Adverse Events Associated with the Clinical Use of Bee Venom: A Review
Abstract
:1. Introduction
2. Results
2.1. Descriptions of Trials
2.2. Adverse Events
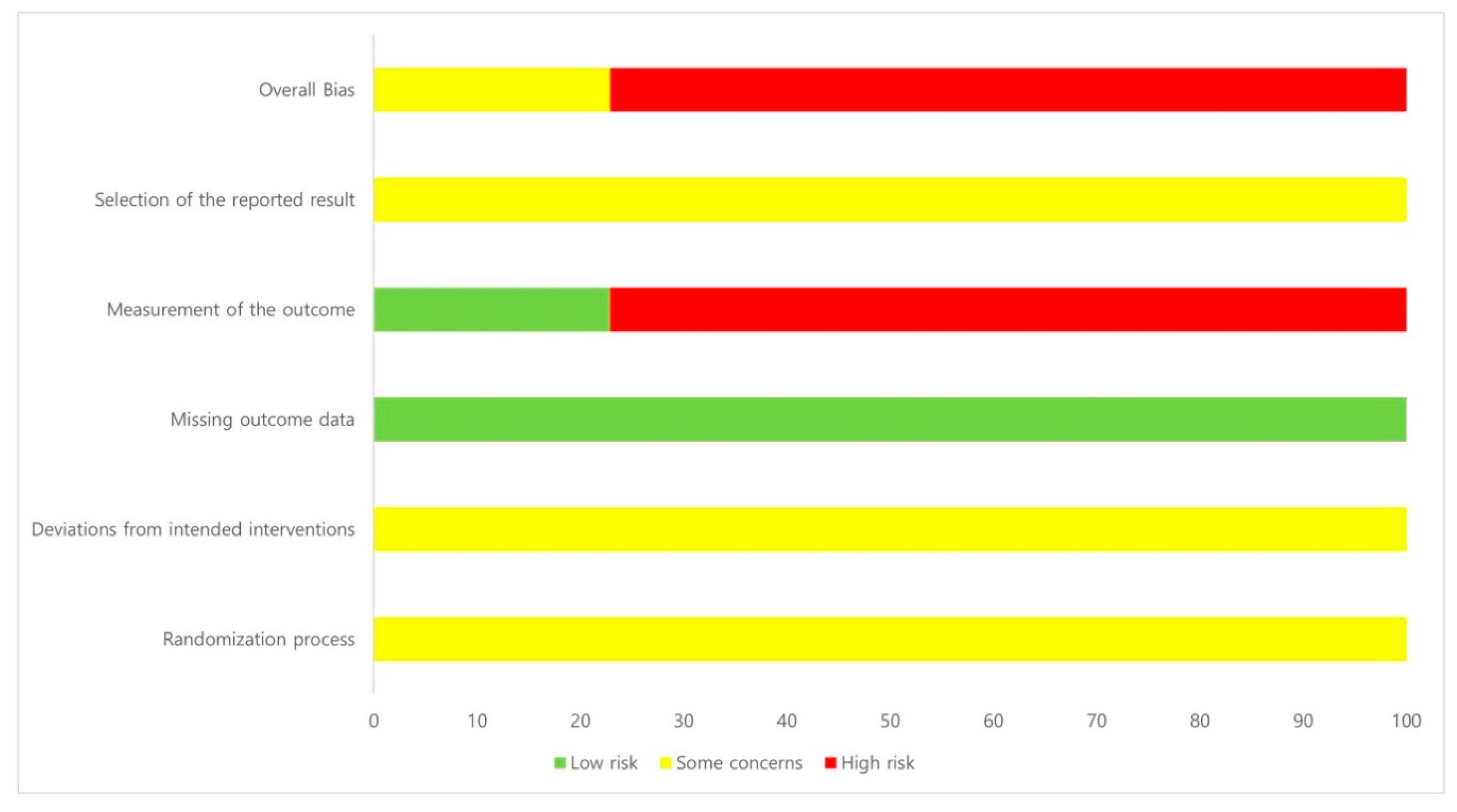
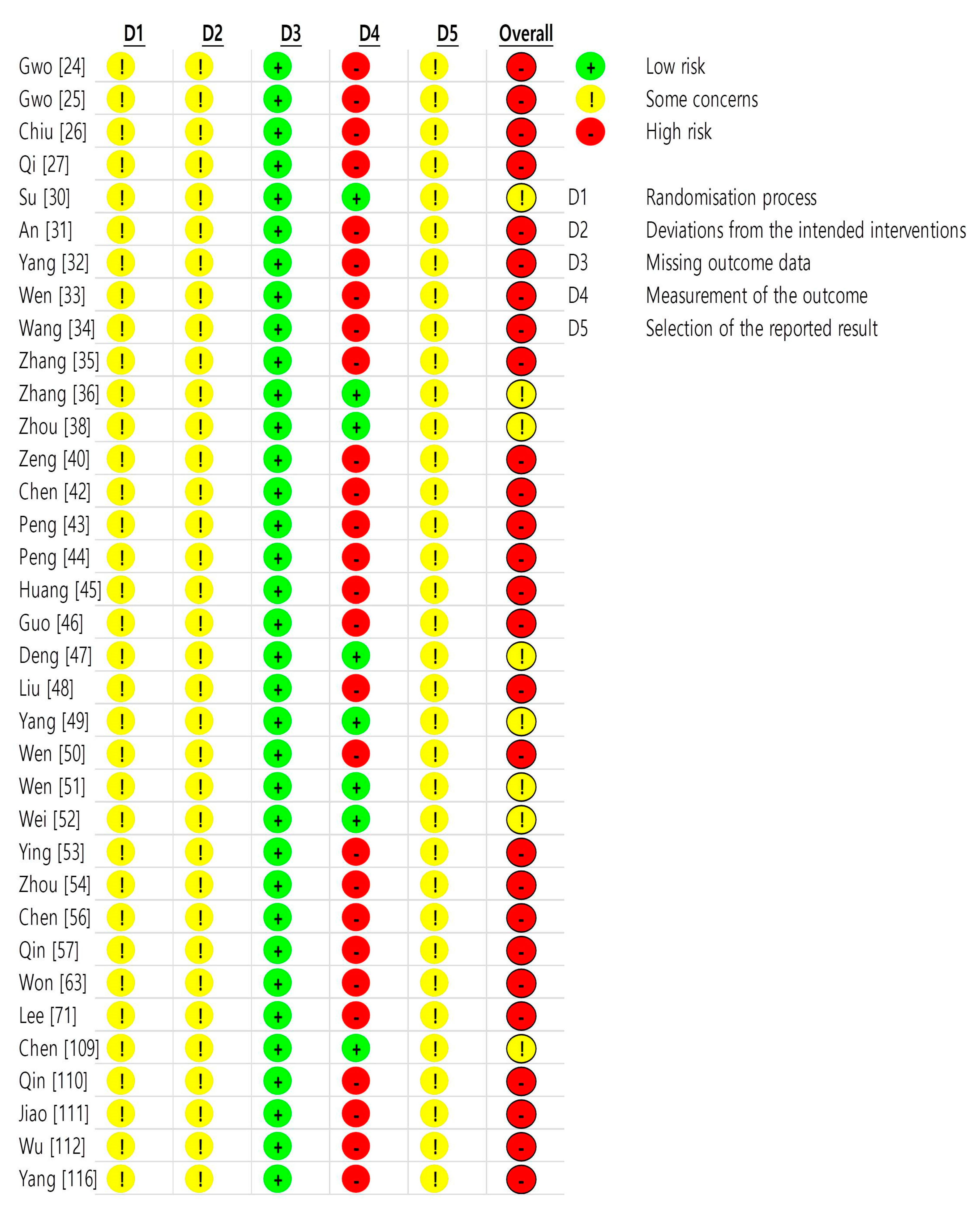
2.3. Risk of Bias in Included Studies
3. Discussion
4. Conclusions
5. Methods
5.1. Search Method for Identifying Studies
5.2. Inclusion Criteria
5.2.1. Types of Studies
5.2.2. Types of Participants
5.2.3. Types of Interventions
5.2.4. Types of Outcome Measures
5.3. Data Selection and Extraction
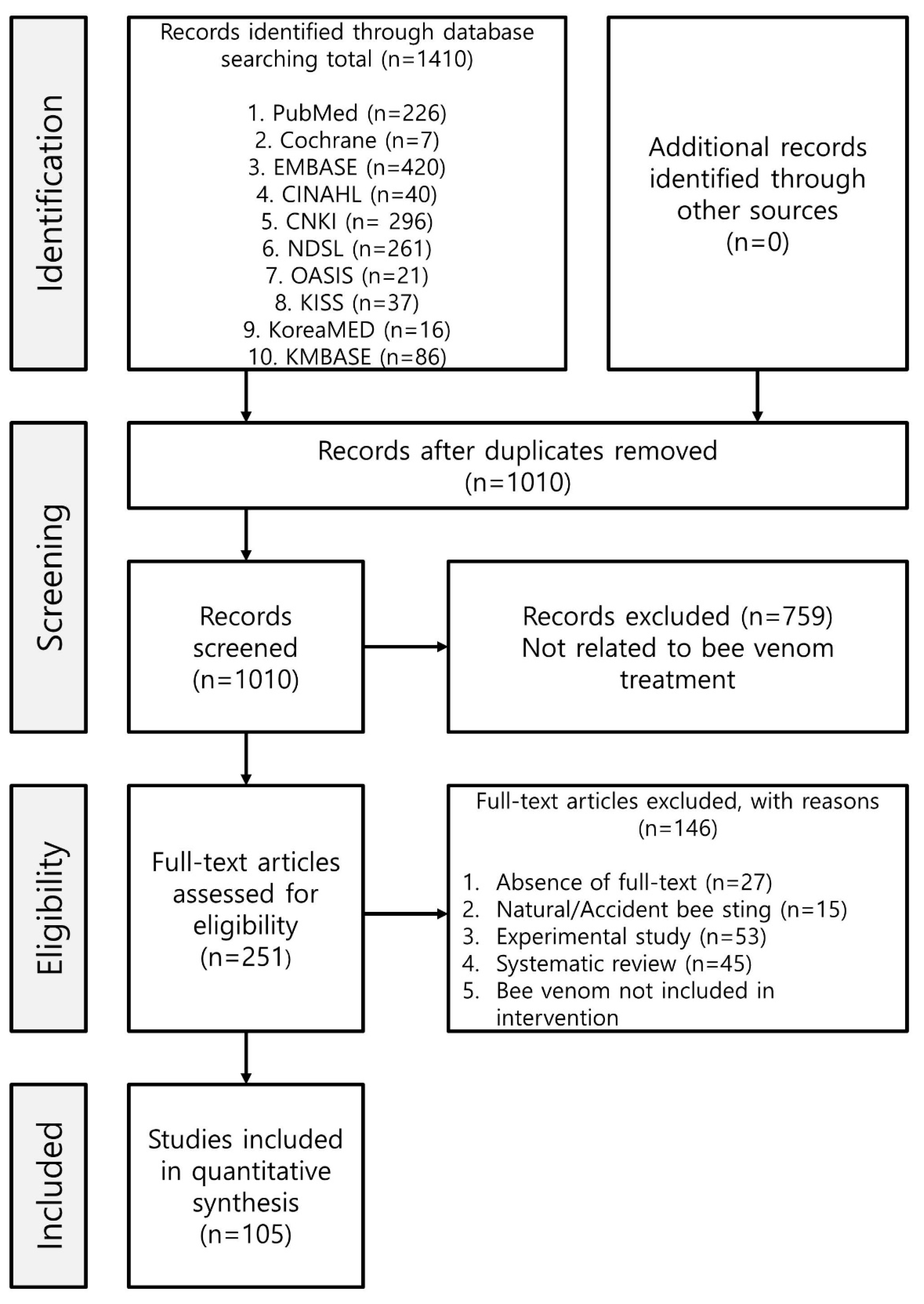
5.3.1. Selection of Studies
5.3.2. Data Extraction
5.3.3. Assessment RCTs
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahn, C.B.; Youn, H.M.; Cho, E.J. An Investigation of Directions of Research on Bee Venom in the Sphere of Oriental Medicine in Korea in Last Decade. Dong-Eui-Han-Eui-Yeon 2011, 5, 23–42. [Google Scholar]
- Karimzadeh, L.; Nabiuni, M.; Kouchesfehani, H.M.; Adham, H.; Bagheri, A.; Sheikholeslami, A. Effect of bee venom on IL-6, COX-2 and VEGF levels in polycystic ovarian syndrome induced in Wistar rats by estradiol valerate. J. Venom. Anim. Toxins Incl. Trop. Dis. 2013, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012, 31, 173–194. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Kim, J.Y.; Kim, W.H.; Gwon, M.G.; Gu, H.M.; Jeon, M.J.; Han, S.M.; Pak, S.C.; Lee, C.K.; Park, I.S.; et al. Therapeutic effects of bee venom and its major component, melittin, on atopic dermatitis in vivo and vitro. Br. J. Pharmacol. 2018, 175, 4310–4324. [Google Scholar] [CrossRef]
- Park, J.H.; Yim, B.K.; Lee, J.-H.; Lee, S.H.; Kim, T.-H. Risk Associated with Bee Venom Therapy: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0126971. [Google Scholar] [CrossRef]
- Jang, S.B.; Kim, K.H. Clinical Effectiveness and Adverse Events of Bee Venom Therapy: A Systematic Review of Randomized Controlled Trials. Toxins 2020, 12, 558. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Shafaghat, F.; Zwiener, R.D. Immunology of Bee Venom. Clin. Rev. Allergy Immunol. 2018, 54, 386–396. [Google Scholar] [CrossRef]
- Ko, S.-H.; Oh, H.-M.; Kwon, D.-Y.; Yang, J.-E.; Kim, B.-J.; Ha, H.-J.; Lim, E.-J.; Oh, M.-S.; Son, C.-G.; Lee, E.-J. Incidence Rate of Bee Venom Acupuncture Related Anaphylaxis: A Systematic Review. Toxins 2022, 14, 238. [Google Scholar] [CrossRef]
- Liberman, P. Anaphylaxis. Med. Clin. N. Am. 2006, 90, 77–95. [Google Scholar] [CrossRef]
- Silva, D.; Singh, C.; Muraro, A.; Worm, M.; Alviani, C.; Cardona, V.; DunnGlvin, A.; Garvey, L.H.; Riggioni, C.; Angier, E.; et al. Diagnosing, managing and preventing anaphylaxis: Systematic review. Allergy 2021, 76, 1493–1506. [Google Scholar] [CrossRef]
- Hernandez, L.; Papalia, S.; Pujalte, G.G.A. Anaphylaxis. Prim. Care 2016, 43, 477–485. [Google Scholar] [CrossRef]
- Han, J.H.; Kim, Y.H.; Bang, C.H.; Lee, J.H.; Kim, T.Y. Skin atrophy occurring at the site of dried honey bee venom (Apitoxin) injection. Ann. Dermatol. 2016, 68, 472–473. [Google Scholar]
- Castro, H.J.; Mendez-Inocencio, J.I.; Omidvar, B.; Omidvar, J.; Santilli, J.; Nielsen, H.S., Jr.; Pavot, A.P.; Richert, J.R.; Bellanti, J.A. A phase I study of the safety of honeybee venom extract as a possible treatment for patients with progressive forms of multiple sclerosis. Allergy Asthma Proc. 2005, 26, 470–476. [Google Scholar] [PubMed]
- Lee, C.H.; Yoon, J.Y.; Shim, S.E.; Kim, J.H.; Kim, J.Y.; Kim, H.N.; Hwang, J.M.; Kim, J.H.; Goo, B.H.; Park, Y.C.; et al. A Retrospective Study on the Clinical Safety of Bee Venom Pharmacopuncture at Craniofacial Acupuncture Points for the Treatment of Facial Disorders. J. Acupunct. Res. 2019, 36, 245–250. [Google Scholar] [CrossRef]
- Jeong, J.K.; Park, G.N.; Kim, K.M.; Kim, S.Y.; Kim, E.S.; Kim, J.H.; Nam, S.K.; Kim, Y.I. The Effectiveness of Ultrasound-guided Bee Venom Pharmacopuncture Combined with Integrative Korean Medical Treatment for Rotator cuff Diseases: A Retrospective Case Series. J. Acupunct. Res. 2016, 33, 165–180. [Google Scholar] [CrossRef]
- Kim, J.M.; Jeon, H.J.; Kim, H.J.; Cho, C.W.; Yoo, H.S. Bee Venom Pharmacopuncture: An Effective Treatment for Complex Regional Pain Syndrome. J. Pharmacopunct. 2014, 17, 66–69. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, C.H. The Clinical Observations of 2 case of Allergic Rhinitis treated with Bee Venom Pharmacopuncture and acupuncture therapy. J. Pharmacopunct. 2009, 12, 99–105. [Google Scholar] [CrossRef]
- Moon, Y.-J.; Chu, H.-M.; Shin, H.-R.; Lee, J.-Y.; Kweon, S.-H.; Kim, C.-H.; Song, B.-K.; Won, J.-H.; Baek, D.-G. A Case Report of Fibromyalgia Improved by Korean Medical Treatment. J. Int. Korean Med. 2019, 40, 192–200. [Google Scholar] [CrossRef]
- Park, O.J.; Kim, S.G.; Jeong, J.L.; Lee, S.M.; Lee, S.J.; Cho, N.G. The Effect of Shinbaro and Bee Venom Pharmacopuncture in Treating Lumbar Disc Herniations. Acupuncture 2013, 30, 41–50. [Google Scholar] [CrossRef]
- An, C.-S.; Kang, K.-S.; Kwon, G.-R. One Case of Systemic Lupus Erythematosus Treated with Traditional Korean Medicine. Korean Pharmacopunct. Inst. 2000, 3, 245–255. [Google Scholar]
- Kim, K.H.; Jeong, H.I.; Lee, G.H.; Jang, S.B.; Yook, T.H. Characteristics of Adverse Events in Bee Venom Therapy Reported in South Korea: A Survey Study. Toxins 2022, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Lim, J.W.; Lee, J.D.; Choi, D.Y.; Lee, S.H. Bee venom treatment for refractory postherpetic neuralgia: A case report. J. Altern. Complement. Med. 2014, 20, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Kam, P.L.; Jeong, W. Clinical observation of bee therapy to reduce bone marrow inhibition after chemotherapy in lung cancer patients. Clin. Rehabil. Oncol. China 2018, 25, 1366–1368. [Google Scholar]
- Gwo, G.H.; Ding, L.H.; Jong, S.W. Clinical observation of bee acupuncture combined with Chinese medicine to treat chronic urticaria with qi-blood deficiency. Guangming Tradit. Chin. Med. 2018, 33, 3196–3198. [Google Scholar]
- Gow, C.Y.; Wang, J.H.; Li, S.L.; Li, L.; Huang, M. Clinical observation of treatment of ankylosing spondylitis by direct acupuncture of bee acupuncture with “Bee venom acupuncture reducing and increasing effect of oral liquid”. Yunnan J. Tradit. Chin. Med. Mater. Med. 2019, 40, 210–213. [Google Scholar]
- Chiu, W.P.; Li, B.; Huang, J.H. Analysis of the curative effect of bee acupuncture therapy on rheumatoid arthritis. Inn. Mong. Tradit. Chin. Med. 2018, 37, 73–74. [Google Scholar]
- Qi, J.-Z. Thinking on the safety evaluation of bee therapy for rheumatoid arthritis. In Proceedings of the First World Bee Therapy Conference, the Second Annual Meeting of the World Union Bee Therapy Committee, Shenzhen, Guangdong, China, 17–21 July 2018; pp. 101–104. [Google Scholar]
- She, R.-T.; Li, W.-Y.; Liu, G.-K.; Lin, Y.-F. Clinical Observation of Spine-pressing and Pivot-relaxing Therapy Combined with Bee-venom Therapy in Treating Ankylosing Spondylitis. J. Guangzhou Univ. Tradit. Chin. Med. 2019, 36, 1012–1017. [Google Scholar]
- Su, X.J.; Wang, H.D.; Li, W.Y. Clinical curative effect of bee acupuncture therapy on ankylosing spondylitis. In Proceedings of the 1st Academic Exchange Conference on Chinese Medicine and Ethnic Medicine, Beijing, China, 6 December 2016; pp. 70–73. [Google Scholar]
- Su, X.H. Clinical Effect of Bee venom Therapy on Hyperplasia of Liver Depression and Stagnation; Guangzhou University of Traditional Chinese Medicine: Guangzhou, China, 2010. [Google Scholar]
- An, X.Z.; Zhang, M.; Zhang, Y.; Sun, Y. Effectiveness of bees on patients with lung cancer combined with cancer pain. J. Bees 2019, 39, 17–20. [Google Scholar]
- Yang, Y. Analysis of Effect of bee Needling Therapy Combined with Traditional Chinese Medicine Therapy in Patients with Rheumatic Arthralgia. China Foreign Med. Treat. 2015, 25, 178–179. [Google Scholar]
- Wen, W.Q.; Huang, S.G.; Che, H.; Tan, N.; Zhou, R.Y.; Zhu, H.J. Bee-Acupuncture Based on Midnight-Noon and Ebb-Flow Doctrine for Ankylosing Spondylitis. J. Anhui Tradit. Chin. Med. Coll. 2011, 30, 40–43. [Google Scholar]
- Wang, D.-Q.; Wang, F. Observation on the clinical efficacy of bee venom injection combined with durogesic in the treatment of advanced cancer pain. Chin. J. Biochem. Pharm. 2012, 33, 878–880. [Google Scholar]
- Zhang, J. Study on the mechanism and clinical comparison of bee acupuncture combined with acupotomy in treating shoulder perivascular inflammation. Clin. Study Tradit. Chin. Med. 2018, 10, 76–77. [Google Scholar]
- Zhang, F. Clinical Study of Acupuncture Combined with Bee Acupuncture in the Treatment of Obstinate Facial Palsy; Yunnan University of Traditional Chinese Medicine: Kunming, China, 2021. [Google Scholar]
- Zhou, R.Y.; Tan, N.; Huang, S.G. Clinical summary of 40 cases of Danqi Buxin Decoction combined with bee acupuncture for ankylosing spondylitis. Chin. Med. Guide 2009, 15, 40–41. [Google Scholar]
- Zhou, Y.F.; Li, W.Y. Effect of bees on HPA axis in rheumatoid arthritis patients. Inn. Mong. Tradit. Chin. Med. 2012, 31, 1–3. [Google Scholar]
- Zhu, H.J.; Huang, S.G.; Tan, N.; Wen, W.Q.; Xu, Z.J. Treatment of 56 patients with ankylosing spondylitis by combining bee acupuncture with renal stasis. Chin. Med. Guide 2009, 15, 33–34. [Google Scholar]
- Zeng, X.Z.; Peng, Q. Clinical observation on thermal treatment combined with bees needle in the treatment of ankylosing spondylitis. Chin. Med. Guide 2012, 9, 98–99. [Google Scholar]
- Chen, L.Y. Evaluation of the Effect of Painless Bee Therapy on Leukocyte Reduction after Chemotherapy of Colorectal Cancer; Guangzhou University of Traditional Chinese Medicine: Guangzhou, China, 2015. [Google Scholar]
- Chen, S.Y.; Zhou, P.; Qin, Y. Clinical Study of Bee Acupuncture Treatment for Rheumatoid Arthritis; Guangzhou University of Traditional Chinese Medicine: Guangzhou, China, 2019. [Google Scholar]
- Peng, H. Clinical Observation of Bee Acupuncture Combined with Tramadol in the Treatment of Moderate Cancer; Hunan University of Traditional Chinese Medicine: Changsha, China, 2011. [Google Scholar]
- Peng, H.; Zhang, Z.F. Observation of Bee Needling Therapy Combined with three-step Analgesic for Cancer Pain. J. Hunan Univ. Tradit. Chin. Med. 2010, 30, 222–225. [Google Scholar]
- Huang, S.G.; Chen, H.; Zhou, R.Y.; Yu, C.; Tan, N.; Zhu, H.J.; Liao, K.H.; Luo, X.G. Effects of Bee Venom Acupuncture on the Grades of Syndromes and Hemorheology on Joint Pain Identified as Wind-cold Pattern. Chin. Med. Guide 2012, 18, 16–18. [Google Scholar]
- Guo, C.Y.; Wang, Z.H.; Li, L.; Li, L.; Huang, M.; Li, S.R. Effect of Panlong’s Lair on the Correlation Indicators in Patients with Ankylosing Spondylitis by Bee Sting Method of Bladder Meridian. In Proceedings of the 3rd World Bee Therapy Conference, the 4th Academic Conference of the World Union Bee Therapy Committee, Beijing, China, 22–24 October 2021; pp. 94–99. [Google Scholar]
- Deng, M. Clinical Observation of Bee Acupuncture Therapy for Rheumatoid Arthritis and Its Effect on HPA Axis; Hunan University of Traditional Chinese Medicine: Changsha, China, 2005. [Google Scholar]
- Liu, X.-D.; Zhang, J.-L.; Zheng, H.-G.; Liu, F.-Y.; Chen, Y. Clinical Randomized Study of Bee-sting Therapy for Rheumatoid Arthritis. Acupunct. Res. 2008, 33, 197–200. [Google Scholar]
- Yang, D.W.; Wu, B.L.; He, M.J.; Guo, X.R. Effect of bee acupuncture on peripheral neuropathy of diabetes. Med. Ujiang 2018, 46, 407–410. [Google Scholar]
- Wen, W.Q.; Huang, S.G.; Zhu, H.J.; Tan, N.; Wang, R.R. Treatment of 41 Postpartum Paralysis by Fumigation of Bee Acupuncture with Chinese Medicine. Sichuan Tradit. Chin. Med. 2011, 29, 114–115. [Google Scholar]
- Wen, Z.F.; Li, R.S.; Zhang, Y.X. Effect of Bee Acupuncture Therapy Combined with Acupoint Injection of Polyinosinic-polycytidylic Acid Injection on Serum T Cell Subsets in Patients with Postherpetic Neuralgia. J. Emerg. Tradit. Chin. Med. 2018, 27, 47–50. [Google Scholar]
- Wei, W.; Kong, L.Q. Treating rheumatoid arthralgia with the Shentong Zhuyu decoction plus bee-sting. Clin. J. Chin. Med. 2018, 10, 61–62. [Google Scholar]
- Ying, C.; Xu, S.L.; Jiang, L.; Chen, Z.L. Curative Effect Observation of Shoulder Periarthritis Treatment with Bee Acupuncture. Acta Chin. Med. 2018, 33, 919–922. [Google Scholar]
- Zhou, B.-X.; Lao, J.-X. Clinical Observation of Bee Venom Acupuncture Combined with Moxibustion with Warming Needle in Treating Neurogenic Tinnitus. J. Guangzhou Univ. Tradit. Chin. Med. 2019, 36, 1749–1752. [Google Scholar]
- Chen, J. Report on 4000 Cases of Treatment of Lumbar Intervertebral Discs by Traditional Chinese Medicine Chuna Redemption Method, Bee Acupuncture Therapy and Compound Bee Poison Injection Therapy. In Proceedings of the “Honeycomb” 2010 National Bee Products Market Information Exchange Conference cum China (Wuhan) Bee Industry Fair, Wuhan, China, 9–12 March 2010; pp. 40–45. [Google Scholar]
- Chen, S.Y.; Zhou, P.; Li, W.Y.; Li, J.J. Clinical Study on Quantitative Effectiveness of Bee Acupuncture on Rheumatoid Arthritis. In Proceedings of the 3rd World Bee Therapy Conference, the 4th Academic Conference of the World Union Bee Therapy Committee, Beijing, China, 22–24 October 2021; pp. 36–43. [Google Scholar]
- Qin, Y.M.; Zhang, W.X.; Kang, P.L.; Zhao, X. Clinical study of bee acupuncture combined with Xianlong granules in the treatment of rheumatoid arthritis. Shandong J. Tradit. Chin. Med. 2021, 40, 1222–1225. [Google Scholar]
- Han, Q.J.; Zheng, Z.; Liu, L.; Zhao, X.Y.; Wang, Y.; Chen, Z.H.; Liu, R.J. Clinical Study of Three-way Treatment of Diabetes by Traditional Chinese Bee Therapy. In Proceedings of the First World Bee Therapy Conference, the Second Annual Meeting of the World Union Bee Therapy Committee, Shenzhen, China, 10 November 2018; pp. 41–44. [Google Scholar]
- Kim, D.H.; Kim, M.Y.; Park, Y.M.; Kim, H.O. A Case of Delayed Type Skin Reaction Induced by Bee Venom Acupuncture. Korean J. Dermatol. 2005, 43, 1237–1240. [Google Scholar]
- Jeong, K.M.; Kwon, S.H.; Baek, Y.S.; Jeon, J.H.; Oh, C.H.; Song, H.J. Cutaneous Mycobacterium massiliense Infection associated with bee venom acupuncture. Ann. Dermatol. 2018, 70, 404. [Google Scholar]
- Lee, E.J.; Ahn, Y.C.; Kim, Y.I.; Oh, M.S.; Partk, Y.C.; Son, C.G. Incidence Rate of Hypersensitivity Reactions to Bee-Venom Acupuncture. Front. Pharmacol. 2020, 11, 545555. [Google Scholar] [CrossRef]
- Yook, T.-H.; Kim, K.-H.; Shin, M.-S. The Clinical Study on the Thermal Changes and Side Effects after Bee Venom Acupuncture Therapy. J. Pharmacopunct. 2001, 4, 7–14. [Google Scholar]
- Won, C.H.; Choi, E.S.; Hong, S.S. Efficacy of Bee Venom Injection for Osteoarthritis Patients. J. Korean Rheum. Assoc. 1999, 6, 218–226. [Google Scholar]
- Kim, S.S. Effects of Bee Venom Acupuncture on Lower Urinary Tract Symptoms Suggestive of Benign Prostatic Hyperplasia; Graduate School of Complementary Alternative Medicine, Pochon CHA University: Pocheon, Korea, 2007. [Google Scholar]
- Kim, J.-H.; Kim, M.-S.; Lee, J.-Y.; Yeom, S.-R.; Kwon, Y.-D.; Kim, D.-W. The Case Report of Analhylaxis after Treated with Bee-Venom Acupuncture. J. Korean Med. Rehabil. 2015, 25, 175–182. [Google Scholar] [CrossRef]
- Li, X.L. Nursing of patients with rheumatoid arthritis treated with bee acupuncture. Chin. J. Nurs. 1994, 523–525. [Google Scholar]
- Ma, H.; Chang, W.Z. Clinical observation of cancer pain controlled by combined morphine sulfate and bee venom injection. Jilin Med. Sci. 2008, 21, 1914–1915. [Google Scholar]
- Yeon, C.-H.; Park, H.-G.; Yi, Y.-S.; Kim, J.-Y.; Chung, S.-H. The Two Cases Report of Bee Venom Injection on Patient with Low Back Pain Maintaining after Heating-Conduction Acupuncture Therapy. J. Korean CHUNA Man. Med. Spine Nerves 2012, 7, 75–81. [Google Scholar]
- Lee, S.J.; Nam, J.H.; Kim, K.W.; Lee, M.J.; Jun, J.Y.; Lim, S.J.; Lee, C.H.; Song, J.H. A Case Study of the Bee Venom Acupuncture Effect for Trigger Finger with Side Effects by Steroid Injection. Acupuncture 2013, 30, 189–196. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kim, K.H. Bee venom acupuncture for circumscribed morphea in a patient with systemic sclerosis: A case report. Medicine 2018, 97, e13404. [Google Scholar] [CrossRef]
- Lee, B.; Seo, B.K.; Kwon, O.J.; Jo, D.J.; Lee, J.H.; Lee, S. Effect of Combined Bee Venom Acupuncture and NSAID Treatment for Non-Specific Chronic Neck Pain: A Randomized, Assessor-Blinded, Pilot Clinical Trial. Toxins 2021, 13, 436. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, Y.W. Clinical research of Bee-venom Acupuncture analgesic effect on Osteoarthritis. J. Acupunct. Res. 1999, 16, 25–37. [Google Scholar]
- Han, S.-H.; Youn, Y.-S.; Kim, S.-S.; Chung, W.-S. A Case Report on Bee Venom Acupuncture for Patient with Osteo-Arthritis of Knee Joint, Diabetic Mellitus, and No Reponse for Steroid Injection. J. Korea CHUNA Man. Med. 2013, 4, 17–28. [Google Scholar]
- Lee, K.H. A Study of the Initial Dose of Sweet Bee Venom for the Treatment of Patients with Lower Back Pain. Korean Acupunct. Moxibustion Med. Soc. 2020, 37, 173–176. [Google Scholar] [CrossRef]
- Lee, M.H.; Son, B.W.; Kim, K.M.; Kim, Y.K. A Case Report on the Effects of Gamiguibi-tang Combined with Sweet Bee Venom to Improve Raynaud’s Disease. Soc. Intern. Korean Med. 2017, 38, 698–708. [Google Scholar] [CrossRef]
- Park, J.-W.; Jeon, J.-H.; Yoon, J.W.; Jung, T.-Y.; Kwon, K.-R.; Cho, C.-K.; Lee, Y.-W.; Sagar, S.; Wong, R.; Yoo, H.-S. Effects of sweet bee venom pharmacopuncture treatment for chemotherapy-induced peripheral neuropathy: A case series. Integr. Cancer Ther. 2012, 11, 166–171. [Google Scholar] [CrossRef]
- Bong, S.M.; Jang, W.S.; Kim, K.H. Effects of Sweet Bee Venom Pharmacopuncture Combined with Korean Medicine Treatment for Acute Low Back Pain Syndrome Patient: A Case Report. Korean J. Acupunct. 2020, 37, 54–62. [Google Scholar] [CrossRef]
- Jo, S.-J.; Yoon, J.-J.; Kim, C.-Y. 11 Cases of Periungual Warts Treated by Korean Medicine. J. Korean Med. Ophthalmol. Otolaryngol. Dermatol. 2019, 32, 224–234. [Google Scholar]
- Castro Neves, A.; Barreira, P.; Moreira Da Silva, J.P. Honeybee immunotherapy in a patient with systemic mastocytosis. Allergy Eur. J. Allergy Clin. Immunol. 2016, 71, 437. [Google Scholar]
- Da Silva, E.N.; Randall, K.L. Pre-treatment with omalizumab allows ultra-rush honey bee venom immunotherapy in patients with mast cell disease. Allergy Eur. J. Allergy Clin. Immunol. 2014, 69, 398–399. [Google Scholar]
- Ekstrom, C.; Salman, S.; Brusch, A. Adverse reactions and modifications to dosing schedule during standard bee venom immunotherapy. Intern. Med. J. 2019, 49, 19. [Google Scholar]
- Fok, J.S.; Heddle, R. Treating honey bee venom allergy in mastocytosis-our experience in Adelaide. Intern. Med. J. 2014, 44, 9. [Google Scholar]
- Gür Çetinkaya, P.; Esenboğa, S.; Uysal Soyer, Ö.; Tuncer, A.; Şekerel, B.E.; Şahiner, Ü.M. Subcutaneous venom immunotherapy in children: Efficacy and safety. Ann. Allergy Asthma Immunol. 2018, 120, 424–428. [Google Scholar] [CrossRef]
- Gür Çetinkaya, P.; Esenboga, S.; Uysal Soyer, Ö.; Yavuz, T.; Tuncer, A.; Sekerel, B.E.; Sahiner, Ü.M. Subcutaneous venom immunotherapy in children: Efficacy and safety. Allergy Eur. J. Allergy Clin. Immunol. 2017, 72, 201. [Google Scholar]
- Kappatou, K.; Brathwaite, N.; Leech, S. Insect venom immunotherapy in children in South East England. Allergy Eur. J. Allergy Clin. Immunol. 2019, 74, 181. [Google Scholar]
- Kempinski, K.; Niedoszytko, M.; Gorska, L.; Kita-Milczarska, K.; Chelminska, M.; Jassem, E. The analysis of safety and effectiveness of allergen immunotherapy for hymenoptera venom allergy. Allergy Eur. J. Allergy Clin. Immunol. 2017, 72, 512. [Google Scholar]
- Kochuyt, A.M.; Stevens, E.A.M. Safety and efficacy of a 12-week maintenance interval in patients treated with hymenoptera venom immunotherapy. Clin. Exp. Allergy 1994, 24, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Kołaczek, A.; Skorupa, D.; Antczak-Marczak, M.; Kuna, P.; Kupczyk, M. Safety and efficacy of venom immunotherapy: A real life study. Postepy Dermatol. I Alergol. 2017, 34, 156–167. [Google Scholar] [CrossRef]
- Mastnik, S.; Rueff, F. Hymenoptera venom immunotherapy in patients with mastocytosis: Management of treatment failure. Allergo J. Int. 2020, 29, 254. [Google Scholar]
- Nittner-Marszalska, M.; Cichocka-Jarosz, E.; Małaczyńska, T.; Kraluk, B.; Rosiek-Biegus, M.; Kosińska, M.; Pawłowicz, R.; Lis, G. Safety of ultrarush venom immunotherapy: Comparison between children and adults. J. Investig. Allergol. Clin. Immunol. 2016, 26, 40–47. [Google Scholar]
- Puebla Villaescusa, A.; Murgadella Sancho, A.; Losa López, L.; Gracia García, B.; San Juan Martinez, N. Effectiveness of omalizumab and bee venom immunotherapy combination: Case report. Eur. J. Hosp. Pharm. 2020, 27, A207. [Google Scholar]
- Rerinck, H.C.; Przybilla, B.; Ruëff, F. Venom Immunotherapy (VIT) in patients with Systemic Mastocytosis (SM) and Hymenoptera Venom Anaphylaxis (HVA): Safety and efficacy of different maintenance doses. J. Allergy Clin. Immunol. 2009, 123, S242. [Google Scholar] [CrossRef]
- Sieber, W.; Pfeifer, M.; Skandra, T.; Siemon, G. Adverse reactions to rush-venom-immunotherapy in hymenoptera sting allergy with Reless and ALK. Atemwegs-Und Lungenkrankh. 1996, 22, 659–666. [Google Scholar]
- Treudler, R.; Tebbe, B.; Orfanos, C.E. Standardized rapid hyposensitization with purified hymenoptera venom in wasp venom allergy. Prospective study of development of tolerance and side-effect profile. Hautarzt 1997, 48, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Vachová, M.; Galanská, R.; Liska, M.; Vítovcová, P.; Vlas, T.; Panzner, P. Comparison of bee and wasp venom allergic patients treated by venom immunotherapy. Allergy Eur. J. Allergy Clin. Immunol. 2017, 72, 682. [Google Scholar]
- Vázquez-Revuelta, P.; González-De-Olano, D.; Padial-Vilchez, A.; Núlñez-Acevedo, B.; Frutos-Reche, M. Omalizumab as adjuvant treatment during venom immunotherapy in mast cell disorders. Allergy Eur. J. Allergy Clin. Immunol. 2017, 72, 684–685. [Google Scholar]
- Wieczorek, D.; Kapp, A.; Wedi, B. Ultra-rush immunotherapy with wasp venom in a high-risk patient and long-term follow-up. Allergo J. Int. 2020, 29, 250–251. [Google Scholar]
- Arzt-Gradwohl, L.; Herzog, S.; Schrautzer, C.; Laipold, K.; Stoevesandt, J.; Trautmann, A.; Vachová, M.; Hawranek, T.; Lang, R.; Alfaya Arias, T.; et al. No effect of antihypertensive drugs on severity of anaphylaxis and adverse events during venom immunotherapy. Allergo J. Int. 2020, 29, 250. [Google Scholar]
- Hanzlikova, J.; Vachova, M.; Gorcikova, J.; Vlas, T.; Panzner, P. Case report of hereditary angioedema with anaphylaxis after hornet sting and consequent venom immunotherapy. Allergy Eur. J. Allergy Clin. Immunol. 2013, 68, 611. [Google Scholar]
- Lanning, J.T.; Kubicz, G.D. Rush therapy for venom anaphylaxis is effective at preventing ID skin reaction. Ann. Allergy Asthma Immunol. 2015, 115, A80. [Google Scholar]
- Nittner-Marszalska, M.; Kowal, A.; Szewczyk, P.; Guranski, K.; Ejma, M. Wasp Venom Immunotherapy in a Patient With Immune-Mediated Inflammatory Central Nervous System Disease: Is it Safe? J. Investig. Allergol. Clin. Immunol. 2017, 27, 127–129. [Google Scholar] [CrossRef]
- Pospischil, I.; Kagerer, M.; Cozzio, A.; Angelova-Fischer, I.; Guenova, E.; Ballmer-Weber, B.; Hoetzenecker, W. Comparison of the safety profiles of three different Hymenoptera venom immunotherapy protocols-a retrospective two-center study of 143 patients. Exp. Dermatol. 2021, 30, e3. [Google Scholar]
- Toldra, S.; Farzanegan, R.; Aleixos, M.; Lanuza, A.; Perez, C.; Prieto, L. Bee venom immunotherapy only tolerated with concurrent treatment with omalizumab. Allergy Eur. J. Allergy Clin. Immunol. 2017, 72, 685. [Google Scholar]
- Goh, J.; MacKey, L.; Johannsen, H.; Ziegler, C. A 20-year retrospective audit of honeybee venom immunotherapy undertaken at the flinders medical centre, South Australia. Intern. Med. J. 2021, 51, 15. [Google Scholar]
- Utani, A.; Hattori, Y. 8 cases of bee venom allergy. Acta Dermatol. 1997, 92, 213–220. [Google Scholar]
- Li, X.Y. Clinical Observation of Allergic Urticaria Caused by Different Doses of Bee Venom Acupuncture Therapy; Guangzhou University of Traditional Chinese Medicine: Guangzhou, China, 2015. [Google Scholar]
- Wen, W.Q.; Lai, D.Y.; Zhu, H.J. 43 Cases of Knee Osteoarthritis Treated by Bee Acupuncture and Bushen Huoxue decoction. Beijing Tradit. Chin. Med. 2003, 13–14. [Google Scholar]
- Wen, W.Q.; Huang, S.G.; Wang, R.R. Bee-Stinging Therapy for Undifferentiated Connective Tissue Disease: A Report of 40 Cases. J. Guangzhou Univ. Tradit. Chin. Med. 2003, 221–223. [Google Scholar]
- Chen, S.Y.; Zhou, P.; Qin, Y. Clinical study of bee acupuncture treatment for rheumatoid arthritis. Acupunct. Study 2018, 43, 251–254, 259. [Google Scholar]
- Qin, X.H. Clinical Curative Effect of Bee Acupuncture on Pain of Shoulder and Hand Syndrome After Stroke; Guangzhou University of Traditional Chinese Medicine: Guangzhou, China, 2010. [Google Scholar]
- Jiao, C.Y. Clinical Curative Effect of Bee Acupuncture on Primary Menstrual Pain; Guangzhou University of Traditional Chinese Medicine: Guangzhou, China, 2011. [Google Scholar]
- Wu, H.B.; Chen, X.H.; Zhang, R.K.; Luo, Q.L.; Cheng, Y.M. Clinical study of Lingnan painless bee therapy combined with McKenzie therapy for lumbar disc herniation. J. Rehabil. 2020, 30, 441–446. [Google Scholar]
- Moon, J.-H.; Yeom, S.D.; Ko, H.S.; Kang, M.J.; Byun, J.W.; Shin, J.H.; Choi, G.S. Therapeutic effect of bee venom solution and fractional CO2 laser on vitiligo. Ann. Dermatol. 2014, 66, 423–424. [Google Scholar]
- Mo, Y.J.; Lu, Y.; Du, S.Y. Evaluation of efficacy of bee venom gel cream on common acne among young volunteers. Cap. Food Med. 2020, 27, 53–54. [Google Scholar]
- Park, B.-R.; Kim, J.-M.; Cho, C.-K.; Shin, S.-H.; Yoo, H.-S. Effect of Bee Venom Ointment Treatment for Chemotherapy-induced Peripheral Neuropathy: A Case Series. Daejeon Univ. Korean Med. Res. Inst. 2014, 22, 111–117. [Google Scholar]
- Yang, J.L.; Cheng, Y.M.; Deng, T.C.; Zhu, J.Y.; Wen, J.Z.; Huang, C.J. Efficacy and safety of bee venom honey spot application in treating acute bacterial tonsillitis in children. Gansu Med. 2014, 33, 820–823. [Google Scholar]
- Lee, Y.; Kim, S.-G.; Kim, I.-S.; Lee, H.-D. Venom Pharmacopuncture Containing Melittin as the Active Ingredient. Evid. Based Complement. Alternat. Med. 2018, 2018, 2353280. [Google Scholar] [PubMed]
- Helden, D.R.V.; Dosen, P.J.; O’Leary, M.A.; Isbister, G.K. Two pathways for venom toxin entry consequent to injection of an Australian elapid snake venom. Sci. Rep. 2019, 9, 8595. [Google Scholar] [CrossRef] [PubMed]
- Nisa, S.A.; Vinu, D.; Krupakar, P.; Govindaraju, K.; Sharma, D.; Vivek, R. Jellyfish venom proteins and their pharmacological potentials: A review. Int. J. Biol. Macromol. 2021, 176, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Zahirovic, A.; Luzar, J.; Molek, P.; Kruljec, N.; Lunder, M. Bee Venom Immunotherapy: Current Status and Future Directions. Clin. Rev. Allergy Immunol. 2020, 58, 326–341. [Google Scholar] [CrossRef]
- Dantzer, J.A.; Wood, R.A. The use of omalizumab in allergen immunotherapy. Clin. Exp. Allergy 2018, 48, 232–240. [Google Scholar] [CrossRef]
- Cortellini, G.; Severino, M.; Francescato, E.; Turillazzi, S.; Spadolini, I.; Rogkakou, A.; Passalacqua, G. Evaluation and validation of a bee venom sting challenge performed by a micro-syringe. Ann. Allergy Asthma Immunol. 2012, 109, 438–441. [Google Scholar] [CrossRef]
- Lee, J.S.; Kwon, G.R.; Choi, H.Y. A Study on Major Components of Bee Venom Using HPLC. J. Korean Acupunct. Moxibustion Soc. 2000, 17, 120–129. [Google Scholar]
- Park, J.S.; Lee, M.J.; Chung, K.H.; Ko, D.K.; Cung, H. Live bee acupuncture (Bong-Chim) dermatitis: Dermatitis due to live bee acupuncture therapy in Korea. Int. J. Dermatol. 2013, 52, 1519–1524. [Google Scholar] [CrossRef]
- Otreba, M.; Marek, L.; Tyczynska, N.; Stojko, J.; Stojko, A.R. Bee Venom, Honey, and Royal Jelly in the Treatment of Bacterial Infections of the Oral Cavity: A Review. Life 2021, 11, 1311. [Google Scholar] [CrossRef]
- Samanci, A.E.T.; Kekecoglu, M. Development of a cream fomulation containing bee venom and other bee products. J. Cosmet. Dematol. 2022. [Google Scholar] [CrossRef]
- Cifuentes, L. Allergy to honeybee not only stings. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Spilker, B. Interpretation of Adverse Reactions. In Guide to Clinical Trials; Raven Press, Ltd.: New York, NY, USA, 1991; pp. 565–587. [Google Scholar]
- Mueller, H.L. Insect Sting Allergy; Gustav Fischer: Stuttgart, Germany, 1990. [Google Scholar]
- World Health Organizations. The Use of the WHO-UMC System for Standardized Case Causality Assessment; World Health Organizations: Uppsala, Sweden, 2005. [Google Scholar]
- Shamseer, L.; Moher, D.; Clarke, M.; Chersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Savovic, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Chapter 8: Assessing risk of bias in a randomized trail. In Cochrane Handbook for Systematic Reviews of Interventions, Version 6.2; Higgins, J.P.T., Thomas, J., Chanderl, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; (Updated February 2021); Cochrane: London, UK, 2021. [Google Scholar]

| First Author | Country | Reason | Paper Type | Number of Cases | Venom Type | Skin Test | Injection Amount | Concomitant Treatment | Adverse Events Symptoms | Adverse Events Severity | Adverse Events Type | Mueller Classification | Causality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Han [12] | Korea | pain prevention therapy | CR | 1 | bees | NR | NR | NR | skin atrophy | severe | SP | Gr1 | probable |
| Castro [13] | U.S.A. | multiple sclerosis | CR | 9 | bees | NR | NR | NR | none | - | - | - | - |
| Lee [14] | Korea | facial palsy | cohort | 108 | bees | tested A: negative B: positive | 0.1–0.2 mL | - | rash pruritus swelling vesicles erythema hives | mild | SP | Gr 1 | probable |
| Jeong [15] | Korea | rotator cuff disease | cohort | 4 | bees | tested (negative) | 0.1~0.5 cc | acupuncture herbal medicine physical therapy | none | - | - | - | - |
| Kim [16] | Korea | CRPS | CR | 1 | bees | NR | 0.15–0.4 mL | anticonvulsant tricyclic antidepressant analgesic | hypersensitivity dyspepsia rash depression | mild | SP SR | Gr1 | possible |
| Kim [17] | Korea | allergic rhinitis | CR | 2 | bees | NR | 0.1~0.3 cc | acupuncture | none | - | - | - | - |
| Moon [18] | Korea | Fibromyalgia | CR | 1 | bees | NR | 0.25 ccx4 | acupuncture pharmacopuncture (hwangryunhaedok-tang) cupping moxibustion herbal medicine | None | - | - | - | - |
| Park [19] | Korea | lumbar disc herniation | cohort | A:12 B:10 | A:- B:bees | tested (negative) | A:1.0 cc B:1.0 cc | A: Shinbaro, acupuncture, cupping, moxibustion, herbal medicine, physical therapy B: acupuncture, cupping, moxibustion, herbal medicine, physical therapy | redness itching | mild | SP | Gr1 | possible |
| An [20] | Korea | Systemic Lupus Erythematosus | CR | 1 | bees | NR | NR | pharmacopuncture acupuncture herbal medicine | None | - | - | - | - |
| Kim [21] | Korea | survey study | cohort | A:132 B:336 | A:bees B:- | tested (negative) | NR | A:- B:NR | point pain redness swelling numbness | mild | SP | Gr1 | possible |
| Lee [22] | Korea | refractory postherpetic neuralgia | CR | 1 | Bees | tested (negative) | NR | NR | none | - | - | - | - |
| Kam [23] | China | lung cancer | nRCT | A:85 B:82 | A:bees B:- | NR | NR | A:- B: granulocyte colony-stimulating factor | NR | NR | NR | NR | NR |
| Gwo [24] | China | chronic urticaria | RCT | A:50 B:50 | A:bees B:- | NR | NR | A: herbal medicine B: acupuncture, herbal medicine | NR | mild | NR | NR | NR |
| Gwo [25] | China | ankylosing | RCT | A:30 B:30 | A:bees | NR | NR | A: Bee’s oral medicine B: western medicine | NR | NR | NR | NR | NR |
| Chiu [26] | China | rheumatoid arthritis | RCT | A:35 B:35 | A:bees B:- | NR | NR | A: methotrexine B: methotrexine, prednisolone acetate | NR | NR | NR | NR | NR |
| Qi [27] | China | rheumatoid arthritis | RCT | A:49 B:49 | A:bees B:- | NR | NR | A: NR B: western medicine | NR | NR | NR | NR | NR |
| She [28] | China | ankylosing | nRCT | A:68 B:38 | A:bees B:- | NR | NR | A: chuna B: oral seraxib capsules | stomachache | mild | SR | Gr2 | possible |
| Su [29] | China | ankylosing | CR | NR | bees | NR | NR | NR | none | - | - | - | - |
| Su [30] | China | enlargement of mammary gland | RCT | A:30 B:30 C:30 | A:bees B:bees C:- | NR | NR | A:- B: acupuncture C: acupuncture | fever urticaria lymphoma cirrhosis bleeding | moderate | SP SR | Gr1 | probable |
| An [31] | China | cancerous pain from lung cancer | RCT | A:39 B:39 | A:bees B:- | NR | NR | A: hydroxycodone tablets B: hydroxycodone tablets | NR | NR | NR | NR | NR |
| Yang [32] | China | rheumatoid arthritis | RCT | A:46 B:46 | A:bees B:- | NR | NR | A: Chinese medicine B: routine treatment | NR | NR | NR | NR | NR |
| Wen [33] | China | ankylosing | RCT | A:40 B:40 | A:bees B:- | NR | NR | A:- B: sulfasalazine, diclofenac | NR | NR | NR | NR | NR |
| Wang [34] | China | cancer pain | RCT | A:44 B:43 | A:bees B:- | NR | NR | A: fentanyl percutaneous patch B: fentanyl percutaneous patch | NR | NR | NR | NR | NR |
| Zhang [35] | China | frozen shoulder | RCT | A:33 B:32 B:32 | A:bees B:- C:- | NR | NR | A: acupotomy B: acupotomy, triamcinolone acetonide C: acupotomy | NR | NR | NR | NR | NR |
| Zhang [36] | China | facial palsy | RCT | A:36 B:35 | A:bees B:- | NR | NR | A: acupuncture B: acupuncture | redness itching | mild | SP | Gr1 | possible |
| Zhou [37] | China | ankylosing | CS | 40 | bees | NR | NR | Chinese medicine | NR | NR | NR | NR | NR |
| Zhou [38] | China | rheumatoid arthritis | RCT | A:40 B:30 C:30 | A:bees | NR | NR | A:- B: electro acupuncture C: western medicine | None | - | - | - | - |
| Zhu [39] | China | ankylosing | CS | 56 | bees | tested (negative) | NR | Chinese medicine | fever itching urticaria pain anaphylaxis | severe | SP SR | Gr4 | probable |
| Zeng [40] | China | ankylosing | RCT | A:54 B:54 | A:bees B:- | NR | NR | A: moxibustion B: acupuncture | NR | NR | NR | NR | NR |
| Chen [41] | China | leukocyte reduction after colorectal cancer chemotherapy | nRCT | A:33 B:33 | A:bees B:- | NR | NR | A:- B: white elm tablets | fever | mild | SP | Gr1 | possible |
| Chen [42] | China | rheumatoid arthritis | RCT | A:30 B:30 | A:bees B:- | NR | NR | A:- B: oral methotrexate, celecoxib | none | - | - | - | - |
| Peng [43] | China | cancer pain | RCT | A:31 B:33 | A:bees B: | NR | NR | A: tramadol 100 mg B: tramadol 100 mg | NR | NR | NR | NR | NR |
| Peng [44] | China | cancer pain | RCT | A:30 B:30 | A:bees B:- | NR | NR | A: pain medicine 3rd phase B: pain medicine 3rd phase (WHO recommended) | NR | NR | NR | NR | NR |
| Huang [45] | China | rheumatoid arthritis | RCT | A:30 B:30 | A:bees B:- | NR | NR | A:- B: hemp tablet | NR | NR | NR | NR | NR |
| Guo [46] | China | ankylosing | RCT | A:36 B:36 | A:bees B:- | NR | NR | A:- B: western treatment | NR | NR | NR | NR | NR |
| Deng [47] | China | RA | RCT | A:20 B:20 C:20 | A:bees B:- C:- | NR | NR | A: metrotrexate B: metrotrexate C: strong metrotrexate | NR | NR | NR | NR | NR |
| Liu [48] | China | RA | RCT | A:50 B:50 | A:bees B:- | NR | NR | A: western medicine B: western medicine | NR | NR | NR | NR | NR |
| Yang [49] | China | diabetic neuropathy | RCT | A:25 B:25 | A:bees B:- | NR | NR | A: epalrestat, methylcobalamin B: epalrestat, methylcobalamin | none | - | - | - | - |
| Wen [50] | China | postpartum pain | RCT | A:41 B:40 | A:bees B:- | NR | NR | A: herbal fumigation B: diclofenac natrium minidose | NR | NR | NR | NR | NR |
| Wen [51] | China | postherpetic neuralgia | RCT | A:36 B:36 | A:bees B:- | NR | NR | A:- B: unknown injection | NR | NR | NR | NR | NR |
| Wei [52] | China | rheumatoid arthritis | RCT | A:30 B:30 | A:bees B:- | NR | NR | A:- B: Chinese medicine | NR | NR | NR | NR | NR |
| Ying [53] | China | shoulder pain | RCT | A:60 B:60 | A:bees B:- | NR | NR | A:- B: massage, acupuncture | NR | NR | NR | NR | NR |
| Zhou [54] | China | neurotic tinnitus | RCT | A:30 B:30 | A:bees B:- | NR | NR | A: heating needle B: flunarizine hydrochloride capsule, mecobalamin minidose | NR | NR | NR | NR | NR |
| Chen [55] | China | lumbar disc herniation | CS | 4000 | bees | NR | NR | chuna | NR | NR | NR | NR | NR |
| Chen [56] | China | rheumatoid arthritis | RCT | A:30 B:30 C:30 | A:bees (high) B:bees (low) C:- | NR | NR | A:- B:- C: methotrexate 10 mg, cerecoxib 0.2 g | NR | NR | NR | NR | NR |
| Qin [57] | China | rheumatoid arthritis | RCT | A:32 B:28 | A:bees B:- | NR | NR | A: xianlong granule B: methotrexate | NR | NR | NR | NR | NR |
| Han [58] | China | diabetes | CS | 80 | bees | NR | NR | Chinese medicine | NR | NR | NR | NR | NR |
| Kim [59] | Korea | NR | CR | 1 | bees | NR | NR | NR | papules crust | moderate | SP | Gr1 | probable |
| Jeong [60] | Korea | NR | CR | 1 | bees | NR | NR | NR | mycobacterium massiliense granulomatous | moderate | SP | Gr1 | probable |
| Lee [61] | Korea | NR | cohort | 8580 | bees | NR | NR | NR | anaphylaxis shock | severe | SR | Gr4 | probable |
| Yook [62] | Korea | effect | nRCT | A:19 B:23 | A:bees B:- | NR | 0.05x4 | A:- B: normal saline | Body ache itching sense redness swelling headache dizziness fatigue nausea | mild | SP SR | Gr2 | possible |
| Won [63] | Korea | osteoarthritis | RCT | A:25 B:26 C:26 D:24 | A,B,C:bees D:- | NR | A:~0.7 mg B:~1.5 mg C:~2.0 mg D:1000 mg | A,B,C:- D: nabumetone | Itching body ache | mild | SP SR | Gr1 | possible |
| Kim [64] | Korea | lower urinary tract symptoms | CS | 41 | bees | NR | NR | NR | none | - | - | - | - |
| Kim [65] | Korea | NR | CR | 2 | bees | NR | NR | NR | (1) hypotension, drowsy mentality, dyspnea, vomiting (2) itching sensation, urticaria, breathlessness, abdominal pain | severe | SP SR | (1) Gr4 (2) Gr3 | probable |
| Li [66] | China | rheumatoid arthritis | CS | 225 | bees | NR | NR | NR | NR | NR | NR | NR | NR |
| Ma [67] | China | cancer pain | CR | NR | bees | NR | 0.5 mg | morphine sulfate | constipation drowsy | mild | SPSR | Gr1 | possible |
| Yeon [68] | Korea | low back pain | CR | 2 | bees | tested (negative) | 0.2 cc | (1) fire needling (2) - | NR | NR | NR | NR | NR |
| Lee [69] | Korea | trigger finger | CR | 1 | bees | tested (negative) | 0.3 cc | NR | none | - | - | - | - |
| Hwang [70] | Korea | systemic sclerosis | CR | 1 | bees | tested (negative) | NR | NR | none | - | - | - | - |
| Lee [71] | Korea | non-specific neck pain | RCT | A:30 B:30 | A:bees B:- | NR | A:NR B:180 mg | A:- B: loxoprofen | none | - | - | - | - |
| Kim [72] | Korea | knee OA | nRCT | A:40 B:NR | A:bees B:- | NR | NR | A:- B: acupuncture | NR | NR | NR | NR | NR |
| Han [73] | Korea | OA with DM | CR | 1 | bees | NR | NR | herbal medicine physical therapy acupuncture | none | - | - | - | - |
| Lee [74] | Korea | lower back pain | cohort | 523 | bees | NR | 0.1–1.2 mL | NR | local hypersensitivity | moderate | SP | Gr1 | possible |
| Lee [75] | Korea | Raynaud’s disease | CR | 1 | bees | NR | NR | herbal medicine (Gamiguibi-tang) | none | - | - | - | - |
| Park [76] | Korea | chemotherapy-induced peripheral neuropathy | CR | 5 | bees | tested (negative) | NR | NR | none | - | - | - | - |
| Bong [77] | Korea | acute low back pain | CR | 3 | bees | NR | NR | acupuncture cupping herbal medicine physical therapy | none | - | - | - | - |
| Jo [78] | Korea | periungual warts | CR | 11 | bees | NR | NR | acupuncture herbal medicine moxibustion | none | - | - | - | - |
| First Author | Country | Reason | Paper Type | Number of Cases | Venom Type | Skin Test | Injection Amount | Concomitant Treatment | Adverse Events Symptoms | Adverse Events Severity | Adverse Events Type | Mueller Classification | Causality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Castro Neves [79] | Turkey | treatment of systematic allergic reactions | CR | 1 | bees | tested (positive) | 100 μg | NR | none | - | - | - | - |
| Da Silva [80] | Australia | treatment of systematic allergic reactions | CR | 2 | bees | tested (positive) | 100 μg | NR | (1) none (2) NR | (1) - (2) NR | (1) - (2) NR | (1) - (2) NR | (1) - (2) NR |
| Ekstrom [81] | Germany | treatment of systematic allergic reactions | CS | A:46 B:68 | bees | tested (positive) | NR | omalizumab (4 cases) | NR | NR | NR | NR | NR |
| Fok [82] | Australia | treatment of systematic allergic reactions | cohort | A:5 B:1 | A:bees B:wasp | tested (positive) | 100 μgx2 | NR | hypotensive systemic reactions | severe | SR | Gr 4 | probable |
| Gür Çetinkaya [83] | Turkey | treatment of systematic allergic reactions | cohort | 107 | wasp | tested (positive) | NR | NR | local reactions systematic reactions | NR | SP SR | NR | possible |
| Gür çetinkaya [84] | Turkey | treatment of systematic allergic reactions | CS | 107 | A:bees B:wasp C:bees,wasp | tested (positive) | NR | NR | NR | NR | SP SR | NR | possible |
| Kappatou [85] | Greece | treatment of systematic allergic reactions | CR | A:8 B:2 | A:wasp B:bees | 6 tested (5 positive) | NR | NR | NR | mild | SP SR | NR | possible |
| Kempinski [86] | Poland | treatment of systematic allergic reactions | CS | 246 | wasp | NR | NR | NR | field stings anaphylaxis | mild severe | SP SR | Gr1 Gr4 | possible probable |
| Kochuyt [87] | Belgium | treatment of systematic allergic reactions | CS | A:128 B:50 | A:wasp B:bees | NR | 100 μg | NR | field re-stings | mild | SP SR | Gr1 | probable |
| Kołaczek [88] | Poland | treatment of systematic allergic reactions | cohort | A:34 B:146 | A:bees B:wasp | NR | NR | NR | NR | mild | SP SR | Gr1 | possible |
| Mastnik [89] | Germany | treatment of systematic allergic reactions | cohort | A:74 B:124 | A:bees B:wasp | NR | A:100~400 μg B:100~200 μg | NR | NR | mild | SR | Gr1 | probable |
| Nittner-Marszalska [90] | Poland | treatment of systematic allergic reactions | cohort | 341 | bees wasp | NR | NR | NR | NR | mild | SR | Gr1 | possible |
| Puebla Villaescusa [91] | Spain | treatment of systematic allergic reactions | CR | 1 | bees | NR | 40~100 μg | omalizumab (300 mg) | none | - | - | - | - |
| Rerinck [92] | Germany | treatment of systematic allergic reactions | cohort | A:4 B:21 C:8 | A:bees B:wasp C:bees,wasp | NR | 100–200 μg | NR | NR | mild | SR | Gr1 | possible |
| Sieber [93] | Germany | treatment of systematic allergic reactions | RCT | A:30 B:30 | A:bees B:wasp | NR | ~100 mg | NR | anaphylaxis | NR | SP SR | Gr1 Gr4 | possible probable |
| Treudler [94] | Germany | treatment of systematic allergic reactions | CS | 20 | wasp | NR | ~210 mg | NR | NR | NR | NR | NR | NR |
| Vachová [95] | Czech | treatment of systematic allergic reactions | nRCT | A:80 B:65 | A:bees B:wasp | NR | NR | NR | anaphylaxis | mild severe | SP SR | Gr1 Gr4 | probable |
| Vázquez-Revuelta [96] | Spain | treatment of systematic allergic reactions | CR | 1 | NR | NR | ~100 μg | NR | chest tightness oxygen desaturation hypotension | severe | SR | Gr4 | probable |
| Wieczorek [97] | Germany | treatment of systematic allergic reactions | CR | 1 | wasp | tested | ~100 μg | NR | none | - | - | - | - |
| Arzt-Gradwohl [98] | Australia | treatment of systematic allergic reactions | cohort | 1425 | bees wasp | NR | NR | NR | NR | NR | NR | NR | NR |
| Hanzlikova [99] | Czech | treatment of systematic allergic reactions | CR | 1 | wasp | tested (positive) | NR | cetirizine 10 mg danazol 200 mg | none | - | - | - | - |
| Lanning [100] | U.S.A. | treatment of systematic allergic reactions | CR | 1 | wasp | NR | 0.1~0.5 mL | NR | rash | mild | SP | Gr1 | possible |
| Nittner-Marszalska [101] | Poland | treatment of systematic allergic reactions | CR | 1 | wasp | NR | NR | NR | none | - | - | - | - |
| Pospischil [102] | Australia | treatment of systematic allergic reactions | cohort | A:54 B:93 | A:bees B:wasp | NR | NR | NR | cluster rash ultra-rush | NR | SP | Gr1 | possible |
| Toldra [103] | France | treatment of systematic allergic reactions | CR | 1 | bees | NR | ~40 μg | omalizumab (300 mg) | anaphylaxis | severe | SR | Gr4 | probable |
| Goh [104] | Australia | treatment of systematic allergic reactions | cohort | 174 | bees | NR | NR | NR | NR | NR | NR | NR | NR |
| First Author | Country | Reason | Paper Type | Number of Cases | Venom Type | Skin Test | Injection Amount | Concomitant Treatment | Adverse Events Symptoms | Adverse Events Severity | Adverse Events Type | Mueller Classification | Causality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Utani [105] | Japan | NR | CR | 8 | bees | NR | NR | - | erythema wheals anaphylaxis | mild severe | SP SR | Gr1 Gr4 | probable |
| Li [106] | China | NR | RCT | A:120 B:120 | bees | NR | A: lower B: higher | - | urticaria | mild | SP | Gr1 | possible |
| Wen [107] | China | knee osteoarthritis | CS | 43 | bees | tested (negative) | NR | Chinese medicine | fever itching urticaria | mild | SP | Gr1 | possible |
| Wen [108] | China | connective tissue disease | CS | 40 | bees | NR | NR | - | rash mild fever | mild | SP | Gr1 | possible |
| Chen [109] | China | rheumatoid arthritis | RCT | A:60 B:60 | A:bees B:- | NR | NR | A:- B: oral methotrexate | none | - | - | - | - |
| Qin [110] | China | shoulder–hand syndrome after CVA | RCT | A:36 B:36 | A:bees B:- | tested (negative) | 1~3 point 1~3 ea | A: citicoline 0.75 g, DW5% or NS250 mL, rehabilitation treatment B: acupuncture, citicoline 0.75 g + DW5% or NS250 mL, rehabilitation treatment | none | - | - | - | - |
| Jiao [111] | China | primary menstrual pain | RCT | A:30 B:30 | A:bees B:- | NR | 1 ea~4 ea | A:- B: oral ibuprofen capsule | none | - | - | - | - |
| Wu [112] | China | lumbar disc herniation | RCT | A:40 B:40 | A:bees B: | NR | 1 ea~10 ea | A: Mckenzie methods, magneto thermal vibration therapy B: Mckenzie methods, magneto thermal vibration therapy | NR | NR | NR | NR | NR |
| First Author | Country | Reason | Paper Type | Number of Cases | Venom Type | Skin Test | Injection Amount | Concomitant Treatment | Adverse Events Symptoms | Adverse Events Severity | Adverse Events Type | Mueller Classification | Causality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moon [113] | Korea | repigmentation of vitiligo | CR | 7 | bees | NR | NR | fractional CO2 laser | itching erythema persisted hyper pigmentation | mild severe | SP | Gr1 | probable |
| Mo [114] | China | acne | CS | 40 | bees | NR | NR | - | burning itching desorption dryness | mild | SP | Gr1 | possible |
| Park [115] | Korea | chemotherapy-induced peripheral neuropathy | CR | 4 | bees | NR | NR | - | none | - | - | - | - |
| Yang [116] | China | tonsillitis | RCT | A:64B:61 | A:beesB:- | NR | NR | A: honey, oral cephaloclonal granules B: oral cephaloclonal granules | NR | NR | NR | NR | NR |
| Mild | Does Not Significantly Impair Daily Activities (Function) Nor Require Additional Medical Intervention |
| Moderate | Significantly impairs daily activities (function) and may require additional medical intervention but resolves afterwards |
| Severe | Serious adverse events that requires intense medical intervention and leaves sequelae |
| Grade Ⅰ | Itch, Urticarial, Malaise, Anxiety |
| Grade Ⅱ | Any of the above plus two or more of the following: angio-oedema, tight chest, nausea, vomiting, diarrhea, abdominal pain, dizziness |
| Grade Ⅲ | Any of the above plus two or more of the following: dyspnea, wheeze, stridor, hoarseness, weakness, feeling of impending doom |
| Grade Ⅳ | Any of the above plus two or more of the following: hypotension, collapse, loss of consciousness, cyanosis |
| Author [Ref] | Condition | Sample Size | Treatment Time | Treatment Period | Intervention | Control | Evaluation Index | Results | p-Value (Significance) |
|---|---|---|---|---|---|---|---|---|---|
| Gwo [24] | chronic urticaria | (A) 50 (B) 50 | NR | NR | (A) -bee venom injection -herbal medicine | (B) -herbal medicine -acupuncture | (1) Efficacy rate (2) Recurrence rate | (1) (A) 96% (B) 90% (2) (A) 14% (B) 38% | (1) p < 0.05 (2) p < 0.05 |
| Gwo [25] | ankylosing spondylitis | (A) 30 (B) 30 | NR | NR | (A) -bee venom injection -bee’s oral medicine | (B) -western medicine | Efficacy rate | (A) 80.00% (B) 66.67% | significant |
| Chiu [26] | rheumatoid arthritis | (A) 35 (B)35 | NR | NR | (A) -bee venom injection -methotrexine | (B) -methotrexine -prednisolone acetate | (1) Efficacy rate (2) Recurrence rate | (1) (A) 96.49% (B) 65.71% (2) (A) 8.57% (B) 14.29% | (1) p < 0.05 (2) p > 0.05 |
| Qi [27] | rheumatoid arthritis | (A) 49 (B) 49 | NR | NR | (A) -bee venom injection | (B) -western medicine | (1) Efficacy rate (2) Recurrence rate | (1) (A) 95.92% (B) 93.88% (2) (A) 4.08% (B) 16.33% | (1) p > 0.05 (2) p < 0.05 |
| Su [30] | enlargement of mammary gland | (A) 30 (B) 30 (C) 30 | 10 | NR | (A) -bee venom injection (B) -bee venom injection -acupuncture | (C) -acupuncture | (1) Efficacy rate (2) Breast pain, menstruation (3) Breast mass (4) Emotional changes | (1) (A) 76.67% (B) 93.33% (C) 66.67% | (1) (A),(C) p > 0.05 (A),(B) p > 0.05 (B),(C) p < 0.05 (2) (A),(B),(C) p < 0.05 (A),(B) p > 0.05(3) (A),(C) p < 0.05 (A),(B) p > 0.05 (B),(C) p < 0.01 (4) (A),(B),(C) p > 0.05 |
| An [31] | cancerous pain from lung cancer | (A) 39 (B) 39 | NR | 20 days | (A) -bee venom injection -hydroxycodone tablets | (B) -hydroxycodone tablets | Efficacy rate | (A) 82.05% (B) 61.54% | p < 0.05 |
| Yang [32] | rheumatoid arthritis | (A) 46 (B) 46 | NR | 30 days | (A) -bee venom injection -herbal medicine | (B) -routine treatment | (1) Efficacy rate (2) Recurrence rate | (1) (A) 97.83% (B) 78.26% (2) (A) 8.70% (B) 28.26% | (1) p < 0.05 (2) p < 0.05 |
| Wen [33] | ankylosing spondylitis | (A) 40 (B) 40 | NR | 12 weeks | (A) -bee venom injection | (B) -sulfasalazine -diclofenac | (1) Efficacy rate (2) Adverse events rate | (1) (A) 77.5% (B) 80.0% (2) (A) 7.5% (B) 25% | (1) p > 0.05 (2) NR |
| Wang [34] | cancer pain | (A) 44 (B) 43 | NR | NR | (A) -bee venom injection -fentanyl percutaneous patch | (B) -fentanyl percutaneous patch | (1) Efficacy rate (2) Quality of life (3) Pain intensity (4) Adverse event rate | NR | (1) p < 0.01 (2) p < 0.05 (3) p < 0.05 (4) p < 0.01 |
| Zhang [35] | frozen shoulder | (A) 33 (B) 32 (C) 32 | NR | NR | (A) -bee venom injection -acupotomy | (B) -acupotomy -triamcinolone acetonide (C) -acupotomy | Efficacy rate | (A) 100% (B) 100% (C) 93.75% | NR |
| Zhang [36] | facial palsy | (A) 36 (B) 35 | NR | 4 weeks | (A) -bee venom injection -acupuncture | (B) -acupuncture | (1) H-B Grade (2) Sunnybrook scale (3) Efficacy rate | (1) NR (2)NR (3) (A) 97.1% (B) 89.9% | (1) p > 0.05 (2) p < 0.05 (3) p < 0.05 |
| Zhou [38] | rheumatoid arthritis | (A) 40 (B) 30 (C) 30 | NR | NR | (A) -bee venom injection | (B) -electro acupuncture (C) -western medicine | Blood test level | NR | NR |
| Zeng [40] | ankylosing spondylitis | (A) 54 (B) 54 | NR | NR | (A) -bee venom injection -moxibustion | (B) -acupuncture | Efficacy rate | (A) 74.07% (B) 42.31% | p < 0.05 |
| Chen [42] | rheumatoid arthritis | (A) 30 (B) 30 | NR | NR | (A) -bee venom injection | (B) -oral methotrexate -celecoxib | (1) Efficacy rate (2) VAS | NR | (1) p < 0.05 (2) p < 0.05 |
| Peng [43] | cancer pain | (A) 31 (B) 33 | NR | NR | (A) -bee venom injection -tramadol 100 mg | (B) -tramadol 100 mg | (1) Pain relief (2) Quality of life (3) Adverse event relief (4) Systemic symptoms | NR | (1) p < 0.01 (2) p > 0.05 (3) p < 0.05 (4) p < 0.05 |
| Peng [44] | cancer pain | (A) 30 (B) 30 | 30 | 30 days | (A) -bee venom injection -pain medicine 3rd phase (WHO recommended) | (B) -pain medicine 3rd phase (WHO recommended) | (1) Efficacy rate (2) Adverse event rate | (1) (A) 96.67% (B) 90.00% (2)NR | (1) p < 0.05 (2) p < 0.05 |
| Huang [45] | rheumatoid arthritis | (A) 30 (B) 30 | 30 | NR | (A) -bee venom injection | (B) -hemp tablets | Efficacy rate | (A) 100% (B) 86.7% | p < 0.01 |
| Guo [46] | ankylosing spondylitis | (A) 36 (B) 36 | NR | NR | (A) -bee venom injection | (B) -western treatment | Efficacy rate | (A) 94.44% (B) 72.22% | significant |
| Deng [47] | rheumatoid arthritis | (A) 20 (B) 20 (C) 20 | NR | 60 days | (A) -bee venom injection -metrotrexate | (B) -metrotrexate (C) -strong metrotrexate | (1) Clinical symptoms (2) Blood test level | NR | NR |
| Liu [48] | rheumatoid arthritis | (A) 50 (B) 50 | NR | 3 months | (A) -bee venom injection -western medicine | (B) -western medicine | (1) Efficacy rate (2) Symptoms (3) Adverse event and recurrence rate | NR | (1) p < 0.05 (2) p < 0.05 (3) p < 0.05 |
| Yang [49] | diabetic neuropathy | (A) 25 (B) 25 | 15 | 15 days | (A) -bee venom injection -epalrestat -methylcobalamin | (B) -epalrestat -methylcobalamin | (1) Neurotransmission speed (2) Hydrogen peroxide enzyme level (3) Glutathione level | NR | (1),(2),(3) p < 0.05 |
| Wen [50] | postpartum pain | (A) 41 (B) 40 | NR | 8 weeks | (A) -bee venom injection -herbal fumigation | (B) -diclofenac natrium minidose | Efficacy rate | (A) 95.2% (B) 77.5% | p < 0.01 |
| Wen [51] | postherpetic neuralgia | (A) 36 (B) 36 | NR | 12 weeks | (A) -bee venom injection | (B) -injection(unknown) | (1) Efficacy rate (2) Blood serum test (3) Adverse event rate | (1) (A) 97.22% (B) 77.78% (2),(3) NR | (1) p < 0.05 (2) p < 0.05 (3) p > 0.05 |
| Wei [52] | rheumatoid arthritis | (A) 30 (B) 30 | NR | NR | (A) -bee venom injection | (B) -Chinese medicine | (1) Efficacy rate (2) VAS (3) Blood serum test (4) Adverse event rate | (1) (A) 90.00% (B) 66.66% (2),(3),(4) NR | (1) p < 0.05 (2) p > 0.05 (3) p < 0.05 (4) p > 0.05 |
| Ying [53] | Shoulder pain | (A) 60 (B) 60 | NR | 4 weeks | (A) -bee venom injection | (B) -massage -acupuncture | (1) Efficacy rate (2) McGill pain scale (3) Constant-Murley score (4) Ridiet analysis | (1) (A) 95.00% (B) 81.67% (2),(3),(4) NR | (1) p < 0.05 (2) p < 0.01 (3) p < 0.01 (4) p < 0.05 |
| Zhou [54] | neurotic tinnitus | (A) 30 (B) 30 | NR | 4 weeks | (A) -bee venom injection -heating needle | (B) -flunarizine hydrochloride capsule -mecobalamin minidose | (1) Hearing impairment threshold level (2) Tinnitus (3) SDS level (4) Efficacy rate | (1),(2),(3) NR (4) (A) 83.33% (B) 66.67% | (1) p < 0.01 (2) p < 0.01 (3) p < 0.01 (4) p < 0.05 |
| Chen [56] | Rheumatoid arthritis | (A) 30 (B) 30 (C) 30 | (A),(B) 24 (C) 56 | 8 weeks | (A) -bee venom injection (high dose) (B) -bee venom injection (low dose) | (C) -methotrexate 10 mg -cerecoxib 0.2 g | Efficacy rate | (A) 86.67% (B) 70.00% (C) 76.67 | p < 0.05 |
| Qin [57] | rheumatoid arthritis | (A) 32 (B) 28 | NR | 3 months | (A) -bee venom injection -xianlong granule | (B) -methotrexate | (1) Efficacy rate (2) TCM syndrome score (3) VAS (4) DAS (5) HAQ score (6) Adverse event rate | (1) (A) 90.6% (B) 85.7% (2),(3),(4),(5),(6) NR | (1) p > 0.05 (2) p > 0.05 (3) p > 0.05 (4) p > 0.05 (5) p < 0.05 |
| Won [63] | osteoarthritis | (A) 25 (B) 26 (C) 26 (D) 24 | 42 | 6 weeks | (A) -bee venom injection(~0.7 mg) (B) -bee venom injection(~1.5 mg) (C) -bee venom injection(~2.0 mg) | (D) -nabumetone 1000 mg | Efficacy rate | NR | (A),(B),(C):(D) p < 0.01 (B),(C):(A) p < 0.01 |
| Lee [71] | non-specific neck pain | (A) 30 (B) 30 | NR | ≥ 3 months | (A) -bee venom injection | (B) -loxoprofen 180 mg | Clinical symptoms | NR | NR |
| Chen [109] | rheumatoid arthritis | (A) 60 (B) 60 | (A) 24 (B) 56 | 8 weeks | (A) -live bee sting | (B) -oral methotrexate | (1) Efficacy rate (2) Morning stiffness, joint pain/edema/tenderness index, grip strength, 15 min walking time, VAS, rheumatoid factor, CRP level | (1) (A) 83.33% (B) 80.00% (2) NR | (1) p > 0.05 (2) p > 0.05 |
| Qin [110] | shoulder–hand syndrome after CVA | (A) 36 (B) 36 | live bee sting 9 acupuncture 18 rehabilitation 18 fluid 21 | 3 weeks | (A) -live bee sting -citicoline 0.75 g -DW5% or NS250 mL -rehabilitation treatment | (B) -acupuncture -citicoline 0.75 g -DW5% or NS250 mL -rehabilitation treatment | (1) Efficacy rate (2) VAS | (1) (A) 93.75% (B) 73.53% (2) NR | (1) p < 0.05 (2) p < 0.01 |
| Jiao [111] | primary menstrual pain | (A) 30 (B) 30 | (A) 10 (B) NR | 3 months | (A) -live bee sting | (B) -oral ibuprofen capsules | (1) Efficacy rate (2) Adverse event rate | (1) (A) 93.3% (B) 76.6% (2) (A) 100% (B) 0% | (1) p < 0.05 (2) p < 0.05 |
| Wu [112] | lumbar disc herniation | (A) 40 (B) 40 | live bee sting, magneto thermal vibration therapy 14 Mckenzie methods 7 | 2 weeks | (A) -live bee sting -Mckenzie methods -Magneto thermal vibration therapy | (B) -Mckenzie methods -Magneto thermal vibration therapy | (1) VAS (2) ODI (3) TCM score (4) Clinical efficacy rate | (1),(2),(3) NR (4) (A) 95% (B) 80% | (1) p < 0.05 (2) p > 0.05 (3) p < 0.05 (4) p < 0.05 |
| Yang [116] | Tonsillitis | (A) 64 (B) 61 | 10 | 5 days | (A) -bee venom externals -honey externals -oral cephaloclonal granules | (B) -oral cephaloclonal granules | (1) Efficacy rate (2) Adverse event rate | (1) (A) 100% (B) 90.2% (2) (A) 3.1% (B) 1.6% | (1) p < 0.05 (2) p > 0.05 |
| Causality Term | Assessment Criteria |
|---|---|
| Certain | -Event or laboratory test abnormality, with plausible time relationship to drug intake -Cannot be explained by disease or other drugs -Response to withdrawal plausible (pharmacologically, pathologically) -Event definitive pharmacologically or phenomenologically (i.e., and objective and specific medical disorder or a recognized pharmacological phenomenon) -Rechallenge satisfactory, if necessary |
| Probable/ Likely | -Event or laboratory test abnormality, with reasonable time relationship to drug intake -Unlikely to be attributed to disease or other drugs -Response to withdrawal clinically reasonable -Rechallenge not required |
| Possible | -Event or laboratory test abnormality, with reasonable time relationship to drug intake -Could also be explained by disease or other drugs -Information on drug withdrawal may be lacking or unclear |
| Unlikely | -Event or laboratory test abnormality, with a time to drug intake that makes a relationship improbable (but not impossible) -Disease or other drugs provide plausible explanations |
| Conditional/ Unclassified | -Event of laboratory test abnormality -More data for proper assessment needed, or -Additional data under examination |
| Unassessable/ Unclassifiable | -Report suggesting and adverse reaction -Cannot be judged because information is insufficient or contradictory -Data cannot be supplemented or verified |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, J.; Lee, G. Adverse Events Associated with the Clinical Use of Bee Venom: A Review. Toxins 2022, 14, 562. https://doi.org/10.3390/toxins14080562
Yoo J, Lee G. Adverse Events Associated with the Clinical Use of Bee Venom: A Review. Toxins. 2022; 14(8):562. https://doi.org/10.3390/toxins14080562
Chicago/Turabian StyleYoo, Jaehee, and Gihyun Lee. 2022. "Adverse Events Associated with the Clinical Use of Bee Venom: A Review" Toxins 14, no. 8: 562. https://doi.org/10.3390/toxins14080562
APA StyleYoo, J., & Lee, G. (2022). Adverse Events Associated with the Clinical Use of Bee Venom: A Review. Toxins, 14(8), 562. https://doi.org/10.3390/toxins14080562





