Intraspecific Differences in the Venom of Crotalus durissus cumanensis from Colombia
Abstract
:1. Introduction
2. Results and Discussion
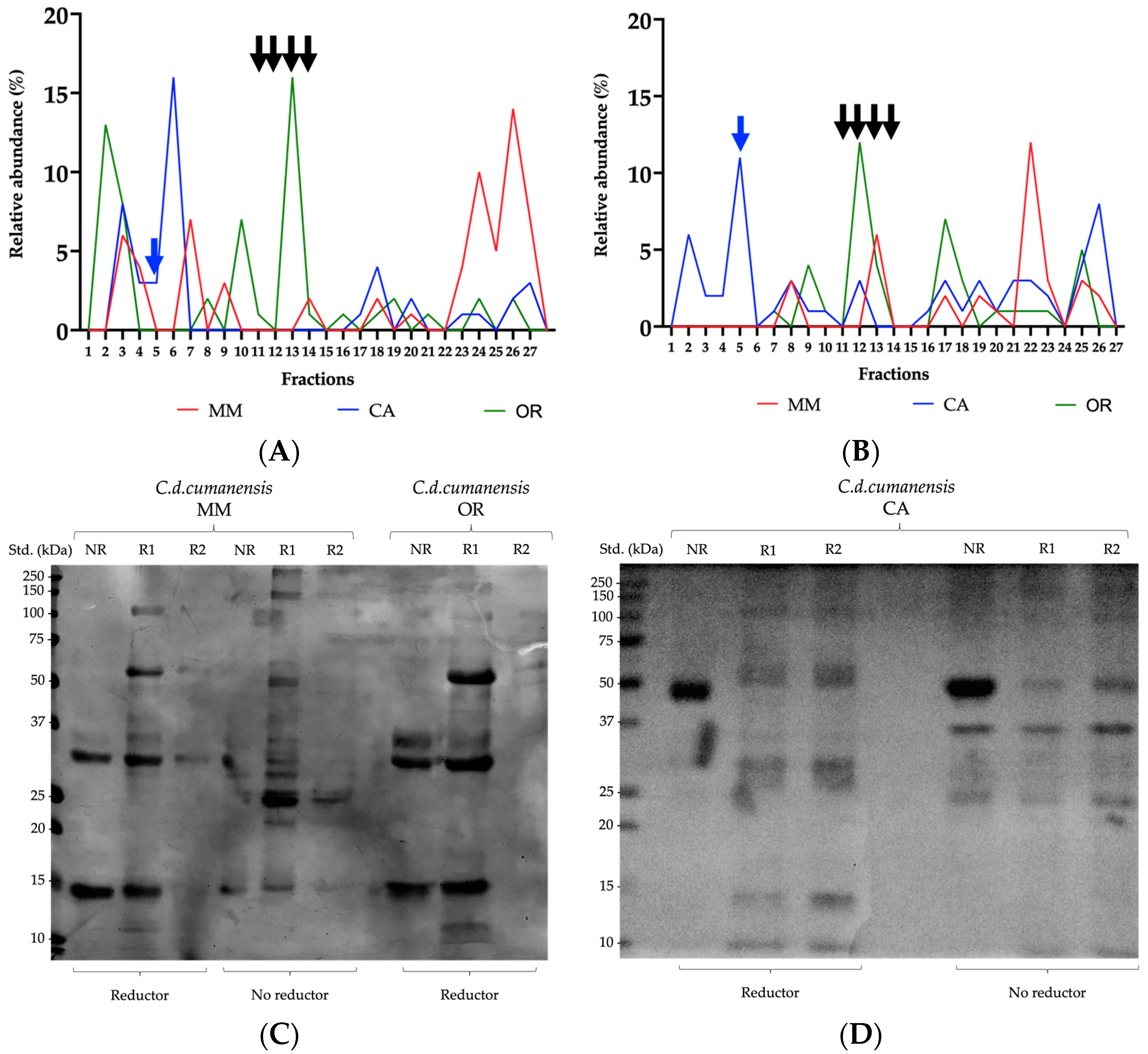
2.1. Phospholipases A2 and Low Molecular Weight Myotoxins as Hallmarks in Protein Profiles
2.2. Heterogeneous Enzyme Activity and Related Cytotoxicity
2.3. Toxic Activity to Reinforce the Differentiation among the Three Venoms
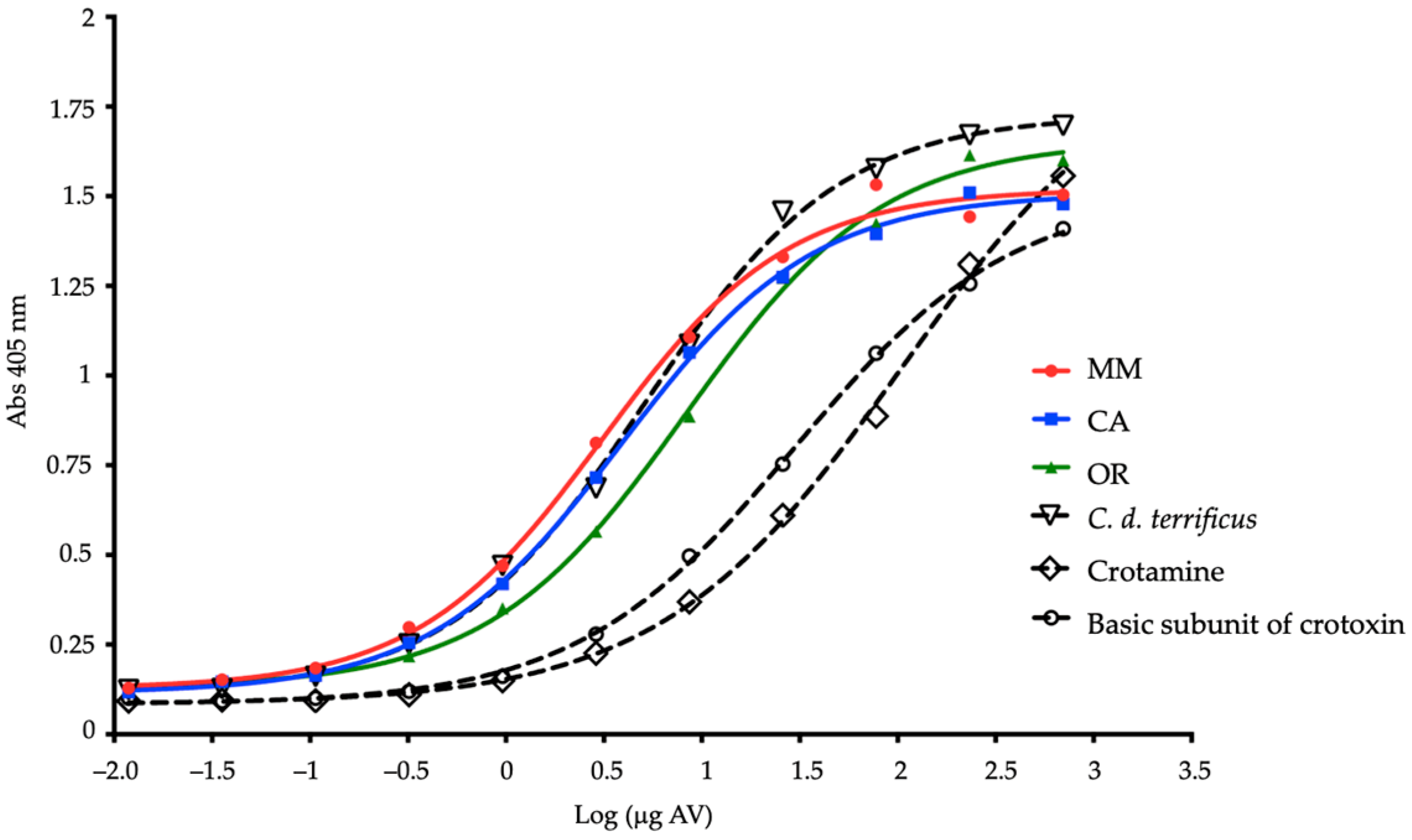
2.4. Recognition of Complete Venoms and Some of Their Fractions by Antivenoms
3. Conclusions
4. Materials and Methods
4.1. Venoms and Antivenoms
4.2. Animals
4.3. Protein Quantification
4.4. Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.5. Reverse Phase High-Performance Liquid Chromatography (RP-HPLC)
4.6. Biological Activities
4.6.1. Median Lethal Dose (LD50)
4.6.2. Minimum Defibrinating Dose (MDD)
4.6.3. Minimum Coagulant Dose (MCD)
4.6.4. Cytotoxicity
4.7. Enzimatic Activities
4.7.1. Phospholipase A2 Activity
4.7.2. Hyaluronidase Activity
4.7.3. Protease Activity
4.8. Antivenom Evaluation
4.8.1. Determination of Median Effective Dose (ED50)
4.8.2. Affinity Chromatography
4.8.3. Western Blotting
4.8.4. Enzyme-Linked Immunosorbent Assay—ELISA
4.9. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uetz, P.; Freed, P.; Aguilar, R.; Hošek, J. The Reptile Database. Available online: https://reptile-database.reptarium.cz/species?genus=Crotalus&species=durissus (accessed on 14 June 2022).
- Lynch, J. El contexto de las serpientes de Colombia con un análisis de las amenazas en contra de su conservación. Rev. Acad. Colomb. 2012, 36, 435–449. [Google Scholar]
- Ayerbe, S. Ofidismo en Colombia. Enfoque, diagnóstico y tratamiento. In Cuidado Intensivo Y Trauma; Ordóñez, C., Ferrada, R., Buitrago, R., Eds.; Distribuna: Bogotá, Colombia, 2009; pp. 1143–1168. ISBN 978-958-8379-14-2. [Google Scholar]
- Lynch, J.; Angarita-Sierra, T.; Ruiz-Gómez, F. Programa Nacional para la Conservación de las Serpientes Presentes en Colombia; Ministerio de Ambiente y Desarrollo Sostenible: Bogotá, Colombia; Universidad Nacional de Colombia, Instituto Nacional de Salud: Bogotá, Colombia, 2014; ISBN 978-958-8901-18-3. [Google Scholar]
- Carbajal-Márquez, R.A.; Cedeño-Vázquez, J.R.; Martínez-Arce, A.; Neri-Castro, E.; Machkour-M’Rabet, S.C. Accessing cryptic diversity in Neotropical rattlesnakes (Serpentes: Viperidae: Crotalus) with the description of two new species. Zootaxa 2020, 4729, 451–481. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J.; Yang, D.C.; op den Brouw, B.; Cochran, C.; Huynh, T.; Kurrupu, S.; Sánchez, E.; Massey, D.J.; Baumann, K.; Jackson, T.N.; et al. Rattling the border wall: Pathophysiological implications of functional and proteomic venom variation between Mexican and US subspecies of the desert rattlesnake Crotalus scutulatus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2018, 205, 62–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segura, Á.; Herrera, M.; Reta Mares, F.; Jaime, C.; Sánchez, A.; Vargas, M.; Villalta, M.; Gómez, A.; Gutiérrez, J.M.; León, G. Proteomic, toxicological and immunogenic characterization of Mexican west-coast rattlesnake (Crotalus basiliscus) venom and its immunological relatedness with the venom of Central American rattlesnake (Crotalus simus). J. Proteom. 2017, 158, 62–72. [Google Scholar] [CrossRef]
- Dowell, N.L.; Giorgianni, M.W.; Griffin, S.; Kassner, V.A.; Selegue, J.E.; Sanchez, E.E.; Carroll, S.B. Extremely Divergent Haplotypes in Two Toxin Gene Complexes Encode Alternative Venom Types within Rattlesnake Species. Curr. Biol. 2018, 28, 1016–1026. [Google Scholar] [CrossRef] [Green Version]
- Neri-Castro, E.; Hernández-Dávila, A.; Olvera-Rodríguez, A.; Cardoso-Torres, H.; Bénard-Valle, M.; Bastiaans, E.; López-Gutierrez, O.; Alagón, A. Detection and quantification of a β-neurotoxin (crotoxin homologs) in the venom of the rattlesnakes Crotalus simus, C. culminatus and C. tzabcan from Mexico. Toxicon X 2019, 2, 100007. [Google Scholar] [CrossRef]
- Faure, G.; Porowinska, D.; Saul, F. Crotoxin from Crotalus durissus terrificus and Crotoxin-Related Proteins: Structure and Function Relationship. In Toxins and Drug Discovery; Gopalakrishnakone, P., Cruz, L.J., Luo, S., Eds.; Springer: Dordrecht, The Netherlands, 2017; Chapter 1; pp. 3–20. ISBN 978-94-007-6452-1. [Google Scholar]
- Yoshida-Kanashiro, E.; Navarrete, L.F.; Rodríguez-Acosta, A. On the unusual hemorrhagic and necrotic activities caused by the rattlesnake (Crotalus durissus cumanensis) in a Venezuelan patient. Rev. Cuba. Med. Trop. 2003, 55, 38–40. [Google Scholar]
- Calvete, J.; Sanz, L.; Cid, P.; De La Torre, P.; Flores-Díaz, M.; dos Santos, M.; Borges, A.; Bremo, A.; Angulo, Y.; Lomonte, B.; et al. Snake Venomics of the Central American Rattlesnake Crotalus simus and the South American Crotalus durissus Complex Points to Neurotoxicity as an Adaptive Paedomorphic Trend along Crotalus Dispersal in South America. J. Proteome Res. 2010, 9, 528–544. [Google Scholar] [CrossRef]
- Silveira, P.V.P.; Nishioka, S.d.A. South american rattlesnake bite in a brazilian teaching hospital. clinical and epidemiological study of 87 cases, with analysis of factors predictive of renal failure. Trans. R. Soc. Trop. Med. Hyg. 1992, 86, 562–564. [Google Scholar] [CrossRef]
- de Azevedo, M.; Hering, S.; Cupo, P. Accidente Crotálico. In Animais Peçonhentos No Brasil2; Cardoso, J., de Siqueira, F., Wen, F., Sant´Ana, C., Haddad, V., Eds.; Sarvier: São Paulo, Brazil, 2009; pp. 108–115. ISBN 978-85-7378-194-6. [Google Scholar]
- Castaño, S. Informe del Evento: Accidente Ofídico, Colombia, Report. 2019; pp. 1–18. Available online: https://www.ins.gov.co/buscador-eventos/Informesdeevento/ACCIDENTE_OFÍDICIO_2019.pdf (accessed on 5 May 2022).
- Urieles, K. Informe del Evento: Accidente Ofídico, Colombia, Report. 2020; pp. 1–19. Available online: https://www.ins.gov.co/buscador-eventos/Informesdeevento/ACCIDENTE%20OFÍDICO_2020.pdf (accessed on 5 May 2022).
- Gómez, J. Informe del Evento: Accidente Ofídico, Colombia, Report. 2021; pp. 1–2. Available online: https://www.ins.gov.co/buscador-eventos/Informesdeevento/ACCIDENTE%20OFIDICO%20PE%20XIII%202021.pdf (accessed on 5 May 2022).
- Chippaux, J.P.; Goyffon, M. Venoms, antivenoms and immunotherapy. Toxicon 1998, 36, 823–846. [Google Scholar] [CrossRef]
- WHO. WHO Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins; WHO, Ed.; World Health Organization Press: Geneva, Switzerland, 2010.
- Isbister, G.K. Antivenom efficacy or effectiveness: The Australian experience. Toxicology 2010, 268, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Salud. Suero Antiofídico Polivalente INS, Colombia, Medication Package Insert. 2017; pp. 1–2. Available online: https://www.ins.gov.co/lineas-de-accion/Produccion/SiteAssets/Paginas/suero-antiofidico-polivalente/Inserto%20Suero%20Antiof%C3%ADdico%20Polivalente.pdf (accessed on 3 February 2022).
- Otero-Patiño, R.; Silva-Hadad, J.; Barona, M.; Toro, M.; Quintana, J.; Díaz, A.; Vásquez, I.; Rodríguez, V.; Delgado, C.; Fernández, M.; et al. Accidente bothrópico en Colombia: Estudio multicéntrico de la eficacia, y seguridad de Antivipmyn-Tri® un antiveneno polivalente producido en México. Iatreia 2007, 20, 244–262. [Google Scholar]
- Instituto Bioclon. Antivipmyn®Tri, México, Full Prescribing Information (FPI). 2016, pp. 1–7. Available online: https://archiveansm.integra.fr/afssaps/content/download/149311/1964979/version/2/file/FINAL_Antivipmyn+Tri+IPP-A_sep2016_ENG.pdf (accessed on 3 February 2022).
- Gómez-Cardona, J.; Gómez-Cabal, C.; Gómez-Cabal, M.L. Sueros Antiofídicos En Colombia: Análisis De La Producción, Abastecimiento Y Recomendaciones Para El Mejoramiento De La Red De Producción. Biosalud 2017, 16, 96–116. [Google Scholar]
- Aguilar, I.; Guerrero, B.; Salazar, A.M.; Giro, M.E.; Peez, J.C.; Sachez, E.E.; Rodrıuez-Acosta, A. Individual venom variability in the South American rattlesnake Crotalus durissus cumanensis. Toxicon 2007, 50, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Salazar, A.; Aguilar, I.; Guerrero, B.; Giron, M.; Lucena, S.; Sanchez, E.; Rodriguez-Acosta, A. Intraspecies differences in hemostatic venom activities of the South American rattlesnakes, Crotalus durissus cumanensis, as revealed by a range of protease inhibitors. Blood Coagul. Fibrinol. 2008, 19, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Garrido Cavalcante, W.L.; Ponce-Soto, L.A.; Marangoni, S.; Gallacci, M. Neuromuscular effects of venoms and crotoxin-like proteins from Crotalus durissus ruruima and Crotalus durissus cumanensis. Toxicon 2015, 96, 46–49. [Google Scholar] [CrossRef]
- Pirela, R.; López-Jonsthon, J.; Hernández, J. Caracterización Toxinológica del Veneno Total de la Serpiente de Cascabel Crotalus durissus cumanensis (VIPERIDAE), presente en la localidad de Porshoure, Guajira Venezolana. Rev. Cient. 2006, 16, 232–238. [Google Scholar]
- Céspedes, N.; Castro, F.; Jiménez, E.; Montealegre, L.; Castellanos, A.; Cañas, C.; Arévalo-Herrera, M.; Herrera, S. Biochemical comparison of venoms from young Colombian Crotalus durissus cumanensis and their parents. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 268–284. [Google Scholar] [CrossRef]
- Arévalo-Páez, M.; Rada-Vargas, E.; Betancur-Hurtado, C.; Renjifo, J.M.; Renjifo-Ibáñez, C. Neuromuscular effect of venoms from adults and juveniles of Crotalus durissus cumanensis (Humboldt, 1811) from Guajira, Colombia. Toxicon 2017, 139, 41–44. [Google Scholar] [CrossRef]
- Quintana-Castillo, J.C.; Vargas, L.J.; Segura, C.; Estrada-Gómez, S.; Bueno-Sánchez, J.C.; Alarcón, J.C. Characterization of the Venom of C. d. cumanesis of Colombia: Proteomic Analysis and Antivenomic Study. Toxins 2018, 10, 85. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Peña, A.; Núñez, V.; Pereañez, J.A.; Rey-Suárez, P. Immunorecognition and Neutralization of Crotalus durissus cumanensis Venom by a Commercial Antivenom Produced in Colombia. Toxins 2022, 14, 235. [Google Scholar] [CrossRef] [PubMed]
- Vargas, L.J.; Quintana, J.C.; Pereañez, J.; Núñez, V.; Sanz, L.; Calvete, J. Cloning and characterization of an antibacterial l-amino acid oxidase from Crotalus durissus cumanensis venom. Toxicon 2013, 64, 1–11. [Google Scholar] [CrossRef]
- Patiño, A.C.; Pereañez, J.; Gutiérrez, J.M.; Rucavado, A. Biochemical and biological characterization of two serine proteinases from Colombian Crotalus durissus cumanensis snake venom. Toxicon 2013, 63, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Castillo, J.C.; Ávila-Gómez, I.C.; Ceballos-Ruiz, J.F.; Vargas-Muñoz, L.J.; Estrada-Gómez, S. Efecto citotóxico de fosfolipasas A2 del veneno de Crotalus durissus cumanensis de Colombia. Rev. Investig. Salud Univ. Boyacá 2017, 4, 16–37. [Google Scholar] [CrossRef] [Green Version]
- Quintana, J.C.; Chacón, A.M.; Vargas, L.; Segura, C.; Gutiérrez, J.M.; Alarcón, J.C. Antiplasmodial effect of the venom of Crotalus durissus cumanensis, crotoxin complex and Crotoxin B. Acta Trop. 2012, 124, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Pereañez, J.A.; Núñez, V.; Huancahuire-Vega, S.; Marangoni, S.; Ponce-Soto, L.A. Biochemical and biological characterization of a PLA2 from crotoxin complex of Crotalus durissus cumanensis. Toxicon 2009, 53, 534–542. [Google Scholar] [CrossRef]
- Mackessy, S. The Field of Reptile Toxinology. In Handbook of Venoms and Toxins of Reptiles; Mackessy, S., Ed.; CRC Press: Boca Raton, FL, USA, 2010; Chapter 1; pp. 1–21. [Google Scholar]
- Hanley, B. Meta-analysis of venom toxicity of 167 most lethal ophidian species provides a basis for estimating human lethal doses. Res. Sq. 2020, 1–31. [Google Scholar] [CrossRef]
- Mejía-Sánchez, M.A.; Clement, H.; Corrales-García, L.L.; Olamendi-Portugal, T.; Carbajal, A.; Corzo, G. Crotoxin B: Heterologous Expression, Protein Folding, Immunogenic Properties, and Irregular Presence in Crotalid Venoms. Toxins 2022, 14, 382. [Google Scholar]
- Roldán-Padrón, O.; Castro-Guillén, J.; García-Arredondo, J.; Cruz-Pérez, M.; Díaz-Peña, L.; Saldaña, C.; Blanco-Labra, A.; García-Gasca, T. Snake Venom Hemotoxic Enzymes: Biochemical Comparison between Crotalus Species from Central Mexico. Molecules 2019, 24, 1489. [Google Scholar] [CrossRef] [Green Version]
- Olaoba, O.T.; Karina dos Santos, P.; Selistre-de-Araujo, H.S.; Ferreira de Souza, D.H. Snake Venom Metalloproteinases (SVMPs): A structure-function update. Toxicon X 2020, 7, 100052. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Escalante, T.; Rucavado, A.; Herrera, C.; Fox, J.W. A comprehensive view of the structural and functional alterations of extracellular matrix by snake venom metalloproteinases (SVMPs): Novel perspectives on the pathophysiology of envenoming. Toxins 2016, 8, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, J.M.; Escalante, T.; Rucavado, A.; Herrera, C. Hemorrhage caused by snake venom metalloproteinases: A journey of discovery and understanding. Toxins 2016, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Bordon, K.C.F.; Perino, M.G.; Giglio, J.R.; Arantes, E.C. Isolation, enzymatic characterization and antiedematogenic activity of the first reported rattlesnake hyaluronidase from Crotalus durissus terrificus venom. Biochimie 2012, 94, 2740–2748. [Google Scholar] [CrossRef] [PubMed]
- Vivas-Ruiz, D.E.; Gonzalez-Kozlova, E.E.; Delgadillo, J.; Palermo, P.M.; Sandoval, G.A.; Lazo, F.; Rodríguez, E.; Chávez-Olórtegui, C.; Yarlequé, A.; Sanchez, E.F. Biochemical and molecular characterization of the hyaluronidase from Bothrops atrox Peruvian snake venom. Biochimie 2019, 162, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, J.P.; Paloschi, M.V.; Pontes, A.S.; Boeno, C.N.; Lopes, J.A.; Setubal, S.S.; Zanchi, F.B.; Soares, A.M. Reptile Venom L-Amino Acid Oxidases—Structure and Function. In Handbook of Venoms and Toxins of Reptiles; Mackessy, S., Ed.; CRC Press: Boca Raton, FL, USA, 2021; Volume 2, Chapter 27; pp. 413–430. [Google Scholar]
- Serrano, S.M.T. The long road of research on snake venom serine proteinases. Toxicon 2013, 62, 19–26. [Google Scholar] [CrossRef]
- Swenson, S.D.; Stack, S.; Markland, F., Jr. Thrombin-Like Serine Proteinases in Reptile Venoms. In Handbook of Venoms and Toxins of Reptiles; Mackessy, S., Ed.; CRC Press: Boca Raton, FL, USA, 2021; Chapter 23; pp. 370–381. [Google Scholar]
- Kang, T.S.; Georgieva, D.; Genov, N.; Murakami, M.T.; Sinha, M.; Kumar, R.P.; Kaur, P.; Kumar, S.; Dey, S.; Sharma, S.; et al. Enzymatic toxins from snake venom: Structural characterization and mechanism of catalysis. FEBS J. 2011, 278, 4544–4576. [Google Scholar] [CrossRef]
- Adade, C.; Carvalho, A.; Tomaz, M.; Costa, T.; Godinho, J.; Melo, P.; Lima, A.P.; Rodrigues, J.; Zingali, R.; Souto-Padrón, T. Crovirin, a Snake Venom Cysteine-Rich Secretory Protein (CRISP) with Promising Activity against Trypanosomes and Leishmania. PLoS Negl. Trop. Dis. 2014, 8, e3252. [Google Scholar] [CrossRef]
- Hamako, J.; Suzuki, Y.; Hayashi, N.; Kimura, M.; Ozeki, Y.; Hashimoto, K.; Matsui, T. Amino acid sequence and characterization of C-type lectin purified from the snake venom of Crotalus ruber. Comp. Biochem. Physiol.-B Biochem. Mol. Biol. 2007, 146, 299–306. [Google Scholar] [CrossRef]
- Walker, J.R.; Nagar, B.; Young, N.M.; Hirama, T.; Rini, J.M. X-ray Crystal Structure of a Galactose-Specific C-Type Lectin Possessing a Novel Decameric Quaternary Structure. Biochemistry 2004, 43, 3783–3792. [Google Scholar] [CrossRef]
- Ferreira, I.G.; Pucca, M.B.; de Oliveira, I.S.; Cerni, F.A.; Jacob, B.d.C.d.S.; Arantes, E.C. Snake venom vascular endothelial growth factors (svVEGFs): Unravelling their molecular structure, functions, and research potential. Cytokine Growth Factor Rev. 2021, 60, 133–143. [Google Scholar] [CrossRef]
- Rivas-Mercado, E.; Garza-Ocañas, L. Disintegrins obtained from snake venom and their pharmacological potential. Med. Univ. 2017, 19, 32–37. [Google Scholar] [CrossRef]
- Angulo, Y.; Castro, A.; Lomonte, B.; Rucavado, A.; Fernández, J.; Calvete, J.; Gutiérrez, J.M. Isolation and characterization of four medium-size disintegrins from the venoms of Central American viperid snakes of the genera Atropoides, Bothrops, Cerrophidion and Crotalus. Biochimie 2014, 107, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Toyama, M.H.; Carneiro, E.M.; Marangoni, S.; Barbosa, R.L.; Corso, G.; Boschero, A.C. Biochemical characterization of two crotamine isoforms isolated by a single step RP-HPLC from Crotalus durissus terrificus (South American rattlesnake) venom and their action on insulin secretion by pancreatic islets. Biochim. Biophys. Acta-Gen. Subj. 2000, 1474, 56–60. [Google Scholar] [CrossRef]
- Marinovic, M.; Dal Mas, C.; Monte, G.; Felix, D.; Campeiro, J.; Hayashi, M. Crotamine: Function Diversity and Potential Applications. In Snake Venoms. Toxinology; Gopalakrishnakone, P., Inagaki, H., Vogel, C., Mukherjee, A., Rahmy, T., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 265–293. ISBN 978-94-007-6409-5. [Google Scholar]
- Sarray, S.; Luis, J.; Ayeb, M.E.; Marrakchi, N. Snake Venom Peptides: Promising Molecules with Anti-Tumor Effects. In Bioactive Food Peptides in Health and Disease; Hernandez, B., Hsieh, C., Eds.; IntechOpen: London, UK, 2013; pp. 219–238. ISBN 978-953-51-0964-8. [Google Scholar]
- Camargo, A.C.M.; Ianzer, D.; Guerreiro, J.R.; Serrano, S.M.T. Bradykinin-potentiating peptides: Beyond captopril. Toxicon 2012, 59, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Lambeau, G.; Lazdunski, M. Receptors for a growing family of secreted phospholipases A2. Trends Pharmacol. Sci. 1999, 20, 162–170. [Google Scholar] [CrossRef]
- Neri-Castro, E.; Lomonte, B.; del Gutiérrez, M.; Alagón, A.; Gutiérrez, J. Intraspecies variation in the venom of the rattlesnake Crotalus simus from Mexico: Different expression of crotoxin results in highly variable toxicity in the venoms of three subspecies. J. Proteom. 2013, 87, 103–121. [Google Scholar] [CrossRef]
- Lomonte, B.; Calvete, J. Strategies in “snake venomics” aiming at an integrative view of compositional, functional, and immunological characteristics of venoms. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 26. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, J.M.; Lomonte, B.; Sanz, L.; Calvete, J.; Pla, D. Immunological profile of antivenoms: Preclinical analysis of the efficacy of a polyspecific antivenom through antivenomics and neutralization assays. J. Proteom. 2014, 105, 340–350. [Google Scholar] [CrossRef]
- Coutinho-Neto, A.; Caldeira, C.A.S.; Souza, G.H.M.F.; Zaqueo, K.D.; Kayano, A.M.; Silva, R.S.; Zuliani, J.P.; Soares, A.M.; Stábeli, R.G.; Calderon, L.A. ESI-MS/MS identification of a bradykinin-potentiating peptide from Amazon Bothrops atrox Snake Venom using a hybrid Qq-oaTOF mass spectrometer. Toxins 2013, 5, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Munawar, A.; Zahid, A.; Negm, A.; Akrem, A.; Spencer, P.; Betzel, C. Isolation and characterization of Bradykinin potentiating peptides from Agkistrodon bilineatus venom. Proteome Sci. 2016, 14, 1. [Google Scholar] [CrossRef] [Green Version]
- Oguiura, N.; Collares, M.A.; Furtado, M.F.D.; Ferrarezzi, H.; Suzuki, H. Intraspecific variation of the crotamine and crotasin genes in Crotalus durissus rattlesnakes. Gene 2009, 446, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Tasima, L.J.; Serino-Silva, C.; Hatakeyama, D.M.; Nishiduka, E.S.; Tashima, A.K.; Sant’Anna, S.S.; Grego, K.F.; De Morais-Zani, K.; Tanaka-Azevedo, A.M. Crotamine in Crotalus durissus: Distribution according to subspecies and geographic origin, in captivity or nature. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cristina, R.; Kocsis, R.; Tulcan, C.; Alexa, E.; Boldura, O.; Hulea, C.; Dumitrescu, E.; Radulov, I.; Muselin, F. Protein structure of the venom in nine species of snake: From bio-compounds to possible healing agents. Braz. J. Med. Biol. Res. 2020, 53, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.W. A brief review of the scientific history of several lesser-known snake venom proteins: L-amino acid oxidases, hyaluronidases and phosphodiesterases. Toxicon 2013, 62, 75–82. [Google Scholar] [CrossRef]
- Bickler, P. Amplification of Snake Venom Toxicity by Endogenous Signaling Pathways. Toxins 2020, 12, 68. [Google Scholar] [CrossRef] [Green Version]
- Sommers, C.L.; Byers, S.W.; Thompson, E.W.; Torri, J.A.; Gelmann, E.P. Differentiation state and invasiveness of human breast cancer cell lines. Breast Cancer Res. Treat. 1994, 31, 325–335. [Google Scholar] [CrossRef]
- Chakrabarty, D.; Sarkar, A. Cytotoxic Effects of Snake Venoms; Springer: Berlin/Heidelberg, Germany, 2017; Volume 111, pp. 1–7. [Google Scholar]
- Girish, K.S.; Kemparaju, K. The magic glue hyaluronan and its eraser hyaluronidase: A biological overview. Life Sci. 2007, 80, 1921–1943. [Google Scholar] [CrossRef]
- Hayashi, M.A.F.; Oliveira, E.B.; Kerkis, I.; Karpel, R.L. Crotamine: A novel cell-penetrating polypeptide nanocarrier with potential anti-cancer and biotechnological applications. Methods Mol. Biol. 2012, 906, 337–352. [Google Scholar] [CrossRef]
- Almeida, J.; Resende, L.; Watanabe, R.; Corassola, V.; Huancahuire-Vega, S.; Caldeira, C.; Coutinho-Neto, A.; Soares, A.; Vale, N.; Gomes, P.; et al. Snake venom peptides and low mass proteins: Molecular tools and therapeutic agents. Curr. Med. Chem. 2016, 23, 1–29. [Google Scholar] [CrossRef]
- Zakraoui, O.; Marcinkiewicz, C.; Aloui, Z.; Othman, H.; Grépin, R.; Haoues, M.; Essafi, M.; Srairi-Abid, N.; Gasmi, A.; Karoui, H.; et al. Lebein, a snake venom disintegrin, suppresses human colon cancer cells proliferation and tumor-induced angiogenesis through cell cycle arrest, apoptosis induction and inhibition of VEGF expression. Mol. Carcinog. 2017, 56, 18–35. [Google Scholar] [CrossRef]
- Lucena, S.; Castro, R.; Lundin, C.; Hofstetter, A.; Alaniz, A.; Suntravat, M.; Anchez, E.S. Inhibition of pancreatic tumoral cells by snake venom disintegrins. Toxicon 2015, 93, 136–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collection, A.T.C. Thawing, Propagating, and Cryopreserving Protocol NCI-PBCFHTB132 (MDA-MB-468) Breast Adenocarcinoma. Am. Type Cult. Collect. 2012, 26, 1–25. [Google Scholar]
- Mackessy, S. Venom Composition in Rattlesnakes: Trends and Biological Significance. In The Biology of Rattlesnakes; Hayes, W.K., Beaman, K.R., Cardwell, M.D., Bush, S.P., Eds.; Loma Linda University Press: Loma Linda, CA, USA, 2008; pp. 495–510. [Google Scholar]
- Lourenço, A.; Zorzella Creste, C.F.; Curtolo de Barros, L.; Delazari dos Santos, L.; Pimenta, D.C.; Barraviera, B.; Ferreira, R.S. Individual venom profiling of Crotalus durissus terrificus specimens from a geographically limited region: Crotamine assessment and captivity evaluation on the biological activities. Toxicon 2013, 69, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Furtado, M.F.D.; Santos, M.C.; Kamiguti, A.S. Age-related biological activity of South American rattlesnake (Crotalus durissus terrificus) venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2003, 9, 186–201. [Google Scholar] [CrossRef]
- Neri-Castro, E.; Ponce-López, R. Variación ontogénica en el veneno de Crotalus simus en México. Árido-Ciencia 2018, 3, 42–47. [Google Scholar]
- Mackessy, S. Thrombin-Like Enzymes in Snake Venoms. In Toxins and Hemostasis: From Bench to Bedside; Kini, M., Clemetson, K., Markland, F., McLane, M.A., Takashi, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 519–557. ISBN 9789048192953. [Google Scholar]
- Alvarez-Flores, M.P.; Faria, F.; de Andrade, S.A.; Chudzinski-Tavassi, A.M. Snake Venom Components Affecting the Coagulation System. In Snake Venoms; Springer: Berlin/Heidelberg, Germany, 2017; pp. 5–6. ISBN 978-94-007-6410-1. [Google Scholar]
- Saravia, P.; Rojas, E.; Arce, V.; Guevara, C.; López, J.C.; Chaves, E.; Velásquez, R.; Rojas, G.; Gutiérrez, J.M. Geographic and ontogenic variability in the venom of the neotropical rattlesnake Crotalus durissus: Pathophysiological and therapeutic implications. Rev. Biol. Trop. 2002, 50, 337–346. [Google Scholar]
- Baudou, F.G.; Litwin, S.; Lanari, L.C.; Laskowicz, R.D.; Damin, C.F.; Chippaux, J.P.; de Roodt, A.R. Antivenom against Crotalus durissus terrificus venom: Immunochemical reactivity and experimental neutralizing capacity. Toxicon 2017, 140, 11–17. [Google Scholar] [CrossRef]
- Borja, M.; Neri-Castro, E.; Pérez-Morales, R.; Strickland, J.; Ponce-López, R.; Parkinson, C.; Espinosa-Fematt, J.; Sáenz-Mata, J.; Flores-Martínez, E.; Alagón, A.; et al. Ontogenetic change in the venom of mexican blacktailed rattlesnakes (Crotalus molossus nigrescens). Toxins 2018, 10, 501. [Google Scholar] [CrossRef] [Green Version]
- Kalil, J.; Fan, H.W. Production and Utilization of Snake. In Toxins and Drug Discovery; Gopalakrishnakone, P., Cruz, L.J., Luo, S., Eds.; Springer: Dordrecht, 2017; Chapter 5; pp. 81–102. ISBN 978-94-007-6452-1. [Google Scholar]
- Walteros, D.; Paredes, A.; León, L. Accidente ofídico; Instituto Nacional de Salud: Bogotá, Colombia, 2017.
- Rodríguez-Vargas, A. Accidente ofídico. In Guía para el Manejo de Emergencias Toxicológicas; Varios, Ed.; Ministerio de Salud y Protección Social: Bogotá, Colombia, 2017; pp. 499–507. ISBN 978-958-5401-33-4. [Google Scholar]
- Gutiérrez, J.M.; Rojas, G.; Rica, U.D.C. El Envenenamiento por Mordedura de Serpiente en Centroamérica; Instituto Clodomiro Picado: San José, Colombia, 2009; ISBN 1529519233. [Google Scholar]
- Bhattacharjee, E.; Mitra, J.; Bhattacharyya, D. L-Amino Acid Oxidase from Venoms. In Toxins and Drug Discovery; Gopalakrishnakone, P., Cruz, L.J., Luo, S., Eds.; Springer: Dordrecht, 2017; Chapter 13; pp. 295–320. ISBN 978-94-007-6452-1. [Google Scholar]
- Yonamine, C.M.; Kondo, M.Y.; Nering, M.B.; Gouvêa, I.E.; Okamoto, D.; Andrade, D.; Alberto da Silva, J.A.; Prieto da Silva, Á.R.; Yamane, T.; Juliano, M.A.; et al. Enzyme specificity and effects of gyroxin, a serine protease from the venom of the South American rattlesnake Crotalus durissus terrificus, on protease-activated receptors. Toxicon 2014, 79, 64–71. [Google Scholar] [CrossRef]
- Antúnez, J.; Fernández, J.; Lomonte, B.; Angulo, Y.; Sanz, L.; Pérez, A.; Calvete, J.; Gutiérrez, J.M. Antivenomics of Atropoides mexicanus and Atropoides picadoi snake venoms: Relationship to the neutralization of toxic and enzymatic activities. J. Venom Res. 2010, 1, 8–17. [Google Scholar]
- Pla, D.; María Gutiérrez, J.; Calvete, J. Second generation snake antivenomics: Comparing immunoaffinity and immunodepletion protocols. Toxicon 2012, 60, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Jackson, T.N.W.; Laustsen, A.H.; Sunagar, K. Causes and Consequences of Snake Venom Variation. Trends Pharmacol. Sci. 2020, 41, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Amazonas, D.R.; Portes-Junior, J.A.; Nishiyama, M.Y., Jr.; Nicolau, C.A.; Chalkidis, H.M.; Mourão, R.H.V.; Grazziotin, F.G.; Rokyta, D.R.; Gibbs, H.L.; Valente, R.H.; et al. Molecular mechanisms underlying intraspecific variation in snake venom. J. Proteom. 2018, 181, 60–72. [Google Scholar] [CrossRef]
- Chippaux, J.P.; Williams, V.; White, J. Snake Venom Variability: Methods of Study, results and interpretation. Toxicon 1991, 29, 1279–1303. [Google Scholar] [CrossRef]
- Durban, J.; Pérez, A.; Sanz, L.; Gómez, A.; Bonilla, F.; Rodríguez, S.; Chacón, D.; Sasa, M.; Angulo, Y.; Gutiérrez, J.M.; et al. Integrated “omics” profiling indicates that miRNAs are modulators of the ontogenetic venom composition shift in the Central American rattlesnake, Crotalus simus simus. BMC Genom. 2013, 14, 234. [Google Scholar] [CrossRef] [Green Version]
- Daltry, J.C.; Wüster, W.; Thorpe, R.S. Diet and snake venom evolution. Nature 1996, 379, 537–542. [Google Scholar] [CrossRef]
- Boldrini-França, J.; Corrêa-Netto, C.; Silva, M.M.S.; Rodrigues, R.S.; De La Torre, P.; Pérez, A.; Soares, A.M.; Zingali, R.B.; Nogueira, R.A.; Rodrigues, V.M.; et al. Snake venomics and antivenomics of Crotalus durissus subspecies from Brazil: Assessment of geographic variation and its implication on snakebite management. J. Proteom. 2010, 73, 1758–1776. [Google Scholar] [CrossRef] [PubMed]
- WHO. Annex 5. Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins; Replacement of Annex 2 of WHO Technical Report Series, No. 964; WHO: Geneva, Switzerland, 2017; pp. 197–388.
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Schägger, H.; von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef]
- ICP. Determinación de Actividades Tóxicas de Venenos de Serpientes y Neutralización por Antivenenos; Manual de Métodos de Laboratorio, Universidad de Costa Rica: San Diego, Costa Rica, 2007. [Google Scholar]
- Spearman, C. The method of ‘right and wrong cases’ (‘constant stimuli’) without Gauss’s formulae. Br. J. Psychol. 1904–1920 1908, 2, 227–242. [Google Scholar] [CrossRef]
- Kärber, G. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Naunyn. Schmiedebergs. Arch. Exp. Pathol. Pharmakol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- de Araújo, A.L.; Radvanyi, F. Determination of phospholipase A2activity by a colorimetric assay using a pH indicator. Toxicon 1987, 25, 1181–1188. [Google Scholar] [CrossRef]
- Memar, B.; Jamili, S.; Shahbazzadeh, D.; Bagheri, P.K. The first report on coagulation and phospholipase A2 activities of Persian Gulf lionfish, Pterois russelli, an Iranian venomous fish. Toxicon 2016, 113, 25–31. [Google Scholar] [CrossRef]
- Cevallos, M.A.; Navarro-Duque, C.; Varela-Julia, M.; Alagon, A.C. Molecular mass determination and assay of venom hyaluronidases by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Toxicon 1992, 30, 925–930. [Google Scholar] [CrossRef]
- Heussen, C.; Dowdle, E.B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal. Biochem. 1980, 102, 196–202. [Google Scholar] [CrossRef]
- Instituto Clodomiro Picado. Manual de Procedimientos para la Determinación de Actividades Tóxicas de Venenos y su Neutralización por Antivenenos; Instituto Clodomiro Picado: San José, Colombia, 2007. [Google Scholar]
- Hermanson, G.T.; Mallia, A.K.; Smith, P.K. Immobilized Affinity Ligand Techniques; Academic Press: San Diego, CA, USA, 1992; ISBN 0123423309. [Google Scholar]
- Burnette, W.N. “Western Blotting”: Electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 1981, 112, 195–203. [Google Scholar] [CrossRef]






| SDS-PAGE | RP-HPLC # Fraction | Possible Related Protein Families | Reference | |||
|---|---|---|---|---|---|---|
| Molecular Weight (kDa) | Relative Intensity (%) by Ecoregion | |||||
| MM | CA | OR | ||||
| 113.1 | 11.4 | 9.5 | 5.0 | 11–14 | LAAO, SVMP, HYA | [33,42,43,44,45,46,47] |
| 83.7 | 8.8 | 6.7 | 3.5 | |||
| 73.2 | 9.9 | 21.2 | 8.3 | |||
| 64.0 | 9.0 | 6.9 | 0.0 | |||
| 57.3 | 4.5 | 0.0 | 0.0 | 9–12 | SVMP, SVSP, CRISP | [31,33,34,42,43,44,48,49,50,51] |
| 54.5 | 0.0 | 4.4 | 0.0 | |||
| 51.4 | 3.6 | 0.0 | 0.0 | |||
| 46.2 | 4.8 | 7.0 | 4.1 | |||
| 41.1 | 5.0 | 0.0 | 0.0 | |||
| 37.2 | 11.8 | 7.6 | 6.6 | |||
| 32.7 | 5.8 | 3.1 | 2.9 | |||
| 29.9 | 5.1 | 5.5 | 2.6 | |||
| 25.7 | 6.0 | 9.6 | 0.0 | |||
| 13.0 | 30.1 | 28.0 | 13.1 | 5–8 | PLA2, CTL, CRISP, growth factors | [35,36,37,51,52,53,54] |
| 11.4 | 9.3 | 14.7 | 4.9 | |||
| 9.8 | 13.5 | 0.0 | 0.0 | 1–6 | DIS, low molecular weight myotoxins, vasoactive peptides | [55,56,57,58,59,60] |
| 8.9 | 16.4 | 19.4 | 0.0 | |||
| 7.8 | 15.1 | 35.6 | 0.0 | |||
| Fraction | RT | RA MM (%) | Molecular Mass (Da) | RA CA (%) | Molecular Mass (Da) | RA OR (%) | Molecular Mass (Da) |
|---|---|---|---|---|---|---|---|
| 1 | 31.0 | 2 | ND | 3 | ND | 2 | ND |
| 2 | 36.6 | 1 | ND | 10 | 4910.9 | 2 | ND |
| 3 | 39.9 | 6 | 1239.1 | 1 | ND | 6 | ND |
| 4 | 45.3 | 2 | ND | 2 | ND | 6 | ND |
| 5 | 47.5 | 18 | ND | 6 | ND | 15 | ND |
| 6 | 49.1 | 5 | ND | 3 | ND | 5 | ND |
| 7 | 55.3 | 18 | 14,395.6 | 19 | ND | 16 | ND |
| 8 | 64.1 | 3 | ND | 1 | ND | 1 | 13,550.0 |
| 9 | 66.2 | 14 | 15,424.5 | 10 | ND | 10 | ND |
| 10 | 69.9 | 4 | ND | 1 | ND | 6 | ND |
| 11 | 73.4 | 4 | 14,439.0 | 1 | ND | 3 | ND |
| 12 | 75.8 | 6 | ND | 8 | ND | 4 | ND |
| 13 | 77.6 | 9 | ND | 12 | ND | 11 | ND |
| 14 | 81.5 | 6 | ND | 24 | ND | 13 | ND |
| Venom | IC50 MCF-7 (μg/mL) | IC50 HTB-132 (μg/mL) | PLA2 (%) 1 | PLA2 (μg/mL) vs. IC50 MCF-7 2 | PLA2 (μg/mL) vs. IC50 HTB-132 2 |
|---|---|---|---|---|---|
| Magdalena Medio (MM) | 0.4 | 9.3 | 44 | 7.7 | 8.3 |
| Caribe (CA) | 0.9 | 3.7 | 32 | 5.0 | 4.2 |
| Orinoquía (OR) | 0.7 | 3.5 | 36 | 8.0 | 7.6 |
| Ecoregion | Median Lethal Dose (LD50, µg/g 1) | Minimum Defibrinating Dose (MDD, µg/g 1) | Minimum Coagulant Dose (MCD, mg/L) |
|---|---|---|---|
| Magdalena Medio (MM) | 0.07 ± 0.009 | 0.08 ± 0.007 | 40.5 ± 2.8 4 |
| Caribe (CA) | 0.10 2,3 ± 0.005 | 0.10 ± 0.007 | 66.7 ± 2.6 |
| Orinoquía (OR) | 0.08 ± 0.007 | 0.11 ± 0.018 | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Vargas, A.; Vega, N.; Reyes-Montaño, E.; Corzo, G.; Neri-Castro, E.; Clement, H.; Ruiz-Gómez, F. Intraspecific Differences in the Venom of Crotalus durissus cumanensis from Colombia. Toxins 2022, 14, 532. https://doi.org/10.3390/toxins14080532
Rodríguez-Vargas A, Vega N, Reyes-Montaño E, Corzo G, Neri-Castro E, Clement H, Ruiz-Gómez F. Intraspecific Differences in the Venom of Crotalus durissus cumanensis from Colombia. Toxins. 2022; 14(8):532. https://doi.org/10.3390/toxins14080532
Chicago/Turabian StyleRodríguez-Vargas, Ariadna, Nohora Vega, Edgar Reyes-Montaño, Gerardo Corzo, Edgar Neri-Castro, Herlinda Clement, and Francisco Ruiz-Gómez. 2022. "Intraspecific Differences in the Venom of Crotalus durissus cumanensis from Colombia" Toxins 14, no. 8: 532. https://doi.org/10.3390/toxins14080532
APA StyleRodríguez-Vargas, A., Vega, N., Reyes-Montaño, E., Corzo, G., Neri-Castro, E., Clement, H., & Ruiz-Gómez, F. (2022). Intraspecific Differences in the Venom of Crotalus durissus cumanensis from Colombia. Toxins, 14(8), 532. https://doi.org/10.3390/toxins14080532






