The In Vitro Cytotoxic Effects of Ionophore Exposure on Selected Cytoskeletal Proteins of C2C12 Myoblasts
Abstract
1. Introduction
2. Results
2.1. The In Vitro Cytotoxic Effect of Monensin, Salinomycin and Lasalocid on C2C12 Myoblasts
2.1.1. The Effect of Ionophore Exposure on Cell Viability
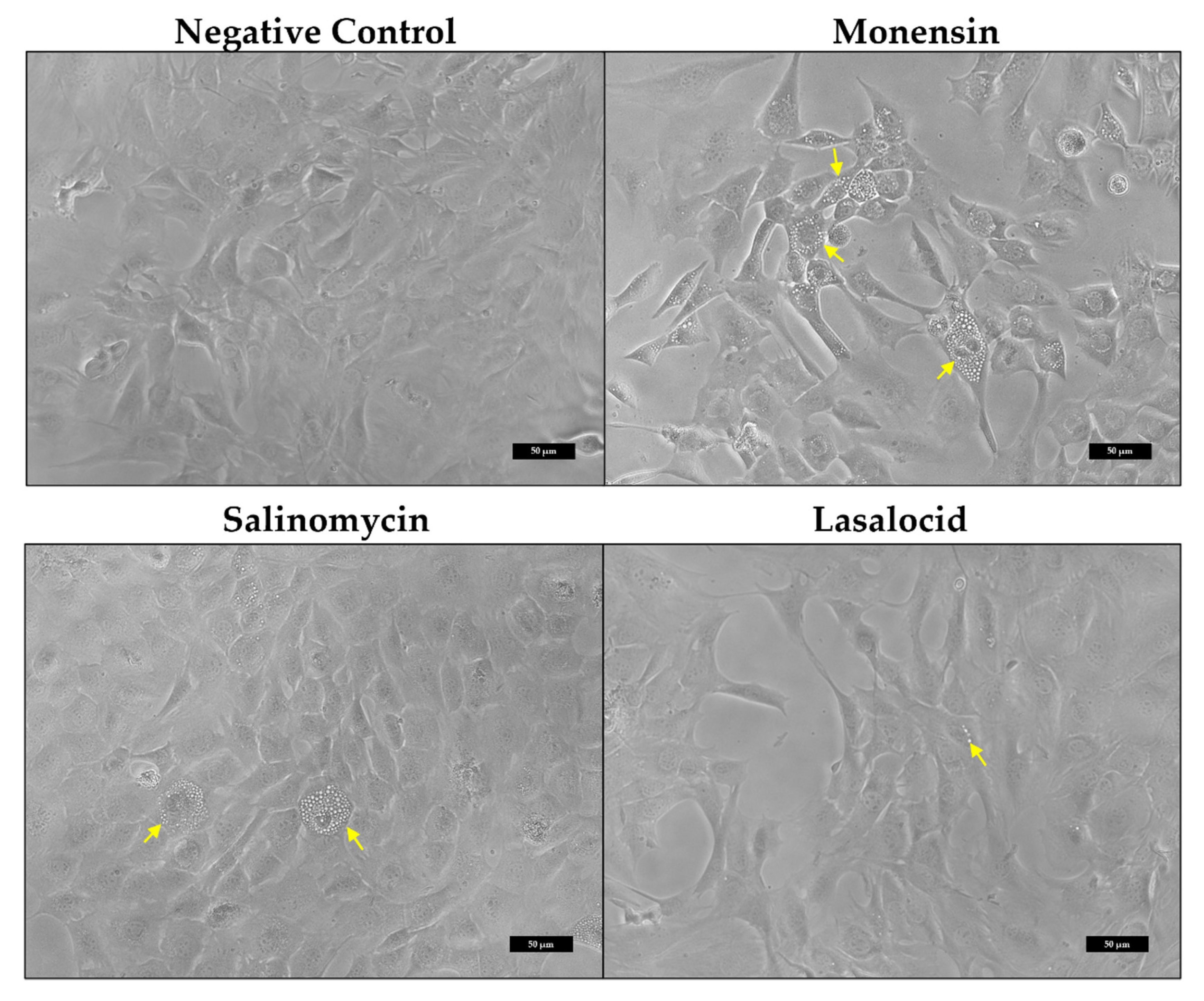
2.1.2. Morphological Alterations of C2C12 Myoblasts
2.2. The In Vitro Effect of Ionophores on Desmin and Synemin in C2C12 Myoblasts
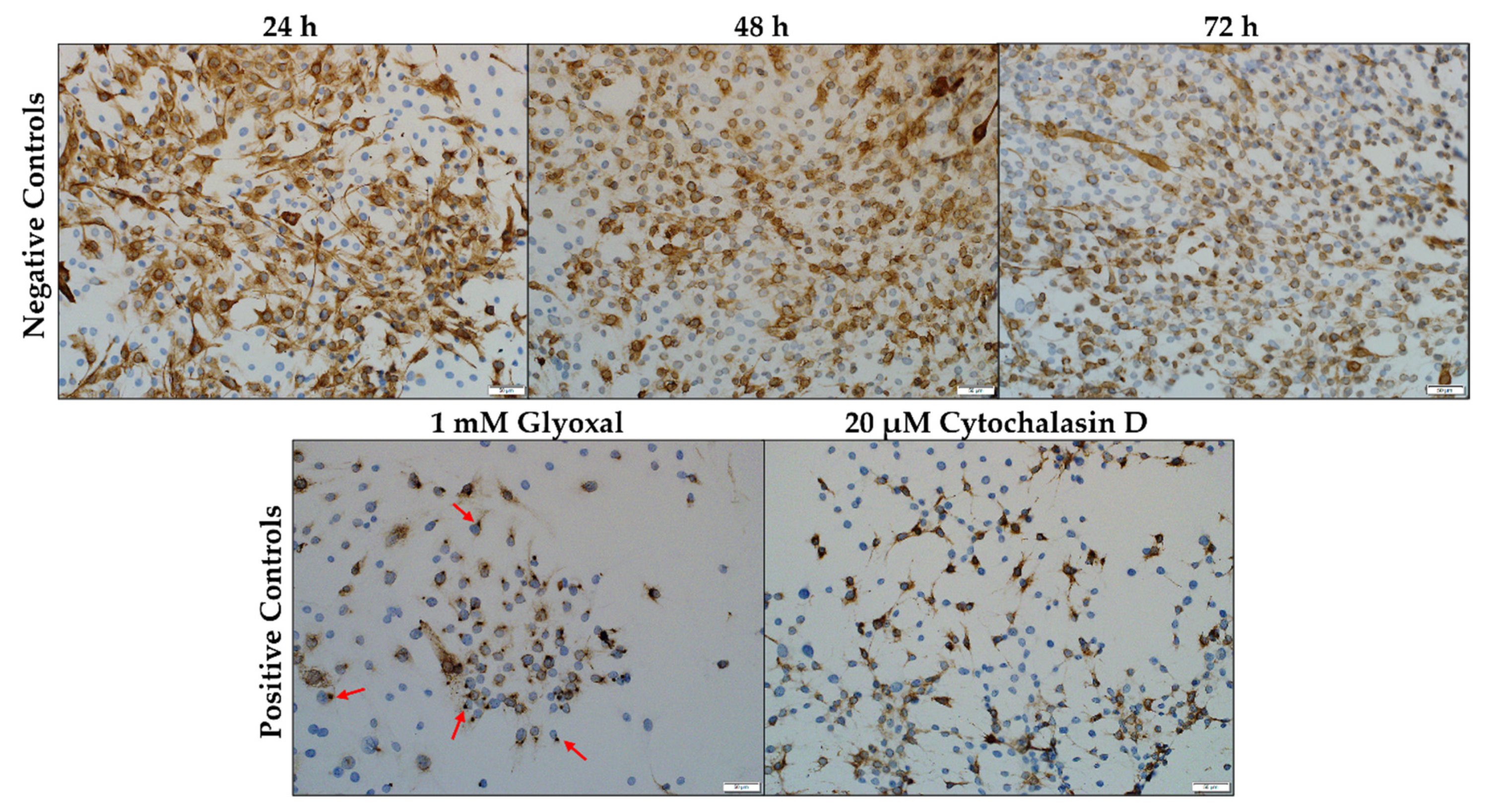
2.2.1. Desmin
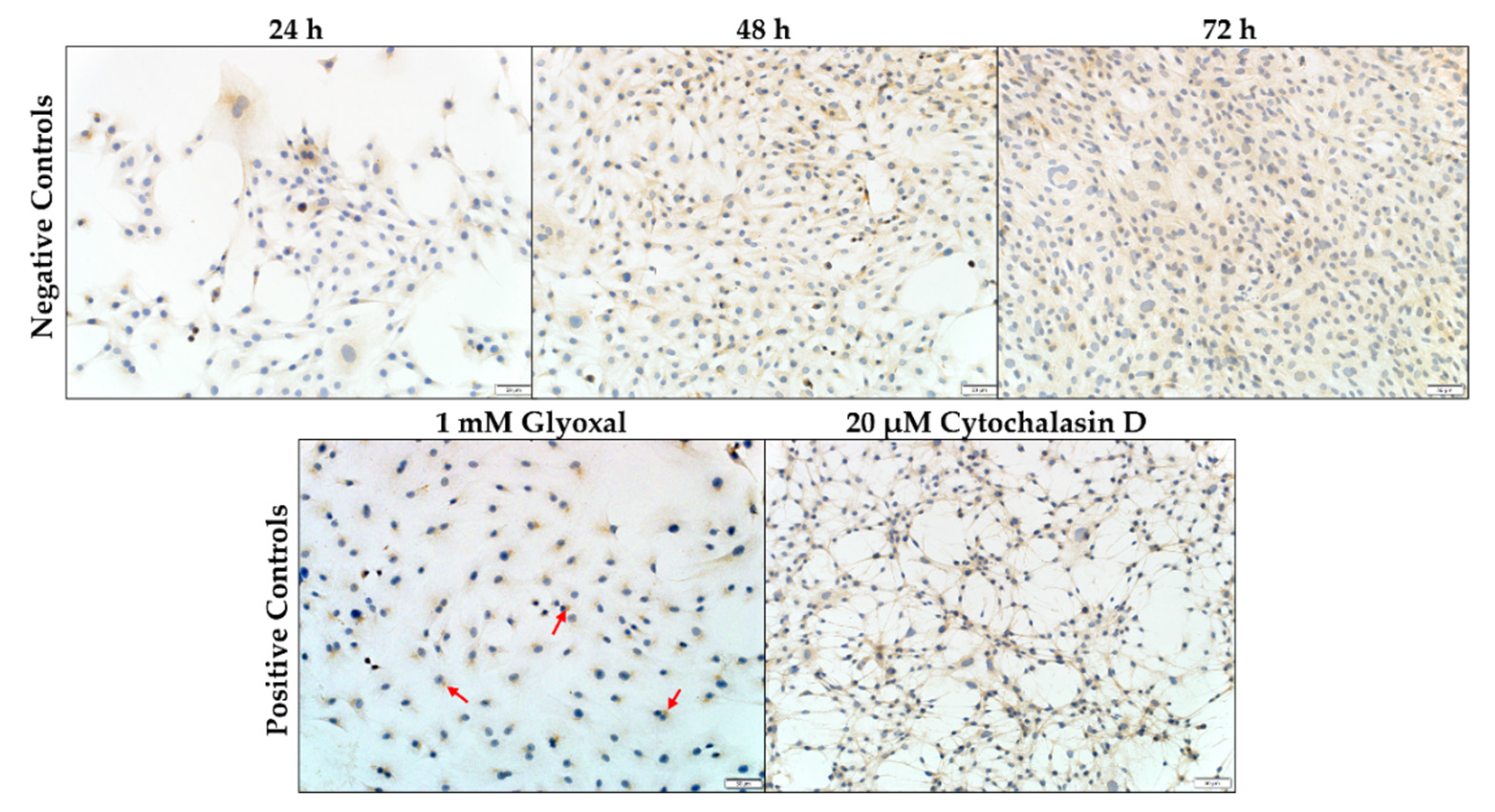
2.2.2. Synemin
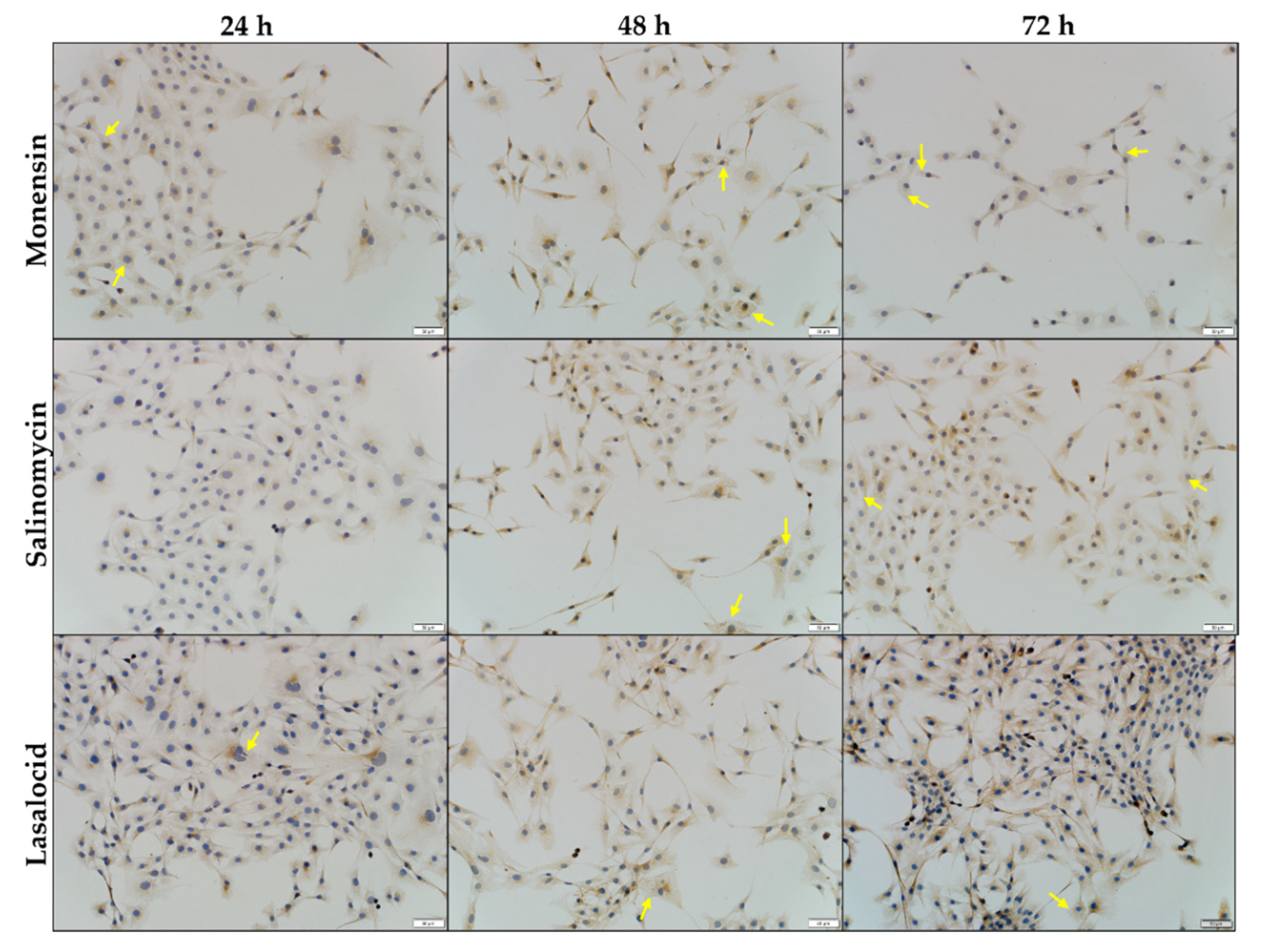
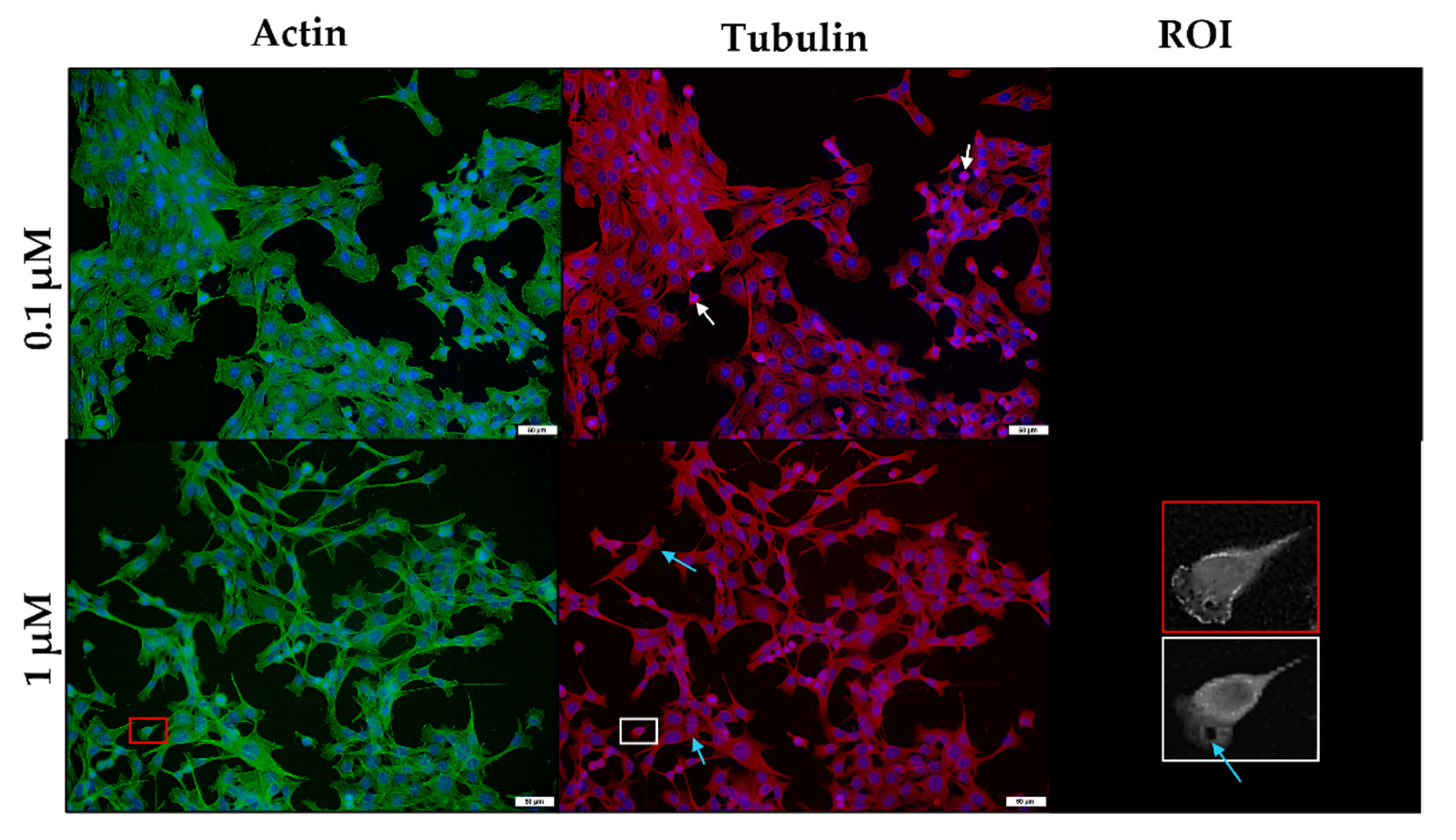
2.3. The In Vitro Effect of the Ionophores on the Microfilament and Microtubule Networks of C2C12 Myoblasts
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cell Culture
5.2. Exposure to the Ionophores
5.3. Cell viability Assay
5.4. Immunocytochemical Detection and Analysis of Desmin and Synemin Intermediate Filaments
5.5. Immunofluorescent Detection of Actin and β-Tubulin
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marques, R.D.S.; Cooke, R.F. Effects of Ionophores on Ruminal Function of Beef Cattle. Animals 2021, 11, 2871. [Google Scholar] [CrossRef]
- Meingassner, J.G.; Schmook, F.P.; Czok, R.; Mieth, H. Enhancement of the Anticoccidial Activity of Polyether Antibiotics in Chickens by Tiamulin. Poult. Sci. 1979, 58, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Novilla, M.N. Ionophores. In Veterinary Toxicology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1073–1092. [Google Scholar]
- Perry, T.W.; Beeson, W.M.; Mohler, M.T. Effect of Monensin on Beef Cattle Performance. J. Anim. Sci. 1976, 42, 761–765. [Google Scholar] [CrossRef]
- Weppelman, R.M.; Olson, G.; Smith, D.A.; Tamas, T.; Van Iderstine, A. Comparison of Anticoccidial Efficacy, Resistance and Tolerance of Narasin, Monensin and Lasalocid in Chicken Battery Trials. Poult. Sci. 1977, 56, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Aleman, M.; Magdesian, K.G.; Peterson, T.S.; Galey, F.D. Salinomycin toxicosis in horses. J. Am. Vet. Med. Assoc. 2007, 230, 1822–1826. [Google Scholar] [CrossRef]
- Ashrafihelan, J.; Eisapour, H.; Erfani, A.M.; Kalantary, A.A.; Amoli, J.S.; Mozafari, M. High mortality due to accidental salinomycin intoxication in sheep. Interdiscip. Toxicol. 2014, 7, 173–176. [Google Scholar] [CrossRef]
- Bastianello, S.S.; McGregor Heather, L.; Mary-Louise, P.; Fourie, N. A chronic cardiomyopathy in feedlot cattle at-tributed to toxic levels of salinomycin in the feed. J. S. Afr. Vet. Assoc. 1996, 67, 38–41. [Google Scholar]
- Britzi, M.; Shimshoni, J.; Edery, N.; Cuneah, O.; Younis, A.; Blech, E.; Oren, P.; Perl, S.; Pozzi, P. Acute salinomycin and maduramicin toxicosis in lactating sows. Isr. J. Vet. Med. 2017, 72, 42–48. [Google Scholar]
- Carpenter, J.A.; Charbonneau, G.; Josephson, G. Tiamulin and narasin toxicosis in nursery pigs. J. Swine Health Prod. 2005, 13, 333–336. [Google Scholar]
- Gy, C.; LeClere, M.; Bélanger, M.; Allano, M.; Beauchamp, G.; Lavoie, J. Acute, subacute and chronic sequelae of horses accidentally exposed to monensin-contaminated feed. Equine Vet. J. 2020, 52, 848–856. [Google Scholar] [CrossRef]
- Oruc, H.H.; Cangul, I.T.; Cengiz, M.; Yilmaz, R. Acute lasalocid poisoning in calves associated with off-label use. J. Vet. Pharmacol. Ther. 2010, 34, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Pakozdy, A.; Challande-Kathman, I.; Doherr, M.; Cizinauskas, S.; Wheeler, S.J.; Oevermann, A.; Jaggy, A. Retrospective study of salinomycin toxicosis in 66 cats. Vet. Med. Int. 2010, 2010, 147142. [Google Scholar] [CrossRef] [PubMed]
- Segev, G.; Baneth, G.; Levitin, B.; Aroch, I.; Shlosberg, I. Accidental poisoning of 17 dogs with lasalocid. Vet. Rec. 2004, 155, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Zavala, G.; Anderson, D.A.; Davis, J.F.; Dufour-Zavala, L. Acute Monensin Toxicosis in Broiler Breeder Chickens. Avian Dis. 2011, 55, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.O. Ionophore use and toxicosis in cattle. Vet. Clin. Food Anim. Pract. 2000, 16, 497–509. [Google Scholar] [CrossRef]
- Fourie, N.; Bastianello, S.S.; Prozesky, L.; Nel, P.W.; Kellerman, T.S. Cardiomyopathy of ruminants induced by the litter of poultry fed on rations containing the ionophore antibiotic, maduramicin. I. Epidemiology, clinical signs and clinical pathology. Onderstepoort J. Vet. Res. 1991, 58, 291–296. [Google Scholar]
- Galitzer, S.J.; Oehme, F.W.; Bartley, E.E.; Dayton, A.D. Lasalocid Toxicity in Cattle: Acute Clinicopathological Changes. J. Anim. Sci. 1986, 62, 1308–1316. [Google Scholar] [CrossRef]
- Gruys, E.; Perreira, C.; Bila, C. Accidental monensin toxicosis in horses in Mozambique. J. S. Afr. Vet. Assoc. 2001, 72, 163–164. [Google Scholar]
- Potter, E.; VanDuyn, R.; Cooley, C. Monensin toxicity in cattle. J. Anim. Sci. 1984, 58, 1499–1511. [Google Scholar] [CrossRef]
- Todd, G.C.; Novilla, M.N.; Howard, L.C. Comparative Toxicology of Monensin Sodium in Laboratory Animals. J. Anim. Sci. 1984, 58, 1512–1517. [Google Scholar] [CrossRef]
- Confer, A.W.; Reavis, D.U.; Panciera, R.J. Light and Electron Microscopic Changes in Cardiac and Skeletal Muscle of Sheep with Experimental Monensin Toxicosis. Vet. Pathol. 1983, 20, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Van Vleet, J.F.; Ferrans, V.J. Ultrastructural alterations in skeletal muscle of pigs with acute monensin myotoxicosis. Am. J. Pathol. 1984, 114, 461–471. [Google Scholar] [PubMed]
- Van Vleet, J.F.; Ferrans, V.J. Ultrastructural alterations in the atrial myocardium of pigs with acute monensin toxicosis. Am. J. Pathol. 1984, 114, 367–379. [Google Scholar]
- Agnetti, G.; Herrmann, H.; Cohen, S. New roles for desmin in the maintenance of muscle homeostasis. FEBS J. 2021, 289, 2755–2770. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.L.; Escaleira, R.; Cataldo, A.; Oliveira, F.; Mermelstein, C.S. Desmin: Molecular interactions and putative functions of the muscle intermediate filament protein. Braz. J. Med. Biol. Res. 2004, 37, 1819–1830. [Google Scholar] [CrossRef]
- Mermelstein, C.; Amaral, L.; Rebello, M.; Reis, J.; Borojevic, R.; Costa, M. Changes in cell shape and desmin intermediate filament distribution are associated with down-regulation of desmin expression in C2C12 myoblasts grown in the absence of extracellular Ca2+. Braz. J. Med. Biol. Res. 2005, 38, 1025–1032. [Google Scholar] [CrossRef]
- Paulin, D.; Li, Z. Desmin: A major intermediate filament protein essential for the structural integrity and function of muscle. Exp. Cell Res. 2004, 301, 1–7. [Google Scholar] [CrossRef]
- Capetanaki, Y. Desmin Cytoskeleton A Potential Regulator of Muscle Mitochondrial Behavior and Function. Trends Cardiovasc. Med. 2002, 12, 339–348. [Google Scholar] [CrossRef]
- Milner, D.J.; Mavroidis, M.; Weisleder, N.; Capetanaki, Y. Desmin cytoskeleton linked to muscle mitochondrial distribution and respiratory function. J. Cell Biol. 2000, 150, 1283–1298. [Google Scholar] [CrossRef]
- Lund, L.M.; Kerr, J.P.; Lupinetti, J.; Zhang, Y.; Russell, M.A.; Bloch, R.J.; Bond, M. Synemin isoforms differentially organize cell junctions and desmin filaments in neonatal cardiomyocytes. FASEB J. 2012, 26, 137–148. [Google Scholar] [CrossRef]
- Mizuno, Y.; Thompson, T.G.; Guyon, J.R.; Lidov, H.G.; Brosius, M.; Imamura, M.; Ozawa, E.; Watkins, S.C.; Kunkel, L.M. Desmuslin, an intermediate filament protein that interacts with α-dystrobrevin and desmin. Proc. Natl. Acad. Sci. USA 2001, 98, 6156–6161. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Guyon, J.R.; Watkins, S.C.; Mizushima, K.; Sasaoka, T.; Imamura, M.; Kunkel, L.M.; Okamoto, K. β-synemin localizes to regions of high stress in human skeletal myofibers. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2004, 30, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Pitre, A.; Davis, N.; Paul, M.; Orr, A.W.; Skalli, O. Synemin promotes AKT-dependent glioblastoma cell proliferation by antagonizing PP2A. Mol. Biol. Cell 2012, 23, 1243–1253. [Google Scholar] [CrossRef]
- Russell, M.A.; Lund, L.M.; Haber, R.; McKeegan, K.; Cianciola, N.; Bond, M. The intermediate filament protein, synemin, is an AKAP in the heart. Arch. Biochem. Biophys. 2006, 456, 204–215. [Google Scholar] [CrossRef]
- Robison, P.; Caporizzo, M.A.; Ahmadzadeh, H.; Bogush, A.I.; Chen, C.Y.; Margulies, K.B.; Shenoy, V.B.; Prosser, B.L. Detyrosinated microtubules buckle and bear load in contracting cardiomyocytes. Science 2016, 352, aaf0659. [Google Scholar] [CrossRef]
- Robison, P.; Prosser, B.L. Microtubule mechanics in the working myocyte. J. Physiol. 2017, 595, 3931–3937. [Google Scholar] [CrossRef] [PubMed]
- Gad, S.; Reilly, C.; Siino, K.; Gavigan, F.; Witz, G. Thirteen Cationic Ionophores: Their Acute Toxicity, Neurobehavioral and Membrane Effects. Drug Chem. Toxicol. 1985, 8, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Cybulski, W.; Radko, L.; Rzeski, W. Cytotoxicity of monensin, narasin and salinomycin and their interaction with silybin in HepG2, LMH and L6 cell cultures. Toxicol. Vitr. 2015, 29, 337–344. [Google Scholar] [CrossRef]
- Kiełbasiński, K.; Peszek, W.; Grabarek, B.O.; Boroń, D.; Wierzbik-Strońska, M.; Oplawski, M. Effect of Salinomycin on Expression Pattern of Genes Associated with Apoptosis in Endometrial Cancer Cell Line. Curr. Pharm. Biotechnol. 2020, 21, 1269–1277. [Google Scholar] [CrossRef]
- Iljin, K.; Ketola, K.; Vainio, P.; Halonen, P.; Kohonen, P.; Fey, V.; Grafström, R.C.; Perälä, M.; Kallioniemi, O. High-throughput cell-based screening of 4910 known drugs and drug-like small molecules identifies disulfiram as an inhibitor of prostate cancer cell growth. Clin. Cancer Res. 2009, 15, 6070–6078. [Google Scholar] [CrossRef]
- Tyagi, M.; Patro, B.S. Salinomycin reduces growth, proliferation and metastasis of cisplatin resistant breast cancer cells via NF-kB deregulation. Toxicol. Vitr. 2019, 60, 125–133. [Google Scholar] [CrossRef]
- Mollenhauer, H.H.; Morré, D.J.; Rowe, L.D. Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity. Biochim. Biophys. Acta BBA Rev. Biomembr. 1990, 1031, 225–246. [Google Scholar] [CrossRef]
- Przygodzki, T.; Sokal, A.; Bryszewska, M. Calcium ionophore A23187 action on cardiac myocytes is accompanied by enhanced production of reactive oxygen species. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2005, 1740, 481–488. [Google Scholar] [CrossRef]
- Olmsted, J.B.; Borisy, G.G. Ionic and nucleotide requirements for microtubule polymerization in vitro. Biochemistry 1975, 14, 2996–3005. [Google Scholar] [CrossRef] [PubMed]
- Weisenberg, R.C. Microtubule Formation in vitro in Solutions Containing Low Calcium Concentrations. Science 1972, 177, 1104–1105. [Google Scholar] [CrossRef]
- Kanje, M.; Edstro¨m, A.; Hanson, M. Inhibition of rapid axonal transport in vitro by the ionophores X-537 A and A 23187. Brain Res. 1981, 204, 43–50. [Google Scholar] [CrossRef]
- Boehmerle, W.; Endres, M. Salinomycin induces calpain and cytochrome c-mediated neuronal cell death. Cell Death Dis. 2011, 2, e168. [Google Scholar] [CrossRef]
- Duncan, C.; Smith, J.; Greenaway, H.C. Failure to protect frog skeletal muscle from ionophore-induced damage by the use of the protease inhibitor leupeptin. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1979, 63, 205–207. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Botha, C.J.; Venter, E.A.; Ferreira, G.C.; Phaswane, R.M.; Clift, S.J. Geigerin-induced disorganization of desmin, an intermediate filament of the cytoskeleton, in a murine myoblast cell line (C2C12). Toxicon 2019, 167, 162–167. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, R. Extensions of DAMAS and Benefits and Limitations of Deconvolution in Beamforming. In Proceedings of the 11th AIAA/CEAS Aeroacoustics Conference, Monterey, CA, USA, 23–25 May 2005; p. 2961. [Google Scholar]
- Alexander, B.; Browse, D.; Reading, S.; Benjamin, I. A simple and accurate mathematical method for calculation of the EC50. J. Pharmacol. Toxicol. Methods 1999, 41, 55–58. [Google Scholar] [CrossRef]





| Monensin | Salinomycin | Lasalocid | ||
|---|---|---|---|---|
| C2C12 cells | 24 h | † (n = 5) | 4.04 ± 1.01 (n = 5) a | 8.68 ± 5.38 (n = 4) a |
| 48 h | 0.04 ± 0.01 (n = 5) c | 0.45 ± 0.13 (n = 5) b,d | 1.38 ± 0.54 (n = 4) b,d | |
| 72 h | 0.02 ± 0.01 (n = 5) c | 0.26 ± 0.06 (n = 5) b,d | 1.46 ± 0.59 (n = 4) b,d | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henn, D.; Venter, A.; Ferreira, G.C.H.; Botha, C.J. The In Vitro Cytotoxic Effects of Ionophore Exposure on Selected Cytoskeletal Proteins of C2C12 Myoblasts. Toxins 2022, 14, 447. https://doi.org/10.3390/toxins14070447
Henn D, Venter A, Ferreira GCH, Botha CJ. The In Vitro Cytotoxic Effects of Ionophore Exposure on Selected Cytoskeletal Proteins of C2C12 Myoblasts. Toxins. 2022; 14(7):447. https://doi.org/10.3390/toxins14070447
Chicago/Turabian StyleHenn, Danielle, Annette Venter, Gezina C. H. Ferreira, and Christo J. Botha. 2022. "The In Vitro Cytotoxic Effects of Ionophore Exposure on Selected Cytoskeletal Proteins of C2C12 Myoblasts" Toxins 14, no. 7: 447. https://doi.org/10.3390/toxins14070447
APA StyleHenn, D., Venter, A., Ferreira, G. C. H., & Botha, C. J. (2022). The In Vitro Cytotoxic Effects of Ionophore Exposure on Selected Cytoskeletal Proteins of C2C12 Myoblasts. Toxins, 14(7), 447. https://doi.org/10.3390/toxins14070447





