins. In Phycotoxins, Chemistry and Biochemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2015; pp. 113–135. [Google Scholar]
- Alonso, E.; Vale, C.; Vieytes, M.R.; Laferla, F.M.; Giménez-Llort, L.; Botana, L.M. 13-Desmethyl spirolide-C is neuroprotective and reduces intracellular Aβ and hyperphosphorylated tau in vitro. Neurochem. Int. 2011, 59, 1056–1065. [Google Scholar] [CrossRef] [PubMed]

| Toxins | Organisms/Source | Toxic Symptoms | Toxic Target | References |
|---|---|---|---|---|
| Nodularin | Nodularia spumigena | PP inactivation | [17,18,19] | |
| Nodularin | Nodularia spumigena | Renal lesions, diarrhea, vomiting, piloerection, weakness, and pallor | Tissue transport and bile anions | [46,47] |
| Nodularin | Nodularia spumigena | Tumor promotion | [18,19] | |
| Oscillatoxin | Schizothrix calcicola and Oscillatoria nigroviridis | Contact irritants | - | [48] |
| Lyngbyatoxin-A | Lyngbya majuscula | Skin irritant, oral and gastrointestinal inflammation | Tumor promotion | [49] |
| Lipopolysaccharide | Most of the cyanobacterial species | Allergic, inflammatory, pyrogenic reactions, fever and septic shock syndrome | Tumor promotion | [50] |
| Aplysiatoxin | Schizothrix calcicola and Oscillatoria nigroviridis | Inflammation, burning ambiances in the throat and mouth, paraesthesia, abdominal pain, vomiting, diarrhea, convulsions, and low blood pressure | Tumor promotion | [51] |
| Aplysiatoxin | Schizothrix calcicola and Oscillatoria nigroviridis | Gastrointestinal symptoms, including diarrhea, nausea, and vomiting | blocked potassium channel Kv1.5 | [52] |
| Aplysiatoxin | Schizothrix calcicola and Oscillatoria nigroviridis | Sodium channel blocked | [51] | |
| Kalkitoxin | Lyngbya majuscula and Trichodesmium spp. | Neurotoxic | Sodium channel blocked | [53] |
| Antillatoxin | Lyngbya majuscula | Ichthyotoxicity and neurotoxicity | Sodium channel blocked, Neurotoxicity | [54] |
| Domoic acid | Pseudo-nitzschia australis, Pseudo-nitzschia calliantha, Pseudo-nitzschia cuspidate, Pseudo-nitzschia delicatissima, Pseudo-nitzschia fraudulenta, Pseudo-nitzschia galaxiae, Pseudo-nitzschia multiseries, Pseudo-nitzschia multistriata, Pseudo-nitzschia pseudodelicatissima, Pseudo-nitzschia pungens, Pseudo-nitzschia seriata, and Pseudo-nitzschia turgidula | Abdominal pains, vomiting, and diarrhea, severe headaches, confusion, agitation, somnolence (sleepiness), memory loss, coma, Ataxia (incoordination), excessive scratching, sleepiness, tremors, heart, Seizures, spells of significant lethargy and inappetence, central blindness, vomiting, blepharospasm, muscular twitching, and aberrant behavior difficulties, convulsions, and mortality | Sodium channel blocked and Glutamate receptors | [55] |
| Saxitoxins | Lyngbya wollei, Cylindrospermopsis raciborskii, Anabaena circinalis, and Aphanizomenon flos-aquae | Respiratory arrest, cardiovascular shock, tickling sensations in the mouth, lips, and tongue, numbness in the extremities, breathing difficulties, gastrointestinal problems, and full paralysis | Sodium channel blocked, Voltage-dependent sodium channel Site 1 | [56,57] |
| Toxins | Organisms/Source | Toxic Symptoms | Toxic Target | References |
|---|---|---|---|---|
| Okadaic acid | Dinophysis sp. and Prorocentrum lima | Incapacitating diarrhea, nausea, vomiting, and abdominal pain | PP inactivation, Oxidative damage, cellular dysfunction, cell cycle, gene expression, inhibit DNA repair mechanism | [76,77,78] |
| Dinophysistoxins Okadaic acids | Dinophysis spp. Prorocentrum spp. | Gastrointestinal illness, nausea, vomiting, and abdominal pain | Ser/thr protein phosphatases | [79,80] |
| Azaspiracid | Protoperidinium crassipes | Severe diarrhea, vomiting, nausea, stomach cramps, and neurotoxicity | Tumor promotion | [81] |
| Ciguatoxin | Gambierdiscus toxicus | Neurological, gastrointestinal, and cardiovascular problems | Sodium channel blocked, Voltage-dependent sodium channel Site 5 | [82,83,84] |
| Saxitoxins | Alexandrium spp. Gymnodinium spp. Pyrodinium spp. | Respiratory arrest, cardiovascular shock, tickling sensations in the mouth, lips, and tongue, numbness in the extremities, breathing difficulties, gastrointestinal problems, and full paralysis | Sodium channel blocked, Voltage-dependent sodium channel Site 1 | [56,57] |
| Brevetoxin | Karenia brevis Gymnodinium breve | Slighter gastroenteritis with neurologic indicators, Nausea, tingling and numbness in the perioral area, loss of motor function, and acute muscular pain | Sodium channel blocked, Voltage-dependent sodium channel Site 5 | [85] |
| Yessotoxins | Gonyaulax spinifera, Lingulodinium polyedrum, and Protoceratium reticulatum | Motor discoordination | Sodium channel blocked | [86] |
| Palytoxin | Ostreopsis mascarenensis, O. siamensis, O. lenticularis, O. fattorussoi, and O. ovata, | Fever, ataxia, inactivity, drowsiness, and limb weakness | Sodium channel blocked | [87,88] |
| Spirolides | Alexandrium ostenfeldii, Alexandrium peruvianum, and Karenia selliformis | Neuron and astrocytes damage | Sodium channel blocked | [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradhan, B.; Kim, H.; Abassi, S.; Ki, J.-S. Toxic Effects and Tumor Promotion Activity of Marine Phytoplankton Toxins: A Review. Toxins 2022, 14, 397. https://doi.org/10.3390/toxins14060397
Pradhan B, Kim H, Abassi S, Ki J-S. Toxic Effects and Tumor Promotion Activity of Marine Phytoplankton Toxins: A Review. Toxins. 2022; 14(6):397. https://doi.org/10.3390/toxins14060397
Chicago/Turabian StylePradhan, Biswajita, Hansol Kim, Sofia Abassi, and Jang-Seu Ki. 2022. "Toxic Effects and Tumor Promotion Activity of Marine Phytoplankton Toxins: A Review" Toxins 14, no. 6: 397. https://doi.org/10.3390/toxins14060397







