Insights into the Underlying Mechanism of Ochratoxin A Production in Aspergillus niger CBS 513.88 Using Different Carbon Sources
Abstract
:1. Introduction
2. Results
2.1. Identification of the OTA-Producing Aptitude by A. niger CBS 513.88
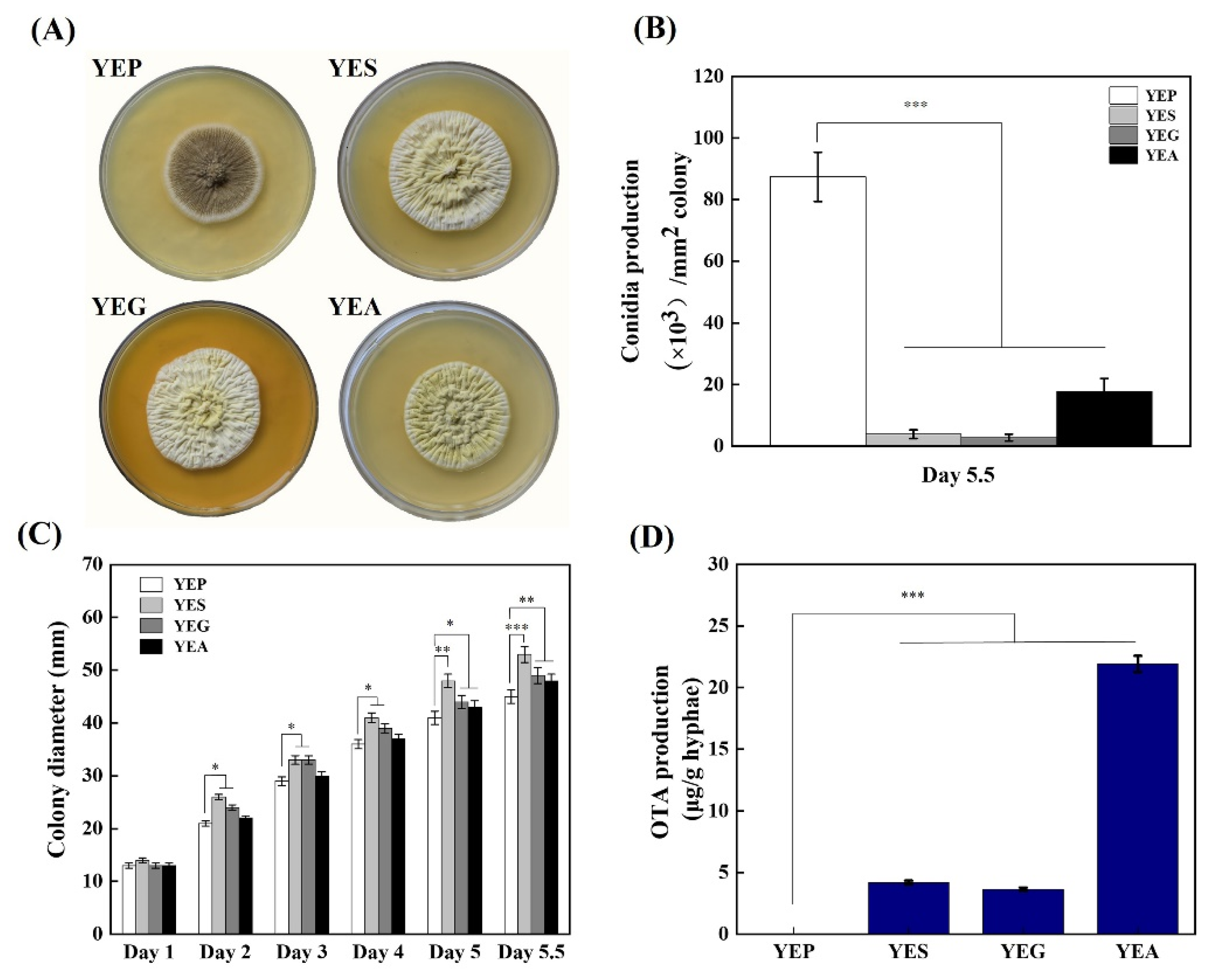
2.2. Influence of Different Carbon Sources on Colony Morphology, Conidia Production, and OTA Biosynthesis of A. niger CBS 513.88
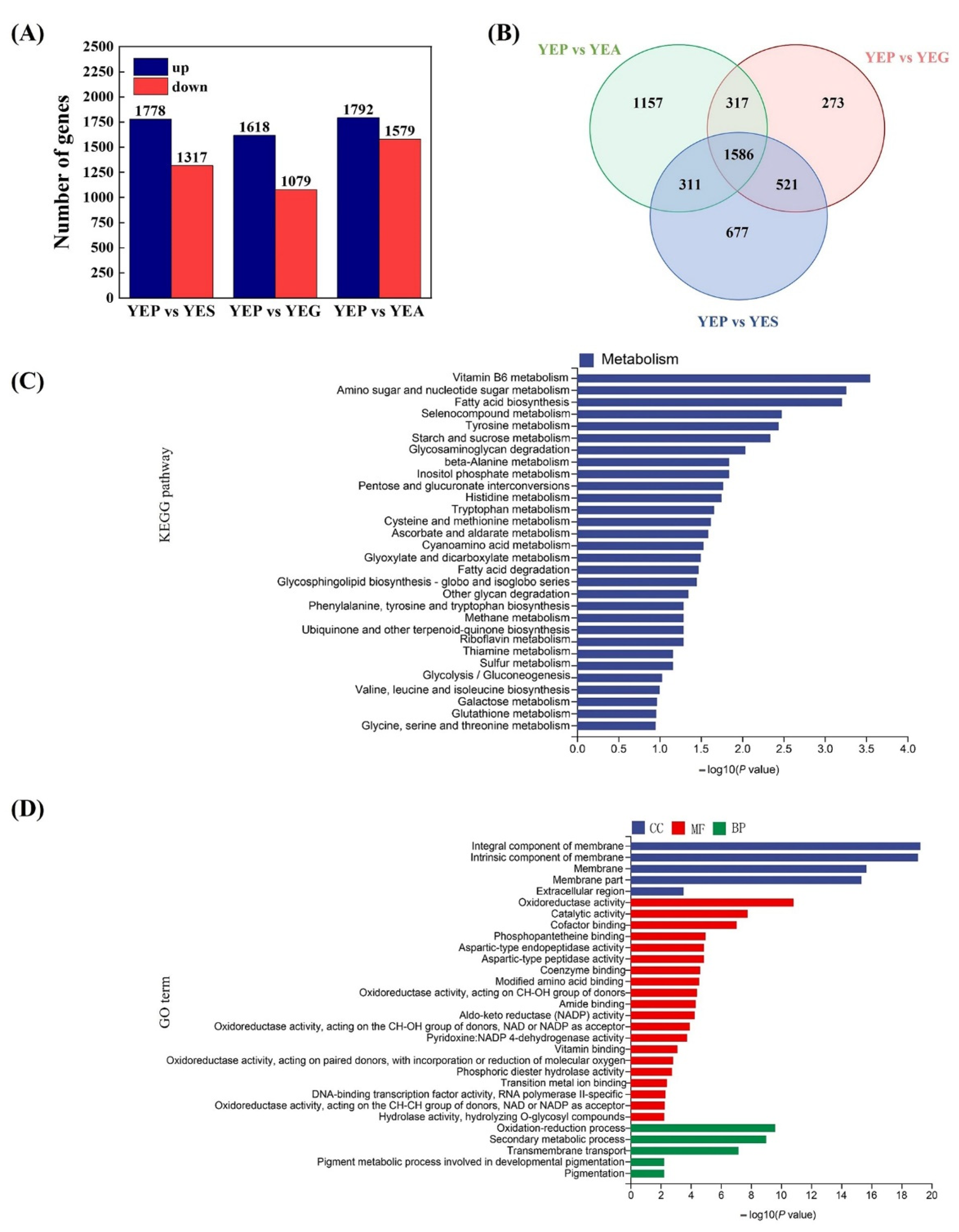
2.3. Transcriptional Profiles and DEGs Analyses of A. niger CBS 513.88 Using Different Carbon Sources
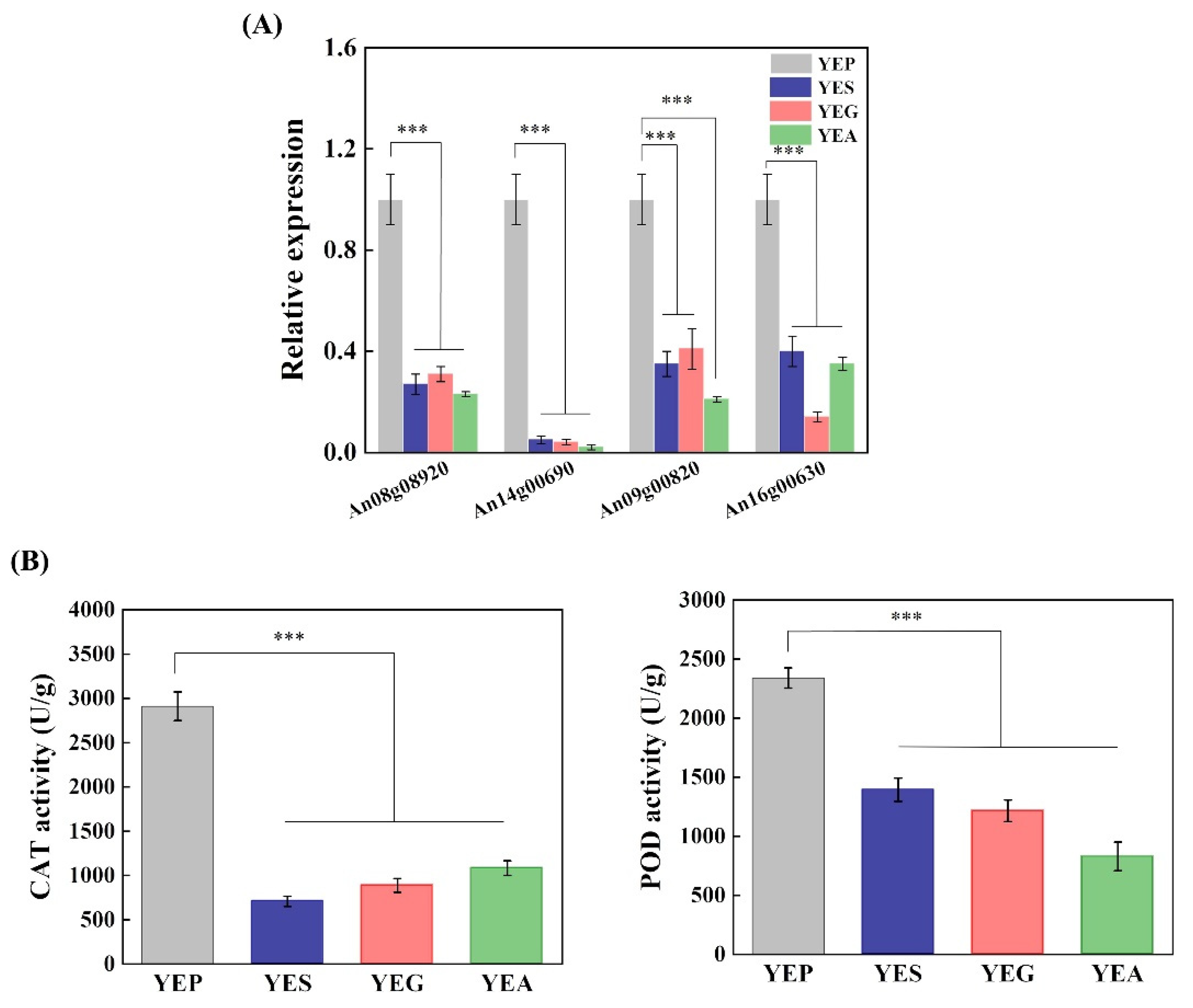
2.4. Carbon Sources Affect the Cellular Redox Homeostasis of A. niger CBS 513.88
2.5. Disruption of a Novel Gal4-like Transcription Factor Affects OTA Biosynthesis
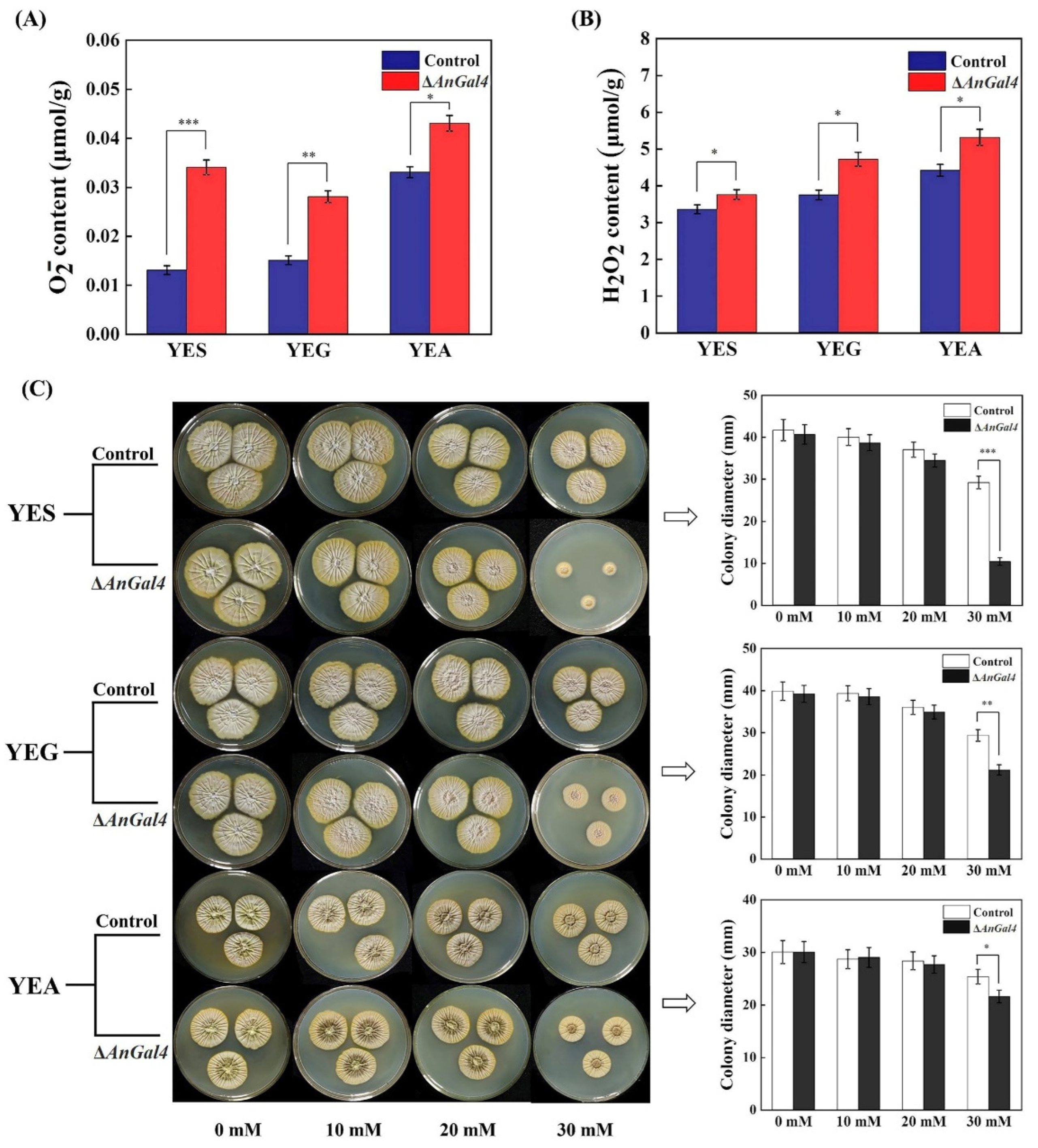
2.6. AnGal4 Affects Cellular Redox Homeostasis of A. niger
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Construction of Strains and Cultivation Conditions
5.2. Colony Morphology, Mycelial Growth, and Conidia Production
5.3. High-Performance Liquid Chromatography (HPLC) Analysis of OTA
5.4. Ultra-High Performance Liquid Chromatography Mass Spectrometry (UHPLC-MS) Analysis
5.5. Transcriptome Analysis
5.6. Real-Time Quantitative PCR (RT-qPCR) Analysis
5.7. Determination of Antioxidant Enzyme Activity, and Superoxide Anion and H2O2 Contents
5.8. Assay of H2O2 Susceptibility
5.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gil-Serna, J.; Vázquez, C.; Patiño, B. Genetic regulation of aflatoxin, ochratoxin A, trichothecene, and fumonisin biosynthesis: A Review. Int. Microbiol. 2020, 23, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Visconti, A.; Perrone, G.; Cozzi, G.; Solfrizzo, M. Managing ochratoxin A risk in the grape-wine food chain. Food Addit. Contam. 2008, 25, 193–202. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Wu, F.; Liu, F.; Wang, Q.; Zhang, X.; Selvaraj, J.N.; Zhao, Y.; Xing, F.; Yin, W.B.; et al. A consensus ochratoxin A biosynthetic pathway: Insights from the genome sequence of Aspergillus ochraceus and a comparative genomic analysis. Appl. Environ. Microbiol. 2018, 84, e01009-18. [Google Scholar] [CrossRef] [PubMed]
- Heussner, A.H.; Bingle, L.E. Comparative ochratoxin toxicity: A review of the available data. Toxins 2015, 7, 4253–4282. [Google Scholar] [CrossRef] [PubMed]
- Pel, H.J.; de Winde, J.H.; Archer, D.B.; Dyer, P.S.; Hofmann, G.; Schaap, P.J.; Turner, G.; de Vries, R.P.; Albang, R.; Albermann, K.; et al. Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat. Biotechnol. 2007, 25, 221–231. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Liu, F.; Wang, Q.; Selvaraj, J.N.; Xing, F.; Zhao, Y.; Liu, Y. Ochratoxin A producing fungi, biosynthetic pathway and regulatory mechanisms. Toxins 2016, 8, 83. [Google Scholar] [CrossRef]
- el Khoury, A.; Atoui, A. Ochratoxin A: General overview and actual molecular status. Toxins 2010, 2, 461–493. [Google Scholar] [CrossRef]
- Gil-Serna, J.; García-Díaz, M.; González-Jaén, M.T.; Vázquez, C.; Patiño, B. Description of an orthologous cluster of ochratoxin A biosynthetic genes in Aspergillus and Penicillium species. A comparative analysis. Int. J. Food Microbiol. 2018, 268, 35–43. [Google Scholar] [CrossRef]
- Passamani, F.R.; Hernandes, T.; Lopes, N.A.; Bastos, S.C.; Santiago, W.D.; Cardoso, M.D.G.; Batista, L.R. Effect of temperature, water activity, and pH on growth and production of ochratoxin A by Aspergillus niger and Aspergillus carbonarius from Brazilian grapes. J. Food Prot. 2014, 77, 1947–1952. [Google Scholar] [CrossRef]
- Abarca, M.L.; Bragulat, M.R.; Castellá, G.; Cabañes, F.J. Impact of some environmental factors on growth and ochratoxin A production by Aspergillus niger and Aspergillus welwitschiae. Int. J. Food Microbiol. 2019, 291, 10–16. [Google Scholar] [CrossRef]
- Wilkinson, J.R.; Yu, J.; Abbas, H.K.; Scheffler, B.E.; Kim, H.S.; Nierman, W.C.; Bhatnagar, D.; Cleveland, T.E. Aflatoxin formation and gene expression in response to carbon source media shift in Aspergillus parasiticus. Food Addit. Contam. 2007, 24, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gong, L.; Jiang, G.; Wang, Y.; Gupta, V.K.; Qu, H.; Duan, X.; Wang, J.; Jiang, Y. Carbon sources influence fumonisin production in Fusarium proliferatum. Proteomics 2017, 17, 1700070. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Li, B.; Tian, S. Effects of carbon, nitrogen and ambient pH on patulin production and related gene expression in Penicillium expansum. Int. J. Food Microbiol. 2015, 206, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, A.; Nakajima, T.; Hirayae, K. Effects of carbon sources and amines on induction of trichothecene production by Fusarium asiaticum in liquid culture. FEMS Microbiol. Lett. 2014, 352, 204–212. [Google Scholar] [CrossRef]
- Li, T.; Jiang, G.; Qu, H.; Wang, Y.; Xiong, Y.; Jian, Q.; Wu, Y.; Duan, X.; Zhu, X.; Hu, W.; et al. Comparative transcriptome analysis of Penicillium citrinum cultured with different carbon sources identifies genes involved in citrinin biosynthesis. Toxins 2017, 9, 69. [Google Scholar] [CrossRef]
- Hashem, A.; Fathi Abd-Allah, E.; Sultan Al-Obeed, R.; Abdullah Alqarawi, A.; Alwathnani, H.A. Effect of carbon, nitrogen sources and water activity on growth and ochratoxin production of Aspergillus carbonarius (Bainier) Thom. Jundishapur J. Microbiol. 2015, 8, e17569. [Google Scholar] [CrossRef]
- Ruiz, B.; Chávez, A.; Forero, A.; García-Huante, Y.; Romero, A.; Sánchez, M.; Rocha, D.; Sánchez, B.; Rodríguez-Sanoja, R.; Sánchez, S.; et al. Production of microbial secondary metabolites: Regulation by the carbon source. Crit. Rev. Microbiol. 2010, 36, 146–167. [Google Scholar] [CrossRef]
- Miyake, T.; Zhang, M.Y.; Kono, I.; Nozaki, N.; Sammoto, H. Repression of secondary metabolite production by exogenous camp in Monascus. Biosci. Biotechnol. Biochem. 2006, 70, 1521–1523. [Google Scholar] [CrossRef]
- Fan, Y.; Gao, Y.; Zhou, J.; Wei, L.; Chen, J.; Hua, Q. Process optimization with alternative carbon sources and modulation of secondary metabolism for enhanced ansamitocin P-3 production in Actinosynnema pretiosum. J. Biotechnol. 2014, 192 Pt A, 1–10. [Google Scholar] [CrossRef]
- Liu, P.; Wang, S.; Li, C.; Zhuang, Y.; Xia, J.; Noorman, H. Dynamic response of Aspergillus niger to periodical glucose pulse stimuli in chemostat cultures. Biotechnol. Bioeng. 2021, 118, 2265–2282. [Google Scholar] [CrossRef]
- Veana, F.; Fuentes-Garibay, J.A.; Aguilar, C.N.; Rodríguez-Herrera, R.; Guerrero-Olazarán, M.; Viader-Salvadó, J.M. Gene encoding a novel invertase from a xerophilic Aspergillus niger strain and production of the enzyme in Pichia pastoris. Enzym. Microb. Technol. 2014, 63, 28–33. [Google Scholar] [CrossRef] [PubMed]
- de Groot, M.J.; Prathumpai, W.; Visser, J.; Ruijter, G.J. Metabolic control analysis of Aspergillus niger L-arabinose catabolism. Biotechnol. Prog. 2005, 21, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Liu, Y.; Wu, M.; Ma, T.; Bai, X.; Hou, J.; Shen, Y.; Bao, X. Disruption of the transcription factors Thi2p and Nrm1p alleviates the post-glucose effect on xylose utilization in Saccharomyces cerevisiae. Biotechnol. Biofuels 2018, 11, 112. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Yang, B.; Zhang, C.; Zhang, H.; Xing, F.; Liu, Y. Carbon catabolite repression gene aocrea regulates morphological development and ochratoxin A biosynthesis responding to carbon sources in Aspergillus ochraceus. Toxins 2020, 12, 697. [Google Scholar] [CrossRef]
- Medina, A.; Mateo, E.M.; Valle-Algarra, F.M.; Mateo, F.; Mateo, R.; Jiménez, M. Influence of nitrogen and carbon sources on the production of ochratoxin A by ochratoxigenic strains of Aspergillus Spp. isolated from grapes. Int. J. Food Microbiol. 2008, 122, 93–99. [Google Scholar] [CrossRef]
- Fountain, J.C.; Bajaj, P.; Pandey, M.; Nayak, S.N.; Yang, L.; Kumar, V.; Jayale, A.S.; Chitikineni, A.; Zhuang, W.; Scully, B.T.; et al. Oxidative stress and carbon metabolism influence Aspergillus flavus transcriptome composition and secondary metabolite production. Sci. Rep. 2016, 6, 38747. [Google Scholar] [CrossRef]
- Abbas, A.; Valez, H.; Dobson, A.D. Analysis of the effect of nutritional factors on OTA and OTB biosynthesis and polyketide synthase gene expression in Aspergillus ochraceus. Int. J. Food Microbiol. 2009, 135, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yang, M.; Bai, Y.; Ge, F.; Wang, S. Antioxidant-related catalase CTA1 regulates development, aflatoxin biosynthesis, and virulence in pathogenic fungus Aspergillus flavus. Environ. Microbiol. 2020, 22, 2792–2810. [Google Scholar] [CrossRef] [PubMed]
- Tolaini, V.; Zjalic, S.; Reverberi, M.; Fanelli, C.; Fabbri, A.A.; Del Fiore, A.; De Rossi, P.; Ricelli, A. Lentinula edodes enhances the biocontrol activity of Cryptococcus laurentii against Penicillium expansum contamination and patulin production in apple fruits. Int. J. Food Microbiol. 2010, 138, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Ponts, N.; Pinson-Gadais, L.; Verdal-Bonnin, M.N.; Barreau, C.; Richard-Forget, F. Accumulation of deoxynivalenol and its 15-acetylated form is significantly modulated by oxidative stress in liquid cultures of Fusarium graminearum. FEMS Microbiol. Lett. 2006, 258, 102–107. [Google Scholar] [CrossRef]
- Montibus, M.; Ducos, C.; Bonnin-Verdal, M.N.; Bormann, J.; Ponts, N.; Richard-Forget, F.; Barreau, C. The bzip transcription factor Fgap1 mediates oxidative stress response and trichothecene biosynthesis but not virulence in Fusarium graminearum. PLoS ONE 2013, 8, e83377. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, M.; Gazzetti, K.; Punelli, F.; Scarpari, M.; Zjalic, S.; Ricelli, A.; Fabbri, A.A.; Fanelli, C. Aoyap1 Regulates Ota Synthesis by Controlling Cell Redox Balance in Aspergillus Ochraceus. Appl. Microbiol. Biotechnol. 2012, 95, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, M.; Punelli, F.; Scarpari, M.; Camera, E.; Zjalic, S.; Ricelli, A.; Fanelli, C.; Fabbri, A.A. Lipoperoxidation affects ochratoxin A biosynthesis in Aspergillus ochraceus and its interaction with wheat seeds. Appl. Microbiol. Biotechnol. 2010, 85, 1935–1946. [Google Scholar] [CrossRef] [PubMed]
- Twumasi-Boateng, K.; Yu, Y.; Chen, D.; Gravelat, F.N.; Nierman, W.C.; Sheppard, D.C. Transcriptional profiling identifies a role for brla in the response to nitrogen depletion and for stua in the regulation of secondary metabolite clusters in Aspergillus fumigatus. Eukaryot. Cell 2009, 8, 104–115. [Google Scholar] [CrossRef]
- Wang, P.; Xu, J.; Chang, P.K.; Liu, Z.; Kong, Q. New Insights of transcriptional regulator AflR in Aspergillus flavus physiology. Microbiol. Spectr. 2022, 10, e0079121. [Google Scholar] [CrossRef]
- Brown, D.W.; Butchko, R.A.; Busman, M.; Proctor, R.H. The Fusarium verticillioides Fum gene cluster encodes a Zn(Ii)2cys6 protein that affects fum gene expression and pumonisin production. Eukaryot. Cell 2007, 6, 1210–1218. [Google Scholar] [CrossRef]
- Bok, J.W.; Chung, D.; Balajee, S.A.; Marr, K.A.; Andes, D.; Nielsen, K.F.; Frisvad, J.C.; Kirby, K.A.; Keller, N.P. Gliz, a transcriptional regulator of gliotoxin biosynthesis, contributes to Aspergillus fumigatus virulence. Infect. Immun. 2006, 74, 6761–6768. [Google Scholar] [CrossRef]
- Abe, Y.; Ono, C.; Hosobuchi, M.; Yoshikawa, H. Functional analysis of MlcR, a regulatory gene for Ml-236b (compactin) biosynthesis in Penicillium citrinum. Mol. Genet. Genom. 2002, 268, 352–361. [Google Scholar] [CrossRef]
- Shimizu, T.; Kinoshita, H.; Nihira, T. Identification and in vivo functional analysis by gene disruption of ctnA, an activator gene involved in citrinin biosynthesis in Monascus purpureus. Appl. Environ. Microbiol. 2007, 73, 5097–5103. [Google Scholar] [CrossRef]
- Kim, J.E.; Jin, J.; Kim, H.; Kim, J.C.; Yun, S.H.; Lee, Y.W. Gip2, a putative transcription factor that regulates the aurofusarin biosynthetic gene cluster in Gibberella zeae. Appl. Environ. Microbiol. 2006, 72, 1645–1652. [Google Scholar] [CrossRef]
- Jekosch, K.; Kück, U. Loss of glucose repression in an Acremonium chrysogenum beta-lactam producer strain and its restoration by multiple copies of the Cre1 gene. Appl. Microbiol. Biotechnol. 2000, 54, 556–563. [Google Scholar] [CrossRef]
- Hajjaj, H.; Niederberger, P.; Duboc, P. Lovastatin biosynthesis by Aspergillus terreus in a chemically defined medium. Appl. Environ. Microbiol. 2001, 67, 2596–2602. [Google Scholar] [CrossRef] [PubMed]
- Vashee, S.; Xu, H.; Johnston, S.A.; Kodadek, T. How do “Zn2 cys6” proteins distinguish between similar upstream activation sites? Comparison of the DNA-binding specificity of the Gal4 protein in vitro and in vivo. J. Biol. Chem. 1993, 268, 24699–24706. [Google Scholar] [CrossRef]
- Yao, Y.; Tsuchiyama, S.; Yang, C.; Bulteau, A.L.; He, C.; Robison, B.; Tsuchiya, M.; Miller, D.; Briones, V.; Tar, K.; et al. Proteasomes, Sir2, and Hxk2 form an interconnected aging network that impinges on the Ampk/Snf1-regulated transcriptional repressor Mig1. PLoS Genet. 2015, 11, e1004968. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Ban, F.; Peng, S.; Xu, D.; Li, H.; Mo, H.; Hu, L.; Zhou, X. Exogenous iron induces nadph oxidases-dependent ferroptosis in the conidia of Aspergillus flavus. J. Agric. Food Chem. 2021, 69, 13608–13617. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Yan, L.; Jia, Y.; Xu, S.; Yan, Z.; Wang, S. Afln Is Involved in tbiosynthesis of aflatoxin and conidiation in Aspergillus flavus. Toxins 2021, 13, 831. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, L.; Chen, H.; Li, M.; Zhu, X.; Gao, Q.; Wang, D.; Zhang, Y. A polyketide synthase encoded by the gene An15g07920 is involved in the biosynthesis of ochratoxin A in Aspergillus niger. J. Agric. Food Chem. 2016, 64, 9680–9688. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Prusova, N.; Dzuman, Z.; Pulkrabova, J.; Hajslova, J. Regulated and non-regulated mycotoxin detection in cereal matrices using an ultra-high-performance liquid chromatography high-resolution mass spectrometry (UHPLC-HRMS) method. Toxins 2021, 13, 783. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, 1–12. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


| Gene ID | Function | Transcriptome Data (log2(Fold Change)) | ||
|---|---|---|---|---|
| YES/YEP | YEG/YEP | YEA/YEP | ||
| DEGs involved in conidiogenesis | ||||
| An01g10540 | Regulatory protein (brlA) | −2.44 *** | −2.18 *** | −2.81 *** |
| DEGs involved in phenylalanine, histidine, tyrosine, tryptophan, and arginine biosynthesis | ||||
| An08g06800 | 5-Dehydroshikimate dehydrase | 1.30 *** | 1.06 *** | 1.71 *** |
| An14g06010 | Chorismate mutase | 1.28 *** | 1.57 *** | 1.90 *** |
| An15g02460 | L-kynurenine/alpha-aminoadipate aminotransfease | 1.45 ** | 1.19 | 1.50 *** |
| An14g07210 | Histidinol dehydrogenase 1 | 3.37 *** | 2.58 * | 2.17 *** |
| An16g02500 | Tryptophan synthase | 4.33 *** | 3.87 *** | 3.40 *** |
| An04g08100 | Shikimate/quinate 5-dehydrogenase | 5.49 *** | 5.66 *** | 4.53 *** |
| An15g02340 | Argininosuccinate synthase | 1.61 *** | 1.05 *** | 1.16 *** |
| DEGs involved in OTA biosynthesis | ||||
| An15g07920 | Polyketide synthase (pks) | 0.01 | 0.19 | 1.7 *** |
| An15g07900 | Oxidoreductase activity (p450) | 3.28 ** | 2.62 | 2.30 ** |
| An15g07910 | Nonribosomal peptide synthase (nrps) | 3.43 * | 2.96 * | 3.28 *** |
| An15g07880 | Oxidoreductase activity (hal) | 3.55 * | 3.19 | 3.59 *** |
| An15g07890 | bZIP transcription factor (bzip) | 2.32 | 2.29 | 1.53 |
| DEGs involved in oxidative stress | ||||
| An08g08920 | Catalase activity (cat) | −1.42 *** | −1.41 *** | −2.15 *** |
| An14g00690 | Catalase activity (cat) | −2.65 *** | −2.04 *** | −3.21 *** |
| An09g00820 | Predicted peroxidase activity (pod) | −2.04 *** | −2.40 *** | −2.75 *** |
| An16g00630 | Predicted peroxidase activity (pod) | −2.16 ** | −3.67 *** | −1.59 * |
| A. niger Strains | Description | Sources |
|---|---|---|
| A.niger CBS 513.88 | Wild-type strain | Provided by Li Pan |
| A. niger CBS 513.88 (kusA−, pyrG−) | A. niger CBS 513.88 derivative, ΔkusA::ptrA, ΔpyrG::hygB | Laboratory constructed |
| A. niger CBS 513.88 (kusA−, pyrG+) (Control) | A. niger CBS 513.88 derivative, ΔkusA, ΔpyrG, pyrG-Com | Laboratory constructed |
| ΔAnGal4 | A. niger CBS 513.88 derivative, ΔkusA, ΔpyrG, ΔAnGal4::pyrG | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, S.; Hu, C.; Nie, P.; Zhai, H.; Zhang, S.; Li, N.; Lv, Y.; Hu, Y. Insights into the Underlying Mechanism of Ochratoxin A Production in Aspergillus niger CBS 513.88 Using Different Carbon Sources. Toxins 2022, 14, 551. https://doi.org/10.3390/toxins14080551
Wei S, Hu C, Nie P, Zhai H, Zhang S, Li N, Lv Y, Hu Y. Insights into the Underlying Mechanism of Ochratoxin A Production in Aspergillus niger CBS 513.88 Using Different Carbon Sources. Toxins. 2022; 14(8):551. https://doi.org/10.3390/toxins14080551
Chicago/Turabian StyleWei, Shan, Chaojiang Hu, Ping Nie, Huanchen Zhai, Shuaibing Zhang, Na Li, Yangyong Lv, and Yuansen Hu. 2022. "Insights into the Underlying Mechanism of Ochratoxin A Production in Aspergillus niger CBS 513.88 Using Different Carbon Sources" Toxins 14, no. 8: 551. https://doi.org/10.3390/toxins14080551
APA StyleWei, S., Hu, C., Nie, P., Zhai, H., Zhang, S., Li, N., Lv, Y., & Hu, Y. (2022). Insights into the Underlying Mechanism of Ochratoxin A Production in Aspergillus niger CBS 513.88 Using Different Carbon Sources. Toxins, 14(8), 551. https://doi.org/10.3390/toxins14080551




