A Pilot Randomized Controlled Trial of Botulinum Toxin Treatment Combined with Robot-Assisted Therapy, Mirror Therapy, or Active Control Treatment in Patients with Spasticity Following Stroke
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Study Design and Paradigm
4.3. Intervention
4.4. Robot-Assisted Training (RT) Group
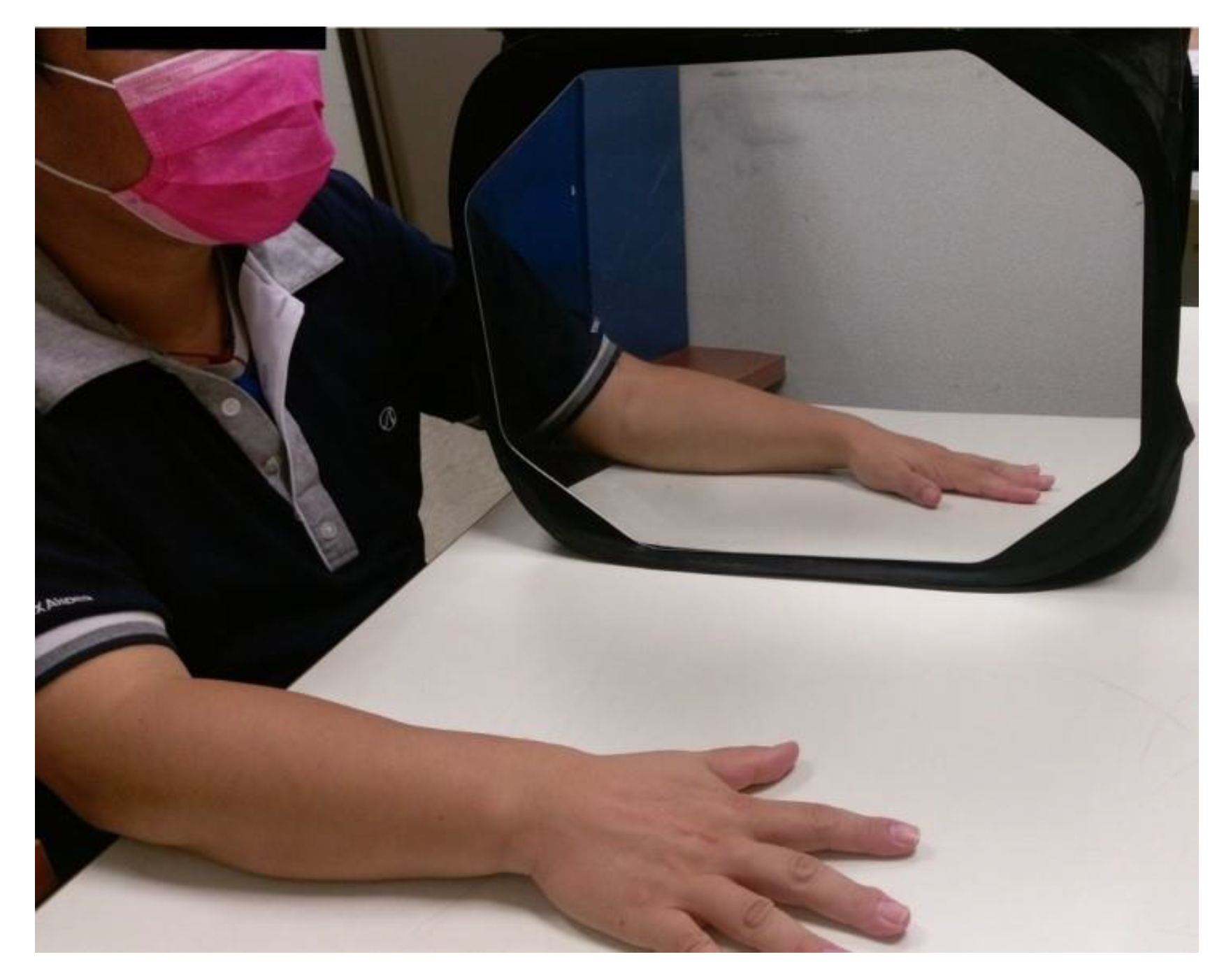
4.5. Mirror Therapy (MT) Group
4.6. Active Control Treatment (AC) Group
4.7. Outcome Measures
4.8. Fugl-Meyer Assessment (FMA)
4.9. Modified Ashworth Scale (MAS)
4.10. Motor Activity Log (MAL)
4.11. Arm Activity Level
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wissel, J.; Schelosky, L.D.; Scott, J.; Christe, W.; Faiss, J.H.; Mueller, J. Early development of spasticity following stroke: A prospective, observational trial. J. Neurol. 2010, 257, 1067–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Paiva, A.; Meunier, F.A.; Molgo, J.; Aoki, K.R.; Dolly, J.O. Functional repair of motor endplates after botulinum neurotoxin type A poisoning: Biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc. Natl. Acad. Sci. USA 1999, 96, 3200–3205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhakta, B.B.; Cozens, J.A.; Chamberlain, M.A.; Bamford, J.M. Impact of botulinum toxin type A on disability and carer burden due to arm spasticity after stroke: A randomised double blind placebo controlled trial. J. Neurol. Neurosurg. Psychiatry 2000, 69, 217–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, S.; Liu, Y.; Shen, L.; Liang, X.; Xu, X.; Wei, Y. Botulinum toxin type a for upper limb spasticity in poststroke patients: A meta-analysis of randomized controlled trials. J Stroke Cerebrovasc. Dis. 2020, 29, 104682. [Google Scholar] [CrossRef]
- Sheean, G.; Lannin, N.A.; Turner-Stokes, L.; Rawicki, B.; Snow, B.J. Botulinum toxin assessment, intervention and after-care for upper limb hypertonicity in adults: International consensus statement. Eur. J. Neurol. 2010, 17 (Suppl. S2), 74–93. [Google Scholar] [CrossRef]
- Foley, N.; Pereira, S.; Salter, K.; Fernandez, M.M.; Speechley, M.; Sequeira, K.; Miller, T.; Teasell, R. Treatment with botulinum toxin improves upper-extremity function post stroke: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2013, 94, 977–989. [Google Scholar] [CrossRef]
- Andringa, A.; van de Port, I.; van Wegen, E.; Ket, J.; Meskers, C.; Kwakkel, G. Effectiveness of botulinum toxin treatment for upper limb spasticity poststroke over different ICF domains: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2019, 100, 1703–1725. [Google Scholar] [CrossRef]
- Ada, L.; O’Dwyer, N.; O’Neill, E. Relation between spasticity, weakness and contracture of the elbow flexors and upper limb activity after stroke: An observational study. Disabil. Rehabil. 2006, 28, 891–897. [Google Scholar] [CrossRef]
- Lindsay, P.; Bayley, M.; Hellings, C.; Hill, M.; Woodbury, E.; Phillips, S. Canadian best practice recommendations for stroke care (updated 2008). CMAP 2008, 179, S1–S25. [Google Scholar] [CrossRef] [Green Version]
- Duncan, P.W.; Zorowitz, R.; Bates, B.; Choi, J.Y.; Glasberg, J.J.; Graham, G.D.; Katz, R.C.; Lamberty, K.; Reker, D. Management of adult stroke rehabilitation care: A clinical practice guideline. Stroke 2005, 36, e100–e143. [Google Scholar] [CrossRef]
- Pollock, A.; Farmer, S.E.; Brady, M.C.; Langhorne, P.; Mead, G.E.; Mehrholz, J.; van Wijck, F. Interventions for improving upper limb function after stroke. Cochrane Database Syst. Rev. 2014, 2014, Cd010820. [Google Scholar] [CrossRef] [PubMed]
- Brewer, B.R.; McDowell, S.K.; Worthen-Chaudhari, L.C. Poststroke upper extremity rehabilitation: A review of robotic systems and clinical results. Top. Stroke. Rehabil. 2007, 14, 22–44. [Google Scholar] [CrossRef] [PubMed]
- Krebs, H.I.; Volpe, B.T.; Williams, D.; Celestino, J.; Charles, S.K.; Lynch, D.; Hogan, N. Robot-aided neurorehabilitation: A robot for wrist rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 327–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwakkel, G.; Kollen, B.J.; Krebs, H.I. Effects of robot-assisted therapy on upper limb recovery after stroke: A systematic review. Neurorehabil. Neural Repair. 2008, 22, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Michielsen, M.E.; Selles, R.W.; van der Geest, J.N.; Eckhardt, M.; Yavuzer, G.; Stam, H.J.; Smits, M.; Ribbers, G.M.; Bussmann, J.B. Motor recovery and cortical reorganization after mirror therapy in chronic stroke patients: A phase II randomized controlled trial. Neurorehabil. Neural Repair. 2011, 25, 223–233. [Google Scholar] [CrossRef]
- Wu, C.Y.; Huang, P.C.; Chen, Y.T.; Lin, K.C.; Yang, H.W. Effects of mirror therapy on motor and sensory recovery in chronic stroke: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2013, 94, 1023–1030. [Google Scholar] [CrossRef]
- Takahashi, C.D.; Der-Yeghiaian, L.; Le, V.; Motiwala, R.R.; Cramer, S.C. Robot-based hand motor therapy after stroke. Brain 2008, 131, 425–437. [Google Scholar] [CrossRef]
- Stevens, J.A.; Stoykov, M.E. Using motor imagery in the rehabilitation of hemiparesis. Arch. Phys. Med. Rehabil. 2003, 84, 1090–1092. [Google Scholar] [CrossRef]
- Gandolfi, M.; Valè, N.; Dimitrova, E.K.; Mazzoleni, S.; Battini, E.; Filippetti, M.; Picelli, A.; Santamato, A.; Gravina, M.; Saltuari, L.; et al. Effectiveness of robot-assisted upper limb training on spasticity, function and muscle activity in chronic stroke patients treated with botulinum toxin: A randomized single-blinded controlled trial. Front. Neurol. 2019, 10, 41. [Google Scholar] [CrossRef] [Green Version]
- Pennati, G.V.; Da Re, C.; Messineo, I.; Bonaiuti, D. How could robotic training and botolinum toxin be combined in chronic post stroke upper limb spasticity? A pilot study. Eur. J. Phys. Rehabil. Med. 2015, 51, 381–387. [Google Scholar]
- Hung, J.W.; Chen, Y.W.; Chen, Y.J.; Pong, Y.P.; Wu, W.C.; Chang, K.C.; Wu, C.Y. The effects of distributed vs. condensed schedule for robot-assisted training with botulinum toxin a injection for spastic upper limbs in chronic post-stroke subjects. Toxins 2021, 13, 539. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.T.; Lin, K.C.; Liu, H.L.; Wu, C.Y.; Wai, Y.Y.; Lee, T.H. Neural correlates of motor recovery after robot-assisted stroke rehabilitation: A case series study. Neurocase 2016, 22, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.L. Improving poststroke recovery: Neuroplasticity and task-oriented training. Curr. Treat. Options Cardiovasc. Med. 2009, 11, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.W.; Wu, C.Y.; Hsieh, Y.W.; Lin, K.C.; Chang, W.Y. Effects of robot-assisted upper limb rehabilitation on daily function and real-world arm activity in patients with chronic stroke: A randomized controlled trial. Clin. Rehabil. 2012, 26, 111–120. [Google Scholar] [CrossRef]
- Kinnear, B.Z.; Lannin, N.A.; Cusick, A.; Harvey, L.A.; Rawicki, B. Rehabilitation therapies after botulinum toxin-A injection to manage limb spasticity: A systematic review. Phys. Ther. 2014, 94, 1569–1581. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.J.; Park, H.K.; Kim, H.J.; Lim, T.; Ku, J.; Cho, S.; Kim, S.I.; Park, E.S. Upper extremity rehabilitation of stroke: Facilitation of corticospinal excitability using virtual mirror paradigm. J. Neuroeng. Rehabil. 2012, 9, 71. [Google Scholar] [CrossRef] [Green Version]
- Deconinck, F.J.; Smorenburg, A.R.; Benham, A.; Ledebt, A.; Feltham, M.G.; Savelsbergh, G.J. Reflections on mirror therapy: A systematic review of the effect of mirror visual feedback on the brain. Neurorehabilit. Neural Repair 2015, 29, 349–361. [Google Scholar] [CrossRef] [Green Version]
- Uswatte, G.; Foo, W.L.; Olmstead, H.; Lopez, K.; Holand, A.; Simms, L.B. Ambulatory monitoring of arm movement using accelerometry: An objective measure of upper-extremity rehabilitation in persons with chronic stroke. Arch. Phys. Med. Rehabil. 2005, 86, 1498–1501. [Google Scholar] [CrossRef]
- Rand, D.; Eng, J.J.; Tang, P.F.; Jeng, J.S.; Hung, C. How active are people with stroke? Use of accelerometers to assess physical activity. Stroke 2009, 40, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R.; Jaasko, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [PubMed]
- Park, S.W.; Wolf, S.L.; Blanton, S.; Winstein, C.; Nichols-Larsen, D.S. The EXCITE Trial: Predicting a clinically meaningful motor activity log outcome. Neurorehabilit. Neural Repair 2008, 22, 486–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, E.L.; Chui, H.C. The Modified Mini-Mental State (3MS) examination. J. Clin. Psychiatry 1987, 48, 314–318. [Google Scholar] [PubMed]
- Grigoriu, A.I.; Dinomais, M.; Rémy-Néris, O.; Brochard, S. Impact of injection-guiding techniques on the effectiveness of botulinum toxin for the treatment of focal spasticity and dystonia: A systematic review. Arch. Phys. Med. Rehabil. 2015, 96, 2067–2078.e2061. [Google Scholar] [CrossRef]
- Doucet, B.M.; Gutman, S.A. Quantifying function: The rest of the measurement story. Am. J. Occup. Ther. 2013, 67, 7–9. [Google Scholar] [CrossRef] [Green Version]
- van der Lee, J.H.; Beckerman, H.; Knol, D.L.; de Vet, H.C.; Bouter, L.M. Clinimetric properties of the motor activity log for the assessment of arm use in hemiparetic patients. Stroke 2004, 35, 1410–1414. [Google Scholar] [CrossRef] [Green Version]
- Pandyan, A.D.; Johnson, G.R.; Price, C.I.; Curless, R.H.; Barnes, M.P.; Rodgers, H. A review of the properties and limitations of the Ashworth and modified Ashworth Scales as measures of spasticity. Clin. Rehabil. 1999, 13, 373–383. [Google Scholar] [CrossRef]
- Schasfoort, F.C.; Bussmann, J.B.; Martens, W.L.; Stam, H.J. Objective measurement of upper limb activity and mobility during everyday behavior using ambulatory accelerometry: The upper limb activity monitor. Behav. Res. Methods 2006, 38, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Uswatte, G.; Giuliani, C.; Winstein, C.; Zeringue, A.; Hobbs, L.; Wolf, S.L. Validity of accelerometry for monitoring real-world arm activity in patients with subacute stroke: Evidence from the extremity constraint-induced therapy evaluation trial. Arch. Phys. Med. Rehabil. 2006, 87, 1340–1345. [Google Scholar] [CrossRef]
- Gebruers, N.; Vanroy, C.; Truijen, S.; Engelborghs, S.; De Deyn, P.P. Monitoring of physical activity after stroke: A systematic review of accelerometry-based measures. Arch. Phys. Med. Rehabil. 2010, 91, 288–297. [Google Scholar] [CrossRef]
- Rand, D.; Eng, J.J.; Tang, P.F.; Hung, C.; Jeng, J.S. Daily physical activity and its contribution to the health-related quality of life of ambulatory individuals with chronic stroke. Health Qual. Life Outcomes 2010, 8, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reale, G.; Giovannini, S.; Iacovelli, C.; Castiglia, S.F.; Picerno, P.; Zauli, A.; Rabuffetti, M.; Ferrarin, M.; Maccauro, G.; Caliandro, P. Actigraphic measurement of the upper limbs for the prediction of ischemic stroke prognosis: An observational study. Sensors 2021, 21, 2479. [Google Scholar] [CrossRef] [PubMed]
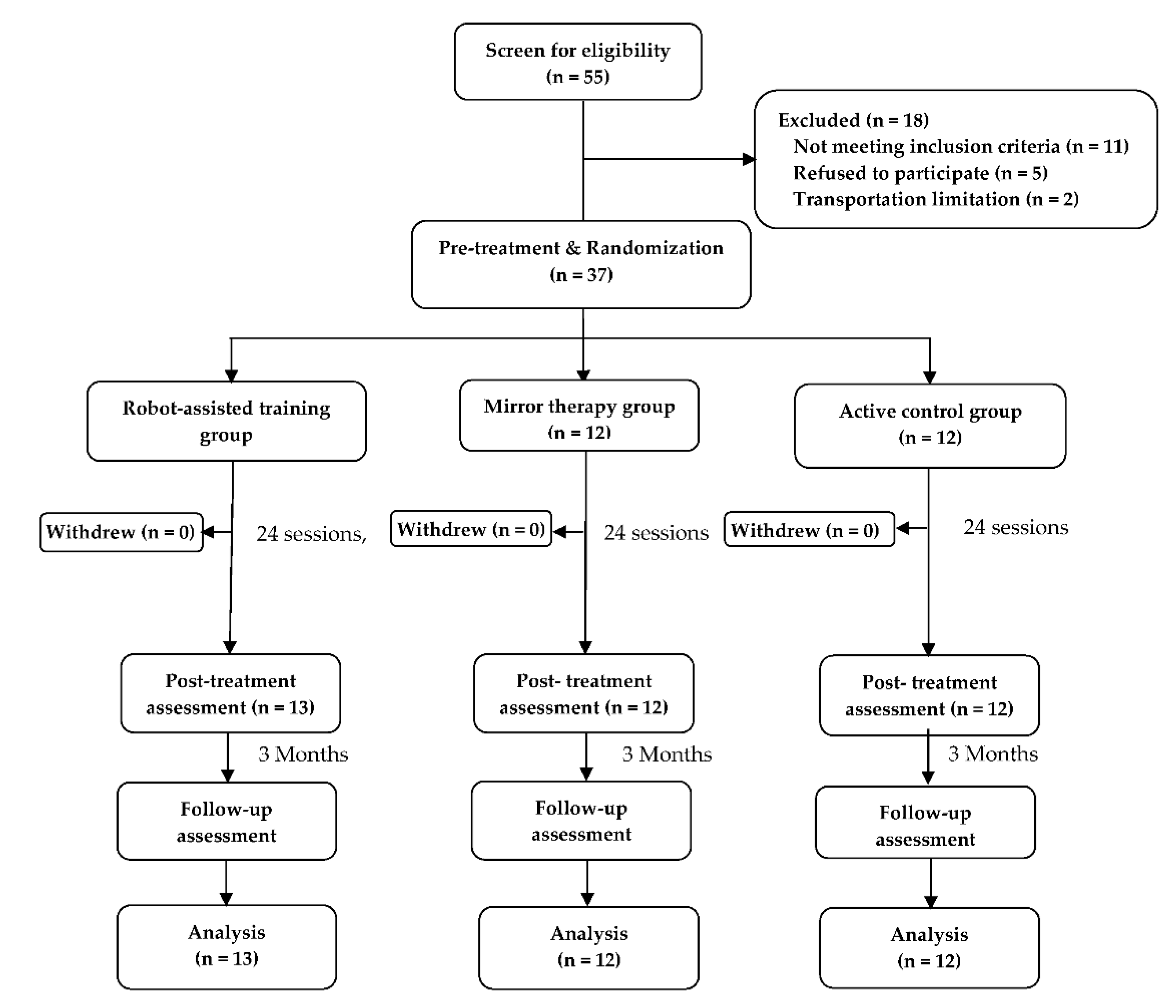

| Variables | RT (n = 13) | MT (n = 12) | AC (n = 12) | F | p† |
|---|---|---|---|---|---|
| Age (year) | 47.68 ± 12.79 | 44.34 ± 10.05 | 49.71 ± 10.86 | 0.687 | 0.510 |
| Gender (male) | 10 (76.9%) | 7 (58.3%) | 7 (58.3%) | 0.523 | |
| Education (year) | 11.00 ± 3.36 | 13.25 ± 2.45 | 10.50 ± 4.50 | 2.072 | 0.142 |
| Brain lesion (Right) | 10 (76.9%) | 9 (75.0%) | 7 (58.3%) | 0.663 | |
| Stroke duration (months) | 33.38 ± 22.71 | 33.08 ± 16.98 | 38.17 ± 25.02 | 0.206 | 0.814 |
| Stroke type | 0.916 | ||||
| Hemorrhagic | 5 (38.5%) | 6 (50.0%) | 5 (41.7%) | ||
| Ischemic | 8 (61.5%) | 6 (50.0%) | 7 (58.3%) | ||
| MMSE | 26.85 ± 2.60 | 28.17 ± 2.20 | 27.08 ± 2.31 | 1.064 | 0.356 |
| RT (n = 13) | MT (n = 12) | AC (n = 12) | F | p | |
|---|---|---|---|---|---|
| FMA | |||||
| UE-proximal | 28.08 ± 5.30 | 28.58 ± 5.07 | 25.42 ± 7.93 | 0.907 | 0.413 |
| UE-distal | 4.85 ± 2.64 | 4.08 ± 4.03 | 4.75 ± 3.79 | 0.171 | 0.844 |
| total | 32.92 ± 7.12 | 32.67 ± 7.92 | 29.67 ± 11.15 | 0.510 | 0.605 |
| MAS | |||||
| Elbow flexor | 1.50 ± 0.28 | 1.45 ± 0.96 | 1.50 ± 0.36 | 0.019 | 0.981 |
| Forearm pronator | 1.69 ± 0.52 | 1.66 ± 0.71 | 1.58 ± 0.70 | 0.030 | 0.970 |
| Wrist flexor | 1.34 ± 0.59 | 1.50 ± 0.73 | 1.67 ± 0.96 | 0.535 | 0.590 |
| Finger PIP flexor | 2.46 ± 0.74 | 2.00 ± 2.00 | 2.08 ± 1.14 | 0.796 | 0.459 |
| MAL | |||||
| AOU | 1.47 ± 0.54 | 1.41 ± 0.55 | 1.01 ± 0.40 | 2.913 | 0.068 |
| QOM | 0.94 ± 0.53 | 0.88 ± 0.64 | 0.52 ± 0.33 | 2.416 | 0.104 |
| Physical Activity | |||||
| Count in the affected side | 402.17 ± 217.57 | 393.20 ± 225.16 | 606.38 ± 228.18 | 3.374 | 0.047 |
| Count in the unaffected side | 1421.48 ± 137.21 | 1155.32 ± 150.31 | 1582.54 ± 137.21 | 2.222 | 0.125 |
| A | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pretreatment | Post-Treatment | Follow-Up | |||||||
| RT | MT | AC | RT | MT | AC | RT | MT | AC | |
| FMA | |||||||||
| UE-proximal | 28.08 ± 5.30 | 28.58 ± 5.07 | 25.42 ± 7.93 | 30.00 ± 5.60 | 30.08 ± 4.01 | 27.50 ± 8.20 | 29.46 ± 5.02 | 29.41 ± 5.16 | 28.41 ± 7.62 |
| UE-distal | 4.85 ± 2.64 | 4.08 ± 4.03 | 4.75 ± 3.79 | 6.46 ± 4.46 | 5.83 ± 3.27 | 5.41 ± 4.18 | 5.46 ± 3.33 | 5.50 ± 3.73 | 5.33 ± 4.03 |
| total | 32.92 ± 7.12 | 32.67 ± 7.92 | 29.67 ± 11.15 | 36.46 ± 8.88 | 35.91 ± 6.48 | 32.91 ± 12.07 | 34.92 ± 7.25 | 34.92 ± 8.49 | 33.75 ± 11.00 |
| MAS | |||||||||
| Elbow flexor | 1.50 ± 0.28 | 1.45 ± 0.96 | 1.50 ± 0.36 | 1.00 ± 0.74 | 1.00 ± 0.56 | 1.13 ± 0.48 | 1.35 ± 0.47 | 1.17 ± 0.44 | 1.29 ± 0.33 |
| Forearm pronator | 1.69 ± 0.52 | 1.67 ± 0.71 | 1.58 ± 0.70 | 1.08 ± 0.84 | 1.21 ± 0.94 | 1.25 ± 0.72 | 1.62 ± 0.58 | 1.46 ± 0.66 | 1.46 ± 0.45 |
| Wrist flexor | 1.34 ± 0.59 | 1.50 ± 0.73 | 1.67 ± 0.96 | 0.69 ± 0.72 | 1.08 ± 0.87 | 0.96 ± 0.50 | 1.12 ± 0.62 | 1.04 ± 0.58 | 1.13 ± 0.53 |
| Finger PIP flexor | 2.46 ± 0.74 | 2.00 ± 1.02 | 2.08 ± 1.14 | 1.85 ± 0.90 | 1.71 ± 0.94 | 1.92 ± 1.31 | 2.31 ± 0.83 | 2.21 ± 0.99 | 2.50 ± 0.80 |
| MAL | |||||||||
| AOU | 1.47 ± 0.54 | 1.41 ± 0.55 | 1.01 ± 0.40 | 1.81 ± 0.70 | 1.98 ± 0.94 | 1.54 ± 0.55 | 1.78 ± 0.80 | 1.69 ± 0.97 | 1.56 ± 0.58 |
| QOM | 0.94 ± 0.53 | 0.88 ± 0.64 | 0.52 ± 0.33 | 1.26 ± 0.74 | 1.35 ± 0.90 | 1.03 ± 0.65 | 1.22 ± 0.82 | 1.24 ± 1.00 | 1.08 ± 0.70 |
| Physical Activity | |||||||||
| Count in the affected side | 402.17 ± 217.57 | 393.20 ± 225.16 | 606.38 ± 228.18 | 398.15 ± 167.70 | 458.39 ± 245.80 | 578.12 ± 307.92 | - | - | - |
| Count in the unaffected side | 1421.48 ± 436.06 | 1155.32 ± 583.02 | 1582.54 ± 410.44 | 1440.98 ± 517.69 | 1193.43 ± 717.09 | 1460.86 ± 361.94 | - | - | - |
| B | |||||||||
| ANCOVA | ANCOVA | ||||||||
| Post-Treatment | Follow-Up | ||||||||
| F | p value | Partial η2 | F | p value | Partial η2 | ||||
| FMA | |||||||||
| UE-proximal | 0.107 | 0.898 | 0.006 | 0.797 | 0.459 | 0.046 | |||
| UE-distal | 0.690 | 0.509 | 0.040 | 0.477 | 0.625 | 0.028 | |||
| total | 0.032 | 0.968 | 0.002 | 0.466 | 0.632 | 0.027 | |||
| MAS | |||||||||
| Elbow flexor | 0.174 | 0.841 | 0.010 | 0.552 | 0.581 | 0.032 | |||
| Forearm pronator | 0.815 | 0.451 | 0.047 | 0.273 | 0.763 | 0.016 | |||
| Wrist flexor | 1.055 | 0.360 | 0.060 | 0.462 | 0.634 | 0.027 | |||
| Finger PIP flexor | 0.269 | 0.765 | 0.016 | 0.561 | 0.576 | 0.033 | |||
| MAL | |||||||||
| AOU | 1.531 | 0.231 | 0.085 | 1.665 | 0.205 | 0.092 | |||
| QOM | 2.839 | 0.073 | 0.147 | 3.785 | 0.033 | 0.187 | |||
| Physical Activity | |||||||||
| Count in the affected side | 0.634 | 0.537 | 0.041 | - | - | - | |||
| Count in the unaffected side | 0.443 | 0.646 | 0.029 | - | - | - | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, J.-W.; Yen, C.-L.; Chang, K.-C.; Chiang, W.-C.; Chuang, I.-C.; Pong, Y.-P.; Wu, W.-C.; Wu, C.-Y. A Pilot Randomized Controlled Trial of Botulinum Toxin Treatment Combined with Robot-Assisted Therapy, Mirror Therapy, or Active Control Treatment in Patients with Spasticity Following Stroke. Toxins 2022, 14, 415. https://doi.org/10.3390/toxins14060415
Hung J-W, Yen C-L, Chang K-C, Chiang W-C, Chuang I-C, Pong Y-P, Wu W-C, Wu C-Y. A Pilot Randomized Controlled Trial of Botulinum Toxin Treatment Combined with Robot-Assisted Therapy, Mirror Therapy, or Active Control Treatment in Patients with Spasticity Following Stroke. Toxins. 2022; 14(6):415. https://doi.org/10.3390/toxins14060415
Chicago/Turabian StyleHung, Jen-Wen, Chu-Ling Yen, Ku-Chou Chang, Wei-Chi Chiang, I-Ching Chuang, Ya-Ping Pong, Wen-Chi Wu, and Ching-Yi Wu. 2022. "A Pilot Randomized Controlled Trial of Botulinum Toxin Treatment Combined with Robot-Assisted Therapy, Mirror Therapy, or Active Control Treatment in Patients with Spasticity Following Stroke" Toxins 14, no. 6: 415. https://doi.org/10.3390/toxins14060415
APA StyleHung, J.-W., Yen, C.-L., Chang, K.-C., Chiang, W.-C., Chuang, I.-C., Pong, Y.-P., Wu, W.-C., & Wu, C.-Y. (2022). A Pilot Randomized Controlled Trial of Botulinum Toxin Treatment Combined with Robot-Assisted Therapy, Mirror Therapy, or Active Control Treatment in Patients with Spasticity Following Stroke. Toxins, 14(6), 415. https://doi.org/10.3390/toxins14060415






