Abstract
Tetrodotoxin (TTX), a potent paralytic sodium channel blocker, is an intriguing marine toxin. Widely distributed in nature, TTX has attracted attention in various scientific fields, from biomedical studies to environmental safety concerns. Despite a long history of studies, many issues concerning the biosynthesis, origin, and spread of TTX in animals and ecosystems remain. This review aims to summarize the current knowledge on TTX circulation inside TTX-bearing animal bodies. We focus on the advances in TTX detection at the cellular and subcellular levels, providing an expanded picture of intra-organismal TTX migration mechanisms. We believe that this review will help address the gaps in the understanding of the biological function of TTX and facilitate the development of further studies involving TTX-bearing animals.
Key Contribution:
Anatomical distribution and accumulation of TTX in animals are reviewed. TTX kinetics in TTX-bearing animals are discussed in detail. This review will help unveil sources of TTX in toxic animals.
1. Introduction
Tetrodotoxin (TTX), one of the deadliest natural toxins, has attracted the interest of researchers from various fields for decades. This non-protein, weakly basic, heat-resistant low-molecular-weight toxin selectively blocks voltage-gated sodium channels along the muscle and nerve cells [1]. Due to the wide distribution of TTX in nature, its ecological and biological characteristics are relevant to date.
Since the isolation and purification of TTX from pufferfish ovaries in the early 1950s [2,3], the search for this toxin in different groups of animals has been ongoing. Most studies have focused on commercial animals and some highly toxic amphibians. However, the search for TTX sources led to the study of the prey and symbiotic microflora of TTX-bearers. Hence, TTX was found in many organisms in addition to commercial fish, including flatworms, nemerteans, annelids, planktonic chaetognaths [4], and bacteria from different taxonomic groups [5]. With progress in the field of TTX detection, the investigations of TTX bearers have improved. In the early works, whole-body and known toxic organ extracts were analyzed. Since the 1990s, studies of tissue and cellular TTX distribution began. Since the 2000s, researchers have actively implemented experimental approaches to study TTX accumulation and excretion in animals. Research progress in the TTX field over the past 20 years has led to the accumulation of a large amount of data, which, however, did not reveal the mechanisms underlying TTX circulation in ecosystems and the bodies of animals. High inter- and intraspecies variances in TTX content and variations in experimental designs and screening studies significantly complicate the search for common patterns in toxin accumulation and usage by animals.
The current review summarizes the data on the intra-organismal distribution of TTX in TTX-bearing animals; its accumulation during animal development; and its accumulation, depletion, and excretion in animals under experimental conditions. Special emphasis is placed on TTX localization at the cellular and subcellular levels.
2. Intra-Organismal Distribution of TTX in TTX-Bearing Animals
2.1. Actinopterygii
The first experiments that led to the discovery of the toxins in the ovaries of fish belonging to the order Tetraodontiformes were conducted from the end of the 19th century to the middle of the 20th century [4]. The isolation of TTX and elucidation of its chemical structure led to the first large-scale toxicity studies of pufferfish inhabiting Japan [6,7]. Species of the genus Takifugu, particularly Takifugu pardalis, Takifugu flavipterus (Takifugu poecilonotus), and Takifugu alboplumbeus (Takifugu niphobles), are highly toxic. In subsequent years, TTX was found among most genera of the family Tetraodontidae dwelling in different countries and regions. High levels of toxicity have been observed in species of the genera Arothron, Amblyrhynchotes, Chelonodon, Dichotomyctere, Lagocephalus, and Sphoeroides [8,9,10,11,12,13]. TTX is also found in the species Yongeichthys criniger (Gobius criniger) of the order Gobiiformes [14,15,16,17].
In previous studies, the authors reported either the general toxicity of the animal [18,19] or the toxicity of its ovaries [20]. Since the 1980s, the toxicity to individual organs and tissues has been studied. Researchers have mainly focused on the skin, muscles, intestines, gonads, and liver (or hepatopancreas, according to Sato et al. [21]); the fins, spleen, blood, and gills have been studied less extensively. Many pufferfish species have high concentrations of TTX in the ovaries, followed by the liver and/or skin; the remaining organs contain small amounts of TTX [7,8,11,22,23,24,25,26,27,28,29,30,31] (Figure 1). In T. alboplumbeus, the ventral skin is more toxic than the dorsal [25]. Comparative toxicity analysis of the fins and skin of Takifugu vermicularis, Takifugu snyderi, and Takifugu porphyreus showed that these tissues in some species had similar levels of toxicity [32]. In Chelonodon patoca, the highest toxicity is found in the skin; the muscles, liver, and ovaries of fish contain lower levels of TTX [27]. The brackish and freshwater species of the genera Dichotomyctere and Pao also have high concentrations of TTX in the skin compared to that in other organs [33,34] High concentrations of TTX can be found in the skin mucus of Arothron meleagris and Sphoeroides lispus [10]. In several species of the genus Lagocephalus, the gastrointestinal tract is the most toxic organ [13,35]. Artificially reared T. rubripes contain low concentrations of TTX accumulated in the ovaries and liver, similar to the observations in wild specimens [30,36]. Sato et al. [37] reported trace amounts of TTX in the guts of cultured T. rubripes. However, some studies reported the absence of toxins in cultured pufferfish [38,39].
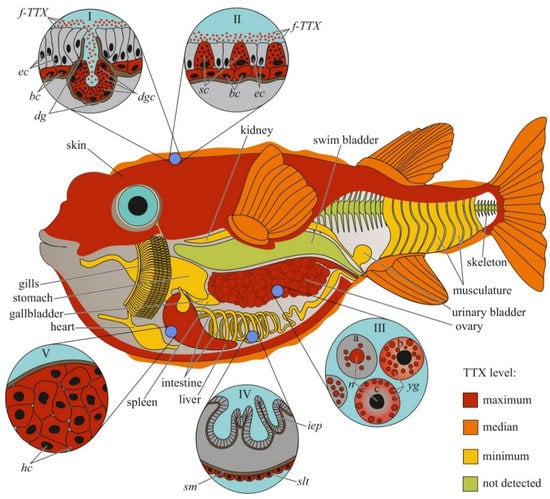
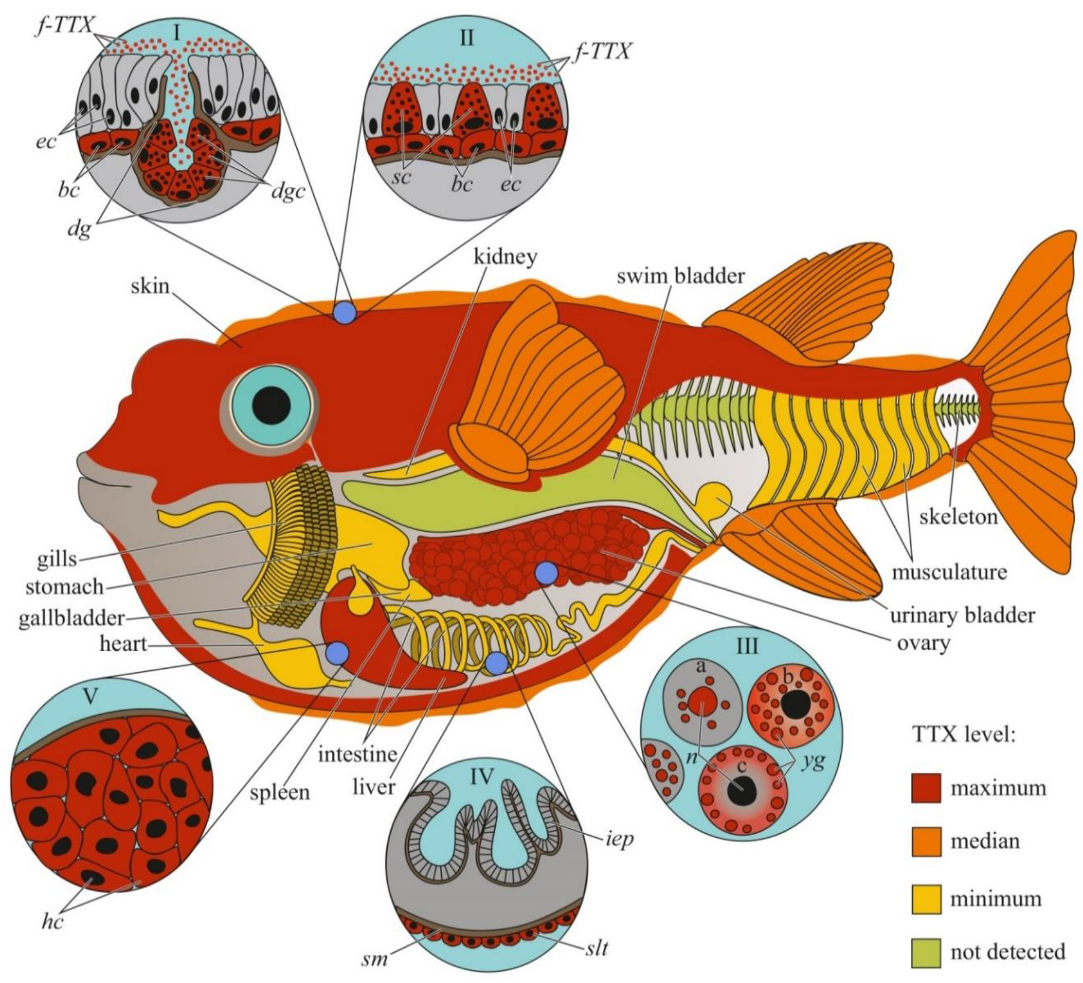
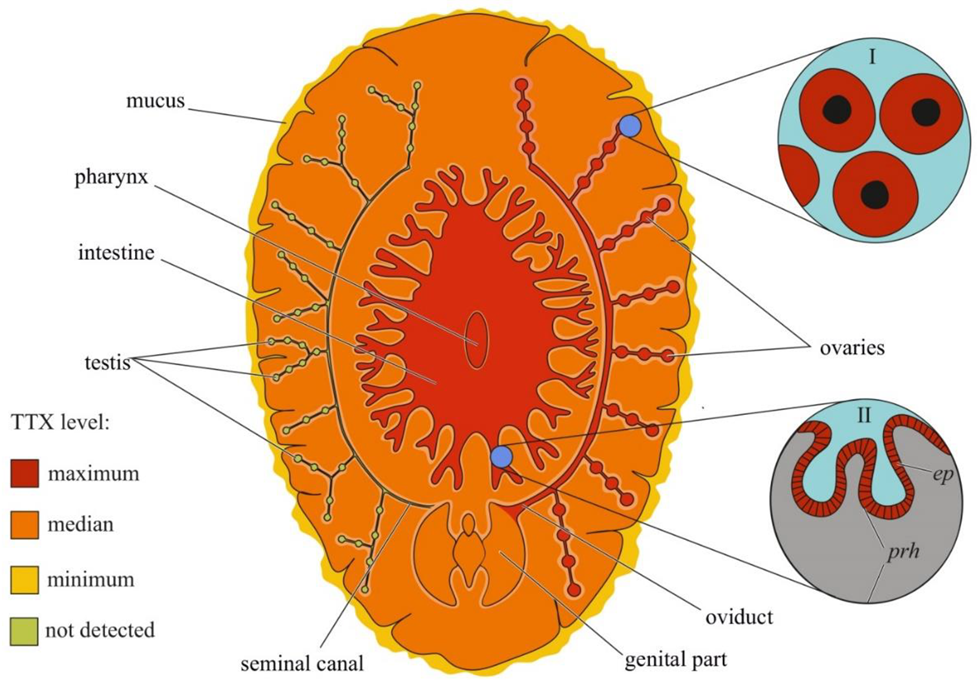
Figure 1.
Schematic illustration of the levels and intra-organismal distribution of tetrodotoxin (TTX) in the adult pufferfish (family Tetraodontidae). Red color on the insets indicates TTX-positive cells. I—Skin with dermal gland (dg). II—Skin with singly scattered succiform cells (sc). III—Oocytes on different maturation stages: a—immature oocyte, b—newly mature oocyte, c—mature oocyte. IV—Intestinal epithelium (iep) and sac-like tissue (slt) outside the serous membrane (sm). V—Liver with hepatocytes (hc). Abbreviations: ec, epithelial cell; bc, basal cell; dg, dermal gland; dgc, dermal gland cell; f-TTX, free TTX; sc, succiform cell; n, nuclei; yg, yolk granules; iep, intestinal epithelium; slt, sac-like tissue; sm, serous membrane; hc, hepatocyte.
The distribution of TTX within the body can vary depending on the habitat of the pufferfish. In Lagocephalus lunaris caught near Thailand [12] and the west coast of Japan [40], TTX is mostly localized in the gonads, while other organs, including the liver, skin, digestive tract, and muscles, contain low concentrations of the toxin. L. lunaris, which inhabits Cambodia, contains substantial concentrations of TTX in the liver, ovaries, and intestines, while the muscles, testes, and skin are less toxic [41]. The TTX content in different organs can also be affected by seasonal changes. In females of several species of the genus Takifugu, Y. criniger, and introduced species of the genera Lagocephalus and Torquigener, caught in the Mediterranean Sea, most of the TTX is contained in the ovaries in the autumn–winter period, during maturation, and in the liver and/or skin in the spring-summer period, after spawning [9,42,43,44,45,46,47,48,49]. Different data were obtained for males of different species of the genus Takifugu. T. flavipterus and T. pardalis do not show seasonal variations in TTX content [42,46]. In male as well as female T. alboplumbeus, an increase in the toxicity level in the pre-spawning and spawning periods was observed, when TTX was localized in the skin and liver [44]. Lagocephalus males contain substantial TTX concentrations in the testes only in the summer; other organs contain trace amounts of the toxin [45]. The concentration of TTX in the organs of Torquigener flavimaculosus males reaches the maximum level in winter and gradually decreases in autumn; in summer, only the levels in the testes and skin increase to winter values [47].
The cellular and intracellular localizations of TTX in pufferfish has been studied in highly toxic organs, including the skin, ovaries, and liver (Figure 1). Kodama et al. [50] detected TTX in secretions collected from the skin gland of T. pardalis and stated that the toxin was produced by the secretory cells of this gland. Later, the localization of TTX in the secretory cells of pufferfish skin was confirmed by immunohistochemistry using anti-TTX antibodies [21,27,51,52]. In T. pardalis [50], T. vermicularis [27], T. flavipterus, and Canthigaster rivulata [21], TTX-secreting cells are located in the glands or gland-like structures. In C. patoca [27], Dichotomyctere ocellatus (Tetraodon steindachneri) [51], and Dichotomyctere nigroviridis (Tetraodon nigroviridis) [52], TTX is located in the so-called succiform cells evenly scattered throughout the skin of the animal. Itoi et al. [53] showed that succiform cells of T. alboplumbeus males stained for TTX more intensely than the succiform cells of the females. TTX has also been found in the undifferentiated basal cells of pufferfish skin [43,51,52,53]. According to electron microscopic studies of D. nigroviridis skin, TTX in the basal cells is associated with membrane-bound granules (presumably lysosomes) [52]. The TTX absorption ability of the basal cells of the skin was demonstrated in experiments with intramuscular, intraperitoneal, and oral administration of toxins to non-toxic cultured pufferfish [54,55,56]. Interestingly, the intensity of staining for TTX in the skin cells depends on the dose of the injected toxin: low concentrations of injected TTX in T. rubripes only resulted in basal cell staining, while an increased dose stained the entire epidermis [56]. In vitro skin slices incubated in a TTX solution showed that TTX was transferred to the basal cells from connective tissue [57]. In addition to succiform and basal cells in the skin of T. alboplumbeus, TTX is found in mucous cells and the flat epithelial cell layer [53]. In the skin of Y. criniger, all epidermal cells, including filament-containing Malpighian [58], basal, and succiform cells, stained positively for TTX [43].
After studying the distribution of TTX in the cellular and subcellular liver fractions of T. pardalis and Takifugu snyderi (Takifugu vermicularis snyderi), Nagashima et al. [59] found that TTX was predominantly associated with the cytosolic fraction of the liver cells. Subsequently, immunocytochemistry with anti-TTX antibodies has been used to detect TTX in the cytoplasm of parenchymal hepatocytes in several species of Takifugu and C. patoca [21,27,53,55,57]. An in vitro experiment with the incubation of T. rubripes liver slices in a TTX solution showed that the toxin was transferred to the parenchymal hepatocytes from the pancreatic exocrine cells [57].
In the first study on the micro distribution of TTX in pufferfish ovaries, TTX was detected in the cytoplasm and membrane-limited yolk granules of pre-ovulated oocytes and the vitellin envelope of the ovulated oocytes [60]. In T. vermicularis ovaries, TTX is found in the yolk granules, vesicles, and nuclei of mature oocytes; immature oocytes do not contain the toxin [27]. In C. patoca, TTX is localised in the connective tissues and nuclei of some oocytes [27]. Itoi et al. found TTX in the oocyte nuclei of T. alboplumbeus [53]. Gao et al. [46] traced the localization of TTX during oocyte maturation in T. pardalis; in early maturation stages, TTX was localized in the nucleus and yolk granules, and late stages, in the cytoplasm and on the periphery of the cell.
Immunohistochemical studies have shown a weak TTX-positive signal in the sac-like tissues outside the serous membrane of the T. flavipterus intestine [21] and in the brain (optic tectum, cerebellum, and medulla oblongata), optic nerve, and olfactory epithelium of T. rubripes juveniles [55].
2.2. Amphibia
Newts are the most popular study animals among terrestrial TTX-bearers. In 1963, a toxin was isolated from the eggs of the newt Taricha torosa (Triturus torosus) [61], which was soon identified as TTX [62,63]. This discovery led to an array of toxicity studies on various members of the Salamandridae family. The first large-scale screening by Wakely et al. [64] showed the presence of TTX in several members of the genera Taricha, Cynops, and Triturus as well as in Lissotriton vulgaris (Triturus vulgaris), Ichthyosaura alpestris (Triturus alpestris), and Notophthalmus viridescens. To date, TTX has been found in 10 genera of Salamandridae residing in different regions of the world: Taricha, Notophthalmus, Cynops, Pachytriton, Paramesotriton, Laotriton, Triturus, Lissotriton, Ichthyosaura, and Ambystoma [64,65,66,67,68]. Differences in TTX content among individuals within populations have been reported for Taricha granulosa [69,70], N. viridescens [71], and Cynops pyrrhogaster [72].
Studies on individual organs and tissues of newts have revealed that TTX is usually localized in the skin and ovaries; other organs, including the muscles, blood, viscera, liver, and testes, contain low concentrations of the toxin [21,64,65,66,73,74] (Figure 2). In some newt species, high TTX concentrations have also been found in the liver [74] and muscles [75].
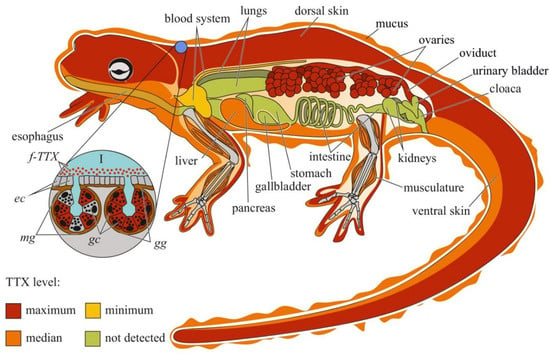
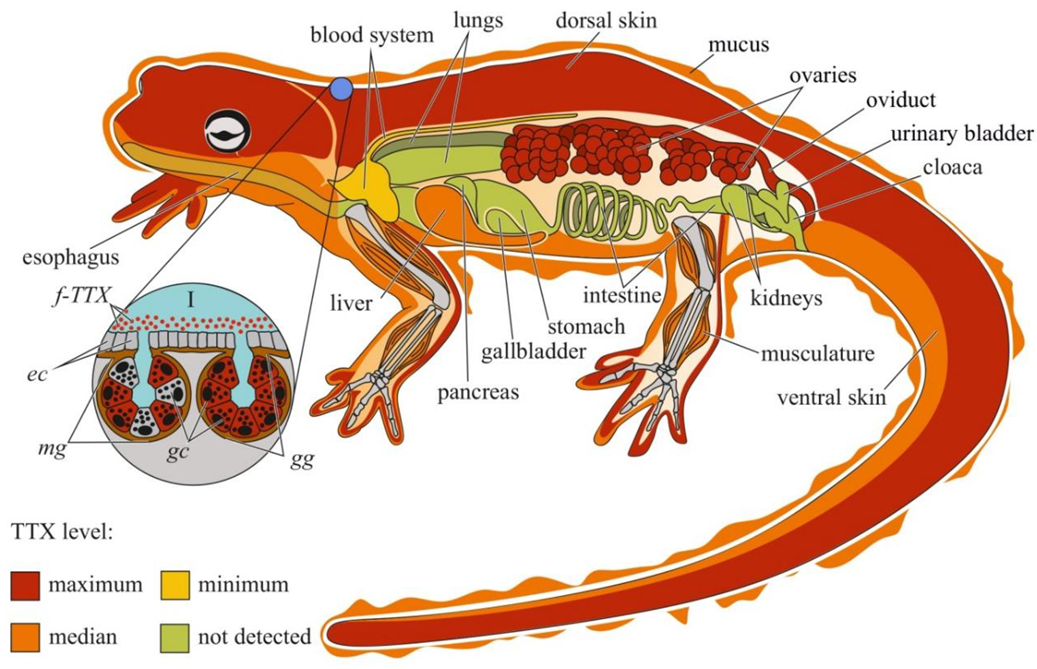
Figure 2.
Schematic illustration of the levels and intra-organismal distribution of tetrodotoxin (TTX) in the adult newt (family Salamandridae). Red color on the insets indicates TTX-positive cells. I—Skin with mature granular (gg) and mixed (mg) glands. Abbreviations: ec, epithelial cell; gc, glandular cell; gg, mature granular gland; mg, mature mixed gland; f-TTX, free TTX.
TTX was first visualized in the skin of C. pyrrhogaster by immunohistochemistry with anti-TTX antibodies [76]. In the juveniles of C. pyrrhogaster, TTX is contained in the glandular cells of immature dermal glands, in adult individuals, in the glandular cells of granular and mixed dermal glands [76]. Sato et al. [21] found TTX-positive cells in the dermal glands of only adult C. pyrrhogaster; TTX-positive structures were absent in the liver, intestines, testes, and ovaries. Mailho-Fontana et al. [77] detected TTX in the dermal glands and blood plasma of dermal capillaries of T. granulosa. The authors also revealed differences in the morphology of TTX-positive glandular cells and the structure and chemical composition of their secretions between individuals from TTX-bearing and non-toxic T. granulosa populations. In N. viridescens, the dermal glands stained the most intensively for TTX; in the connective tissues, liver, intestinal epithelium, ovaries, testes, and kidneys, the TTX-positive labelling was moderate [74]. Spicer et al. [78] hypothesized that similar to other newt species, N. viridescens might possess an increased number of granular glands in brightly pigmented spots on the dorsal skin, which might be associated with increased levels of TTX. Although no differences in TTX concentration were found between the red spots and neighboring skin without spots, juveniles with more dorsal spots possessed higher TTX levels. Uniform TTX-staining was observed in the tissues of nematodes, trematodes, and cestodes parasitizing the intestinal cavity of N. viridescens [79].
In the order Anura, TTX has been found in four genera: Atelopus (family Bufonidae) [80,81,82,83,84,85,86,87], Colostethus (family Dendrobatidae) [84], Brachycephalus (family Brachycephalidae) [88,89,90], and Polypedates (family Rhacophoridae) [91]. TTX in frogs was first reported by Kim et al. [80] in 1975. The authors detected TTX in the skin of Atelopus varius and Atelopus chiriquiensis; internal organs, muscles, and bones did not contain TTX. A similar TTX distribution has been observed in Colostethus inguinalis [84] and Polypedates sp. [91]. Later, TTX was also found in the liver and ovaries of the Anura representatives. In Brachycephalus ephippium, TTX is contained in the skin, liver, and ovaries [88,90], whereas in Brachycephalus pernix, only in the skin and liver [90]. However, the TTX concentration in the ovaries of B. ephippium was three times lower than that in the skin [90]. In contrast, the ovaries of A. chiriquiensis [81] and Atelopus glyphus [86] contained more toxins than the skin. Immunohistochemical studies of Atelopus hoogmoedi showed the presence of TTX in the hepatocytes, granular skin glands, and epithelial skin cells [87].
2.3. Mollusca
The data on intra-organismal TTX distribution in octopuses, gastropods, and bivalves are summarized in Figure 3. TTX in molluscs was first discovered in 1978 when a toxin isolated from the posterior salivary glands of the octopus Hapalochlaena maculosa was identified [92]. Subsequently, TTX in H. maculosa was found not only in the salivary glands, but also in all body parts, including the arms, cephalothorax, and abdomen [93,94]. In Hapalochlaena fasciata, TTX has been found in the anterior and posterior salivary glands, arms, mantle, digestive gland, gonads, brachial heart, nephridia, gills, oviducal gland, and ink sac [95,96,97]. Hapalochlaena lunulate contains TTX in its salivary glands, gonads, mantle, arms, and ink [95,97,98]. However, the posterior salivary glands were the most toxic organs in both species. Immunofluorescence microscopy of the micro distribution of TTX in the tissues of H. lunulate and H. fasciata showed that TTX was concentrated in the cells lining the secretory tubules within the posterior salivary gland [97]. In the mantle and arms of H. lunulate, TTX was concentrated beneath the integumentary epidermis and in the channels of the circulatory system running through the dermis.
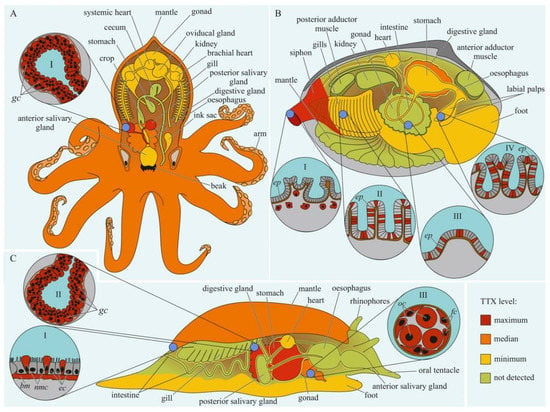
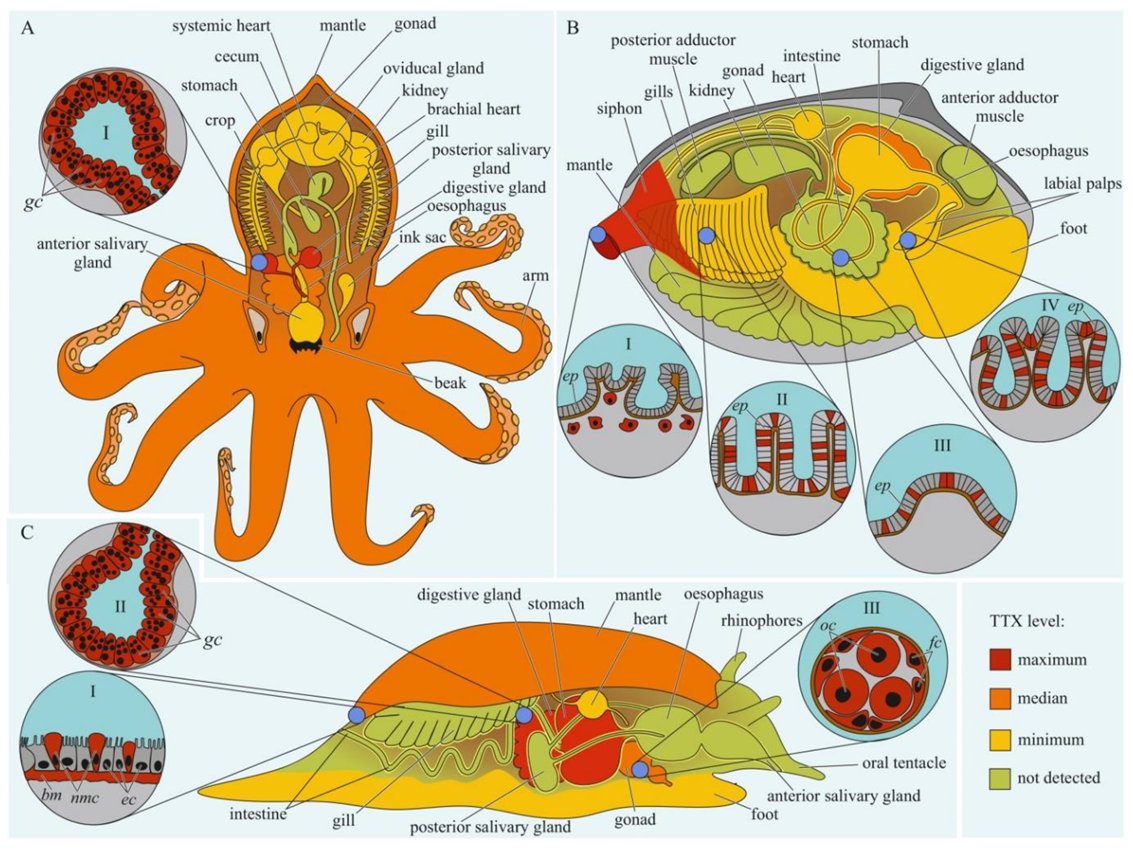
Figure 3.
Schematic illustration of the levels and intra-organismal distribution of tetrodotoxin (TTX) in molluscs. Red color on the insets indicates TTX-positive cells. (A)—Blue-ringed octopus (genus Hapalochlaena). I—Posterior salivary gland with glandular cells (gc). (B)—Marine gastropod (genus Pleurobranchaea). I—The epidermis of the mantle. II—Digestive gland with glandular cells (gc). III—Oocytes (oc) surrounded by follicles (fc). (C)—Marine bivalve mollusc (genus Paphies). I—Siphon with TTX-positive cells located under the epithelium (ep). Gills (II), intestine (III), and labial palp (IV) showing epithelium (ep). Abbreviations: gc, glandular cells; bm, basement membrane; ec, epithelial cells; nmc, neutral mucin cells; oc, oocytes; fc, follicles; ep, epithelium.
In 1981, a substance with a neuroparalytic effect and physicochemical properties similar to TTX was isolated from the digestive glands of the sea snails, Charonia lampas (Charonia sauliae) and Babylonia japonica [99,100]. Further studies revealed TTX in sea snails Tutufa bufo (Tutufa lissostoma) [101], Buccinum undatum [102], Patella depressa, and Nucella lapillus [103], as well as in some members of the families Naticidae [104,105], Trochidae [106,107], Nassariidae [108,109,110,111,112,113,114,115,116,117], and Olividae [118,119]. In most studies, TTX has been detected in the digestive glands. In the lined moon snail Tanea lineata (Natica lineata), the muscles are the most toxic part of the body, followed by the remaining parts, including the salivary gland, brain, and mouth organs; the digestive gland contains the least amount of TTX [120]. Hwang et al. [105] found that when seawater was released from the mantle cavity of T. lineata, TTX was secreted into the water. In another study, Nassarius conoidalis (Niotha clathrata) released TTX into the water in response to the first electric shock treatment; repeated stimulation did not cause toxin secretion [121].
TTX was also found in sea slugs Pleurobranchaea maculata [122], Onchidella celtica, and Aplysia depilans [103]. Wood et al. [123,124] reported high TTX concentrations in the stomachs of P. maculata, while the mantle and gonads of the mollusc were less toxic. A detailed study of the tissues and cells of P. maculata revealed the presence of TTX in the neutral mucin cells and basement membrane in the mantle, oocytes, and follicles in the gonads, and digestive gland [125]. TTX was found in P. maculate egg clutches [124,125].
The well known TTX-bearing bivalve mollusc is Paphies australis [126]. In P. australis, the highest level of TTX is contained in the siphon [127]. Immunohistochemical studies have shown that TTX was localized in the cells of the inner and outer epithelia of the siphon, in the inside cells of the epithelium of the intestine and rectum, and in the cytoplasm of some epithelial cells of the labial palps and gills [128]. Since 2008, TTX has been detected in many commercial bivalves living in European waters [102,103,119,127,129,130,131,132,133,134].
2.4. Echinodermata
The undigested body parts of starfish, found in the contents of the digestive glands of marine TTX-bearing gastropods, served as a starting point for the search for TTX in echinoderms. TTX has been found in several starfish species of the genus Astropecten [135,136,137,138] and in Ophidiaster ophidianus as well as in sea urchins Echinus esculentus [103] and Fellaster zelandiae (Arachnoides zelandiae) [139]. Lin et al. showed that the internal organs of A. scoparius had the highest toxicity, whereas the gonads and body wall were less toxic [106,140]. The tissue and cell distribution of TTX in echinoderms has not been studied.
2.5. Nemertea
For the first time, TTX was detected in two species of marine ribbon worms, Lineus fuscoviridis, and Tubulanus punctatus in 1988 [141], although pyridine compounds with a neurotoxic effect were previously found in extracts of Amphiporus angulatus [142]. In the nemertean Cephalothrix simula (in the article by Cephalothrix linearis), both the body and proboscis were found to be highly toxic, with the proboscis being the most toxic [143]. In addition, TTX was released into the mucus on the surface of the animal’s body during mechanical stimulation. Subsequently, TTX and its analogs have been found in representatives of the genera Cephalothrix [144,145,146,147,148,149,150,151], Lineus [147,149,150,152,153], Ramphogordius [149], Riseriellus [149], Amphiporus [149], Yininemertes [154], Quasitetrastemma [150], and Collarenemertes [150].
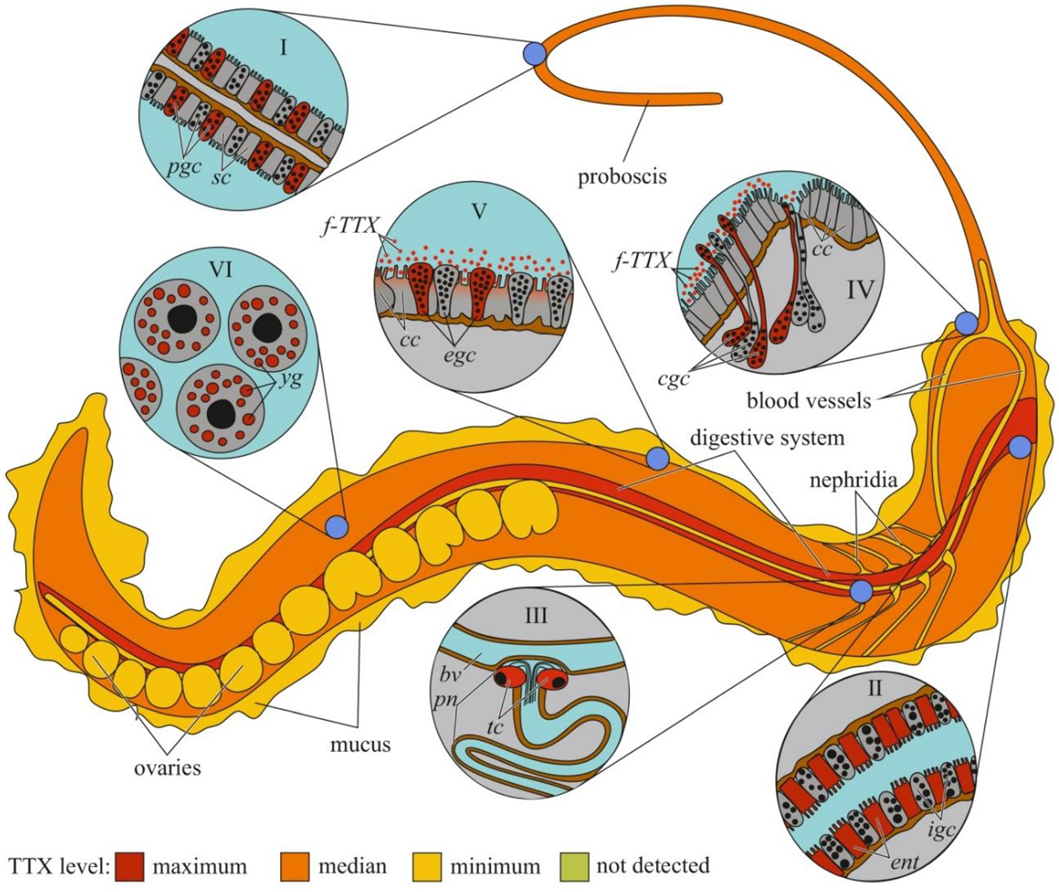
The cellular and tissue localizations of TTX has been well studied in highly toxic nemerteans of the genus Cephalothrix [155,156] (Figure 4). In an early study on C. simula (Cephalothrix sp.), TTX was detected in the vesicles of the epidermal bacillary cells, basal lamina, granular cells of the proboscis epithelium, rhynchocoel epithelium, and vesicles of the intestinal epithelial cells located near the blood vessels and rhynchocoel [155]. The excretory system (protonephridia) and eggs also contain TTX. Vlasenko and Magarlamov found higher TTX content in the anterior region of the body of C. cf. simula than in the posterior one [151]. High TTX concentrations were detected in the intestine, body wall, proboscis, and mucus on the surface of the animal body. According to immunohistochemical studies, the main sites of TTX accumulation in C. cf. simula are the secretory cells of the integument, epidermal ciliary cells, mucous cells of the cephalic glands, glandular epithelium of the proboscis, enterocytes, and terminal cells of the protonephridia [156]. In the secretory and glandular cells, TTX is associated with secretory granules, in the ciliary cells, with microvilli, in the enterocytes, with phagosomes. In the protonephridial cells, TTX is located in the cytoplasm. In Dushia atra and Micrura verrilli, TTX was found in dermal glandular cells, intestinal epithelium, and outer proboscis epithelium, including pseudocnidae-containing cells [157]. In Kulikovia alborastrata (Lineus alborostratus), TTX was present in type-I subepidermal bacillary gland cells in the cutis and pseudocnidae-containing and mucoid gland cells in the proboscis [152]. Within glandular cells, TTX is associated with the nuclear envelope, the endoplasmic reticulum membrane, and secretory granules. Moreover, the glandular cells of the cutis in K. alborostrata released TTX-containing mucus in response to external stimuli [153].
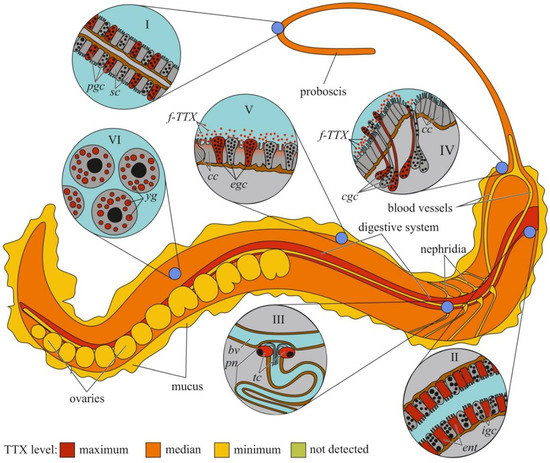
Figure 4.
Schematic illustration of the levels and intra-organismal distribution of tetrodotoxin (TTX) in the marine ribbon worm (genus Cephalothrix). Red color on the insets indicates TTX-positive cells. I—Glandular epithelium of proboscis. II—Intestinal epithelium. III—Protonephridium (pn) associated with the blood vessel (bv). IV—Cephalic gland. V—Integument. VI—Oocytes. Abbreviations: pgs, proboscidial gland cell; sc, supportive cell; ent, enterocyte; igs, intestinal glandular cell; bv, blood vessel; pn, protonephridium; tc, terminal cell; cc, ciliary cell; cgc, cephalic gland cell; f-TTX, free TTX; egc, epidermal gland cells; yg, yolk granules.
2.6. Platyhelminthes
Although the toxicity of flatworms was reported in early naturalistic works from the 18th and 19th centuries, the first scientific work noting the presence of neurotoxins in marine and terrestrial flatworms dates back to 1943 [158]. After 40 years, TTX was found in extracts of the marine flatworms Planocera multitentaculata [159,160] and Planocera reticulata (type A) [161,162]. Subsequent studies showed the presence of TTX in several marine [125,163,164] and terrestrial [165] flatworms. In P. multitentaculata, the most toxic organs are the oviducts filled with mature eggs and organs of the digestive (including the pharynx) and reproductive systems; a weak neurotoxic effect of the mucus enveloping the worm was observed [160,166]. High TTX concentrations have also been found in the pharynx and eggs of Planocerid sp. 1 [163]. Detailed immunohistochemical studies have detected TTX in the cytoplasm of the ova and epithelial cells of the pharynx of Stylochoplana sp. [167] and the ova of P. reticulata [155]. In the terrestrial flatworms Bipalium adventitium and Bipalium kewense, most of the TTX was contained in the head and eggs; the anterior and posterior parts of the body contained smaller amounts of the toxin [165]. Figure 5 summarizes the intra-organismal TTX distribution in marine flatworms.
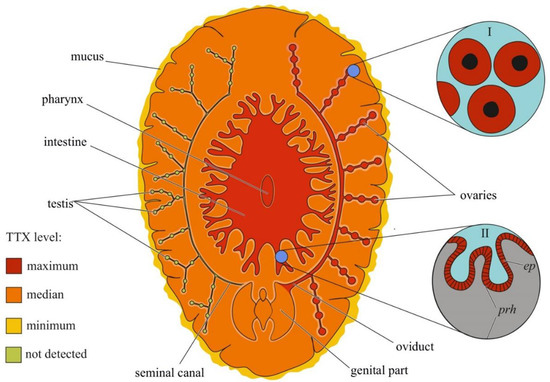
Figure 5.
Schematic illustration of the levels and intra-organismal distribution of tetrodotoxin (TTX) in the marine flatworm (order Polycladida). Red color on the insets indicates TTX-positive cells. I—Oocytes. II—Pharynx showing epithelial cells (ep) and parenchyma (prh).
2.7. Annelida
In 1986, TTX was found in the annelid Pseudopotamilla occelata [168]. In their review, Miyazawa and Noguchi [4] mentioned that TTX was present in Lepidonotus helotypus, Halosydna brevisetosa, Hermenia acanthopeltis, and Harmothoe imbricata.
2.8. Chaetognatha
In 1988, TTX was found in planktonic chaetognaths Parasagitta elegans [169]; the sodium-channel-blocking activity of extracts of chaetognaths of the families Eukrohniidae (genus Eukrohnia), Sagittidae (genus Flaccisagitta), and Spadellidae (genus Spadella) was also reported; however, physicochemical methods of TTX detection were not used. Interestingly, only fractions containing chaetognath heads were toxic; the bodies did not contain TTX.
2.9. Arthropoda
TTX is found in three classes of arthropods: Malacostraca, Merostomata, and Copepoda. Among Malacostraca, TTX is detected in several crab species of the family Xanthidae [168,170,171,172,173,174,175,176]. Xanthid crabs contain TTX throughout their body, but the most toxic organs differ among species. In Lophozozymus pictor [171] and Demania reynaudi [174], the cephalothorax and viscera were found to be more toxic, whereas, in Atergatis floridus, the viscera and appendages were more toxic than other organs [174]. Saito et al. showed that the high toxicity of A. floridus was associated with the muscles of the chelipeds, particularly the muscles of the palm and carpus [177]. In some specimens, the muscles of the walking legs, gills, and ovaries were found to be toxic. In Demania cultripes, the viscera are more toxic than the appendages [176].
Among Merostomata, TTX is found in the horseshoe crab Carcinoscorpius rotundicauda [178]. In C. rotundicauda, TTX was first detected in the eggs [178]. Subsequent studies showed the presence of TTX in almost all organs and tissues of crabs of this species; however, most of the toxins are located in the eggs, muscles, viscera, and hepatic caecum [179,180,181].
Ikeda et al. first revealed the presence of TTX in the ectoparasitic copepod Caligus fugu (Pseudocaligus fugu) that parasitizes the pufferfish T. alboplumbeus [179]. In the same year, TTX was detected in another parasite of T. alboplumbeus, Taeniacanthus sp. [180]. Anti-TTX antibody-based immunohistochemical studies have shown that TTX in C. fugu was accumulated in all tissues of the body, intestines, and appendages except for the epicuticle and reproductive system, including the ovaries, oviduct, uterus, and egg sacs [179]. TTX appears at an early stage of chalimus development, and at chalimus stage IV and in adult copepods, it is present in all tissues and organs, except for the reproductive system [181].
2.10. Concluding Remarks
Data on TTX localization in animals can help elucidate the biological and physiological significance of the toxin. Most studies have focused on the anatomical distribution of TTX in animals, and only a small percentage of studies have focused on the intratissue and intracellular localization of TTX. A common tendency for TTX accumulation in the skin, ovaries, and endodermal organs (digestive system and liver) can be traced among different taxonomic groups of animals. The cellular distribution of TTX in these organs has mostly been studied in pufferfish (Figure 1) and nemerteans (Figure 4). Data on the cellular localization of TTX in other TTX-bearing animals are either limited or absent. More studies implementing high-resolution microscopy, such as laser scanning microscopy and electron microscopic immunocytochemistry, are required to trace the cellular and subcellular TTX distribution. These studies allow significantly expand the understanding of the cellular mechanisms of TTX sorption and migration within the body of a TTX-bearing animal. However, molecular mechanisms involved in TTX retention are yet to be explored. Studying the resistance of toxic pufferfish and arthropods to TTX, TTX-binding molecules in these animals were supposed [25,182,183,184]. TTX-binding proteins and high-molecular weight substances were isolated from the plasma of some pufferfish species, shore crab, and toxic gastropods [185,186,187,188,189] Nevertheless, the manner in which these molecules bind and transport TTX remains unclear. Future studies of the transcription factors involved in the regulation of TTX transport and accumulation in TTX-bearing animals are needed.
3. TTX in Animal Development: From Egg Maturation to Hatched Larvae
3.1. Actinopterygii
TTX was first isolated from the ovaries of T. rubripes in 1950 [190]. The high toxicity of the ovaries and the absence or low concentrations of TTX in the testes of most marine pufferfish species [191] led to studies of TTX kinetics in the maturation period. Studies on the seasonal changes in the TTX distribution in the tissues of T. alboplumbeus and T. flavipterus showed that TTX content in the ovaries increased sharply during egg maturation, and the total TTX concentration in the body of animals of both sexes was higher during maturation/spawning than in other months [42,44]. The ovaries of Lagocephalus sceleratus are toxic throughout the year, whereas the testes are toxic only in spring and autumn [45]. In T. flavimaculosus, the gonads are toxic in all seasons, except summer, and females are more toxic than males [47].
Using an indirect competitive enzyme immunoassay, TTX concentrations were calculated in T. alboplumbeus oocytes before and after ovulation [60]. The ovulated oocytes contained half as much TTX as pre-ovulated oocytes. Detailed studies of TTX distribution in the ovaries during oocyte maturation and spawning have been conducted on Y. criniger [43] and T. pardalis [46]. TTX accumulation in the ovaries of Y. criniger starts at the yolk globule stage, accounting for 45% of the total toxin in the body [43]. During the spawning period, the total toxicity of the female increases by up to eight times, where 73% of TTX is present in the ovaries. At the end of spawning, the toxicity of the female remains the same, but the TTX is mostly contained in the skin, and the contribution of the ovaries does not exceed 2%. Similar results were obtained for T. pardalis: during egg maturation, most of the TTX was concentrated in the ovaries, and after spawning, TTX content in the ovaries showed a sharp decline [46]. TTX is localized in the oocyte nucleus in the perinucleolar phase, redistributed to the yolk vesicles and globules during maturation, and partially transferred to the egg membrane close to spawning [46]. A similar study on T. vermicularis revealed TTX in the nuclei of oocytes in the perinucleolar phase, but in the yolk vesicles and granules at the final stage of yolk formation [27]. In the same study, the ovaries of C. patoca contained TTX only in the connective tissue and nuclei of some oocytes during the perinucleolar phase. In mature females of T. alboplumbeus, intense immunoreactivity against TTX in the oocyte nuclei was observed in both the perinucleolar and during yolk formation phases [53,60] whereas, in T. flavipterus, the oocytes stained intensively for TTX in the perinucleolar phase and weakly during maturation [21].
In 1998, Matsumura investigated TTX accumulation during the early development of T. alboplumbeus from fertilized eggs to hatched larvae [192]. After fertilization, TTX concentration in the developing embryos continuously increased. Immunohistochemical studies of T. rubripes and T. alboplumbeus larvae with anti-TTX antibodies showed even distribution of TTX over the body surface; no specific reaction was observed in internal organs [193,194]. Predation experiments with fertilised eggs and larvae of the above-mentioned pufferfish species and juveniles of different fish species showed that, despite low TTX content in eggs and larvae, predators promptly spat out the swallowed prey [193,194]. Nagashima et al. [195] traced the dynamics of TTX content during the development of T. rubripes for up to 98 days after hatching. The concentration of TTX per gram of body weight decreased during the growth and development of the animal, while the total content of the toxin increased.
3.2. Amphibia
TTX accumulation during the ontogeny of amphibians has mostly been studied in the Salamandridae family. The presence of a substance with a neuroparalytic effect in the embryos and eggs of newts was discussed as early as the 1930s [196,197,198]. While studying the growth of the eyes in newts, an accidental discovery was made: the transplantation of the eye vesicles of T. torosa embryos into Ambystoma tigrinum embryos led to temporary paralysis of the latter [197]. Parabiosis experiments with the embryos of two species of newts showed that the paralytic effect of the eggs decreased when the T. torosus yolk was eliminated and the effect completely disappeared when self-feeding juveniles formed. The authors also reported the paralytic effect of the extracts of the embryos, larvae, eggs, and blood of T. torosus females. The genus Taricha remains the most widely studied genus with respect to TTX accumulation during embryonic development. TTX has been found in the ovaries, testes (trace amounts), eggs, and embryos of T. torosa [64,65,199]; eggs of T. granulosa [65,200,201,202,203]; and Taricha rivularis [65]. TTX has also been found in the ovaries of Cynops ensicauda [66], egg clutches of Cynops orientalis [204], and eggs of Paramesotriton hongkongensis and N. viridescens [65]. Hanifin et al. [202] noted a positive correlation between the TTX contents in the dorsal skin and the egg clutches of T. granulosa. TTX concentrations in egg clutches differ between specimens [202], but clutches produced by the same female at different times contained eggs with similar levels of toxicity [200]. Moreover, TTX in the egg clutches of T. granulosa is unevenly distributed, and the amount of TTX decreases from the beginning to the end of the clutch [201].
The total amount of TTX in T. granulosa eggs does not significantly change during embryonic development, and the toxin is mostly concentrated in the embryo and not in the jelly coat [201]. Gall et al. analyzed the TTX content at different developmental stages of the T. granulosa larvae and evaluated their palatability to the predatory dragonfly nymphs Anax junius [203]. TTX concentration is the highest in newly hatched newt larvae, declines at 4 weeks of age, and remains relatively constant until the end of development (28 weeks). The palatability of newts was correlated with toxicity, given that older larvae and juveniles were preyed on more frequently than the new hatchlings [203]. Another study suggested that TTX secreted by newt skin could act as a deterrent signal, allowing larvae to avoid cannibalism from adults [205]. Behavioural experiments showed that adult T. torosa skin secretions or pure TTX triggered the avoidance of predation of the larvae. Similar behavioral responses were mostly observed in 3–5-week-old larvae and disappeared by the seventh week of development. Based on electrophysiological recordings and the lack of response to TTX in T. torosa larvae with a blocked nasal cavity, the authors claimed that the olfactory epithelium of the larvae possessed TTX-sensitive cells. Sato et al. [21] found TTX-positive immunoreaction in the pharynx and stomach of C. pyrrhogaster hatched larvae. In C. pyrrhogaster, artificially grown from eggs, TTX was detected only up to 22 weeks after hatching; older newts (from 36 to 70 weeks) did not possess TTX [206]; however, wild newts of the same age contained TTX. In a similar study involving C. orientalis, TTX was detected in adult wild newts and their egg clutches, but not in artificially reared larvae [204].
3.3. Mollusca
TTX has been found in the eggs, egg sacs, and larvae of the gastropod P. maculata [122,123,125,139,167] and blue-ringed octopuses [96,207,208,209]. An early study revealed low TTX concentrations in the egg sacs and 2-week-old larvae of P. maculata [122]. Furthermore, Wood et al. showed that the egg-laying season of P. maculata corresponded with the seasonal peaks of TTX concentrations (June–August) [124]. In another study, the authors revealed a correlation between TTX concentration in first-laid egg masses and adults of P. maculata; TTX concentration on a per wet weight basis in the egg masses released at the beginning of spawning was always higher than that in the spawning individuals [123]. In the egg sacs of P. maculata, TTX was localised in the eggs rather than in the surrounding matrix [167].
After the identification of TTX as the main component of the venom of H. maculosa [92], a TTX-like substance was isolated from the eggs of the octopus [207]. Williams et al. traced TTX concentrations at different developmental stages of the octopus H. lunulata, from undifferentiated eggs to hatched paralarvae [96,208]. TTX was present at all studied stages, increasing in concentration from the last stages of embryonic development (2–3 weeks after egg deposition) to the hatching of the formed paralarva (4 weeks after egg deposition). No correlation was observed between maternal TTX levels and larval developmental stage. TTX has also been found in the paralarvae of H. fasciata [209].
3.4. Others
High TTX concentrations, comparable to those in adults, have been found in the eggs and larvae of the marine flatworm P. multitentaculata [16,160,166,210,211,212] and eggs of Stylochoplana sp. [125], Planocerid sp. [163], and the terrestrial flatworm B. adventitium [165]. In P. multitentaculata larvae, TTX was found to be distributed over the body surface [212]. In P. reticulate eggs, the TTX concentration was not calculated, but the toxin was detected using immunohistochemistry with monoclonal anti-TTX antibodies [155]. In two immunocytochemical studies on marine ribbon worms, TTX was found in the cytoplasm of C. simula [155] and C. cf. simula eggs [156]. TTX has also been found in the eggs of the horseshoe crab C. rotundicauda [178,213,214,215,216,217] and the crab A. floridus [177]. However, the fate of TTX during embryogenesis in these animals remains unknown.
The pattern of TTX content in larval development has been described for the ectoparasitic copepod C. fugu: TTX is absent in planktonic larvae; at chalimus stage I and during subsequent feeding on the mucus of the host body, TTX appears in all tissues of the larva except the cuticle, gut, and some muscles; at chalimus stage IV and in adult animals, TTX is retained in the whole body except the reproductive system [181].
3.5. Concluding Remarks
Studies of TTX dynamics during individual development of TTX-bearing animals aim to clarify the role of the toxin in the offspring survival. Numerous studies showing high levels of TTX in the ovaries and eggs of TTX-bearing animals during maturation/spawning season suggest the participation of the maternal toxin in a fate of offspring. Predation and behavioral experiments with newts and pufferfish showed that TTX might functioning as a chemical defense against predators and, in some cases, cannibalism by older individuals. These studies revealed a positive correlation between the survival rate and TTX content in larvae or juveniles of TTX-bearing species. However, immunohistochemical studies did not reveal specialized cellular or subcellular structures allowing embryos or larvae of TTX-bearers retain TTX, indicating that the maternal toxin accumulated in the yolk during eggs maturation could be lost in the course of individual development. TTX dynamics studies show that concentration of the toxin in pufferfish and newts remains stable or increases during embryogenesis, and sharply decreases after hatching. The resorption of yolk during larval development can explain a rapid decrease in TTX level, but mechanisms retaining the toxin remain unclear. In this context, investigations of molecular basis of retention and maturation-dependent transportation of TTX are required. On the other hand, TTX appearance and role in the individual development of other TTX-bearing animals remains to be elucidated.
4. TTX Accumulation and Depletion Studies
The aim of TTX accumulation and depletion studies is to unveil the sources of the toxin. These studies usually involve oral or injectional administration of TTX to non-toxic lab-reared TTX-bearing animals (Table 1).

Table 1.
Summary of the experimental studies on tetrodotoxin (TTX) administration to non-toxic individuals of TTX-bearing animals.
4.1. Actinopterygii
The first feeding experiment with non-toxic cultured pufferfish was conducted in 1981 [218]. Toxic pufferfish ovaries, methanol extract, and crystalline TTX were used as sources of TTX for non-toxic T. rubripes. The liver, skin, muscles, gonads, spleen, kidneys, and gallbladder of fish fed ovaries became toxic on the fifth day of the experiment, and by the 20th day, the toxicity gradually increased. In the fish of the methanol extract-feeding group, organ toxicity was lower than that in the other groups, but they became toxic by the end of the experiment. Interestingly, the fish fed crystalline TTX remained nontoxic. However, T. alboplumbeus juveniles fed crystalline TTX acquired toxicity and retained 30% of the administered toxin for 5 months [232]. TTX was found in the liver, skin, and ovaries of animals. In the work of Honda et al. T. rubripes of different ages accumulated TTX by ingesting both pufferfish tissues and their extracts and purified TTX [220]. The accumulated TTX was retained in pufferfish tissues for at least 45 days after the TTX-containing diet was completed. T. alboplumbeus juveniles fed highly toxic pufferfish livers accumulated up to 70% of the administered dose of the toxins [233]. The pattern of accumulation of TTX and its analog in the liver, skin, and intestines varied significantly. In the liver, the concentration of TTX accumulated during 30 days of the TTX-containing diet gradually decreased over the next 210 days of diet without TTX, whereas the content of 4,9-anhydro-TTX remained stable throughout the experimental period. In the skin, the contents of both toxins on the 30th day of the experiment were low and gradually increased toward the end of the observation period. In the intestine, TTX and 4,9-anhydroTTX were maintained at the same level throughout the experiment. Moreover, the selective pattern of TTX or paralytic shellfish toxins accumulation from the diet of pufferfish species naturally bearing one or the other toxin was revealed [235]. When T. pardalis was administered TTX and decarbamoylsaxitoxin (dsSTX), TTX accumulated in the liver, ovaries, and skin of the animal, and only trace amounts of dsSTX were detected in the intestine. In a similar experiment with TTX and STX, STX had accumulated in the ovaries and skin of the freshwater pufferfish Pao suvattii.
Intraperitoneal injection of tritiated TTX into T. rubripes lead to TTX accumulation in most tissues within an hour, with the highest level in the ventral skin [224]. The TTX levels in the liver and muscles were significantly low. On the sixth day of the experiment, TTX radioactivity levels decreased in most tissues, except for the skin and gallbladder. After intramuscular injection of TTX, 4-epiTTX, and 4,9-anhydro-TTX, T. alboplumbeus juveniles retained 34–40% of the toxins on day 16 after administration [234]. T. rubripes juveniles intramuscularly injected with pure TTX or toxic ovary extract retained approximately 60% of the injected toxin after 1–4 h [54]. After 8–12 h, TTX concentration decreased and again increased to 60–80% after 24–168 h. Low TTX concentrations were retained in the liver, whereas most of the toxin accumulated in the skin and was localized in the basal cells of the epidermal layer. Several subsequent studies have shown that in the first hours after intramuscular injection of TTX to pufferfish, most of TTX accumulated in the liver, and then a substantial part of the toxin has been transported to the skin and/or ovaries [227,230,231]. As the concentration of TTX in the liver decreased, the toxin appeared in the gallbladder, indicating excretion by the bile ducts [227]. Tatsuno et al. showed that TTX concentration in the skin and liver of T. rubripes increased with an increase in the dose of intramuscularly administered toxin, but the TTX accumulation ratio (ratio of accumulated TTX in each tissue to the administered dose) differed significantly between the tissues [56]. In the liver, the TTX accumulation ratio did not depend on the administered dose, whereas in the skin, it decreased with an increase in the dose.
Tatsuno et al. [221] found that the TTX distribution profile in T. rubripes in the first 24 h after toxin administration was dependent on the developmental stage of the liver. Individuals with a high hepatosomatic index retained 84% of the administered TTX, which mostly accumulated in the liver. Younger fish with an undeveloped liver retained only 31% of the administered TTX, mostly in their skin. In 3-month-old T. obscurus juveniles, up to 73% of accumulated TTX was localized in the skin, whereas less than 20% of the toxin occurred in the liver [236]. In vitro experiments with liver tissue slices incubated in TTX solution demonstrated that, unlike other fish, the pufferfish liver was able to accumulate TTX [118,241,242,243]. However, no difference was observed in TTX uptake between the livers of juvenile and adult pufferfish [244]. A recent study examined the TTX uptake ability of the liver, skin, and intestines of young (8 months old) and adult (20 months old) T. rubripes individuals [57]. The TTX uptake ability of the liver and intestines did not differ significantly between the two age groups, but the skin of young fish accumulated almost twice as much TTX as the skin of the adult.
In most experiments, other than pure TTX, toxic pufferfish organs or their extracts were used, and in a few studies, other TTX-bearing organisms were used as a source of the toxin (Table 1). In an early experiment, cultured T. rubripes (10 individuals) and T. alboplumbeus (three individuals) were fed a diet enriched with the TTX-producing bacterium Shewanella putrefaciens [219]. However, after 30 days of feeding, only one T. rubripes individual contained TTX in its liver. The successful toxification of T. alboplumbeus was achieved in a predation experiment with the flatworm P. multitentaculata [210]. TTX was detected in the liver, skin, and intestines of the pufferfish. Interestingly, the fish fed a weakly toxic planocerid ingested almost all the TTX, whereas the fish fed a highly toxic worm ingested only approximately 20% of the toxin. Zhang et al. did not reveal a direct effect of TTX concentration in Nassarius semiplicata on toxin accumulation in T. obscurus fed molluscs [236]. The accumulation ratio of TTX ranged from 35.76% in pufferfish fed moderately toxic molluscs to 40.20% when fed low-toxicity molluscs.
4.2. Amphibia
Studying the distribution of TTX in the tissues and organs of several species of newts, Wakely et al. found similar TTX levels in the body of T. torosa kept in the laboratory for 1 year and newly caught animals [64]. Several subsequent studies have shown that newts can retain TTX for a long period in captivity. Thus, the weight and general toxicity of T. granulosa kept in captivity for 167 days decreased, but the toxicity per gram of body weight remained almost the same [245]. In a study by Hanifin et al., TTX levels in the skin of T. granulosa not only remained unchanged, but also increased by an average of 20% after a year of maintenance in laboratory [73]. Wild-caught T. granulosa continued to produce eggs containing substantial amounts of TTX for 3 years in captivity [200]. In lab-reared T. granulosa juveniles kept on a non-toxic diet for 3 years, slow TTX accumulation in the skin during the first 2 years was observed [246]. In the third year, there was a sharp increase in toxicity. However, the authors noted that wild-caught T. granulosa juveniles were more toxic. Opposite results were obtained by Yotsu-Yamashita et al. in their experiments with N. viridescens [71]. Six years in captivity led to a complete loss of TTX, and only trace amounts of the toxin were detected in newts after 3 years. Long-term TTX retention under experimental conditions has also been observed in Atelopus frogs. Atelopus oxyrhynchus and Atelopus subornatus contained TTX, 4-epiTTX, and 4,9-anhydroTTX after more than 3 years in captivity [83,85]. At the same time, A. varius reared in the laboratory for more than 2 years did not contain TTX [247].
Juveniles of C. pyrrhogaster fed toxic pufferfish ovary extracts accumulated substantial amounts of TTX and retained them for a week, while the levels of the accumulated toxin differed between populations [72]. Recent studies on C. pyrrhogaster fed TTX and/or its putative biosynthetic intermediates showed that ingested compounds accumulated in the animals, but their conversion to other TTX analogs did not occur [21,237]. TTX has been detected in the tail, intestine, liver, skin, and ovaries of experimental animals [21]. Immunohistochemical studies of C. pyrrhogaster juveniles after feeding showed the presence of TTX in the epithelial cells of the intestine, mucoid skin cells, and ovaries; in the tail, TTX was localized in the mucoid skin cells and dermis [21].
4.3. Mollusca
Narita et al. conducted the first feeding experiment with the non-toxic gastropod Charonia sauliae [238]. The molluscs were fed TTX-bearing specimens of the starfish, A. polyacanthus. The digestive glands became toxic after a week of feeding, and the average TTX accumulation was 33%. An increase in the dose of TTX led to an increase in the total amount of accumulated toxin. When toxified molluscs were subsequently maintained on a TTX-free diet, no TTX metabolism and/or excretion from the body was observed. In a feeding experiment with P. maculata, TTX was detected in all the tissues within an hour of feeding [139]. The molluscs initially accumulated about 32% of the administered TTX, and the percentage of accumulated TTX gradually decreased to 9% on the 39th day of the experiment. High TTX concentrations were detected in the mantle of the molluscs. Biessy et al. conducted an interesting feeding experiment with P. australis using encapsulated TTX [240]. The bivalves fed agar-gelatine capsules with TTX bound to humic acid for 13 days actively accumulated toxins at concentrations exceeding the allowable norms in food products. However, the level of accumulated TTX did not exceed 0.5–1% of that contained in the food. When non-toxic marine snails Nassarius (Pliarcularia) globosus and Reticunassa festiva were fed toxic ovaries of T. vermicularis, the TTX accumulation ratios were 4% and 2%, respectively [239]. The non-toxic mussels Mytilus galloprovincialis were toxified after feeding on toxic P. multitentaculata larvae; TTX was detected only in the intestines of the animals [212]. Long-term maintenance of molluscs on a non-toxic diet after a week of feeding on planocerid larvae resulted in a sharp decrease in the TTX concentration.
In a study by McNabb et al. [122], toxic P. maculata specimens maintained in aquaria retained TTX for 26 d. In a large-scale study with P. maculata kept on a TTX-free diet for 126 days, the average TTX concentrations in the molluscs declined over time [123]. The depuration time differed among organs, with the fastest depuration observed in the heart and the slowest, in the gonads. The foot, mantle, and stomach were depleted of TTX at the same rate. Depuration of TTX during long-term maintenance without the toxin (150 days) has also been found in P. australis [248]. The siphons had the highest TTX concentrations during the entire observation period. The lowest concentrations of TTX and fast depuration were detected in the digestive glands, whereas after 21 days, only trace amounts of the toxin remained.
4.4. Non-TTX-Bearing Animals
An early study of the skin toxicity of T. granulosa revealed its activity against various vertebrates, including mammals of the orders Rodentia, Carnivora, and Eulipotyphla; birds of the order Passeriformes; reptiles of the order Squamata; and the newt itself [249]. As a result of forced or voluntary feeding on newt body parts or injection of the newt skin extract, all tested animals showed symptoms characteristic of TTX poisoning. However, snakes of the genus Thamnophis were resistant to TTX. Williams et al. [250] suggested that snakes that fed on highly toxic newts had substantial amounts of TTX in their tissues. Thamnophis sirtalis that fed on toxic T. granulosa specimens for 5 weeks retained TTX in the liver for 7 weeks and in the kidneys for 3 weeks [250]. Moreover, TTX was detected in the liver of T. sirtalis one month after ingestion of only one newt. In an experiment of oral TTX administration to newly hatched T. sirtalis, the half-life of TTX excreted from the liver was 8 days [97]. The authors found that 99% of a single TTX dose in the experiment would be eliminated from the snake’s body within 61 days.
Gall et al. [251] examined the ability of aquatic macroinvertebrates sympatric with T. granulosa to consume toxic newt eggs. Among the animals tested, only caddisfly Limnephilus flavastellus larvae could consume substantial quantities of toxic eggs, without this affecting their further development. Another study involving wild-caught L. flavastellus larvae and lab-reared larvae additionally fed with T. granulosa eggs showed higher toxin levels in the latter [252]. Interestingly, wild-caught larvae kept on a TTX-free diet retained the toxin for up to 134 days, even during metamorphosis and adult stage.
4.5. Concluding Remarks
Despite numerous studies elucidating the ways of TTX accumulation in marine and terrestrial animals and ecosystems, the problem of TTX biogenesis remains unresolved. Additionally, if the generally accepted hypothesis of TTX origin in nature is its bacterial production, as a source of toxin in animals, symbiotic microflora (endogenous), diet (exogenous), or combination of both factors are considered. TTX production by symbiotic and free-living bacteria and problems concerning the contribution of the microbiome in the toxification of the animal have been widely discussed in other reviews [1,5,191,253] and are not considered in this work. Here, we discuss challenges facing authors in the interpretation of experimental studies revealing TTX origin in animals.
Most scientific evidence indicates different toxification patterns between terrestrial and marine animals. Experiments with the long-term maintenance of wild-caught TTX-bearing newts on a TTX-free diet revealed endogenous acquisition of the toxin. In the similar experiments with marine molluscs, rapid TTX depuration was observed. Difficulties occur comparing results of this type of studies even within amphibias. Thus, species of the genus Taricha and some Atelopus frogs kept in laboratory for a long period retain or even increase TTX level, while N. viridescens and A. varius lose the toxicity. The main problem facing researchers is the measurement of the initial TTX concentration in the experimental animal. The average concentration of TTX within a given population or the toxin content in a small patch of skin are usually used as a starting point for investigation. At the same time, TTX content can significantly vary both within population and on different skin parts of one animal. As a consequence, it is difficult to trace actual depuration rate of TTX.
Feeding experiments held on marine and terrestrial animals and results of observations clearly indicate that TTX-bearers can accumulate exogenous TTX, through the food web. Using different methods of TTX administration, researches traced TTX kinetics inside animal body. All of these studies are based on the unique experimental designs different in basic parameters, such as time of TTX administration, observation period, and even organs investigated. Disparate data, on the one hand, significantly expand the understanding of TTX migration inside animals, and on the other hand, do not provide the understanding of common patterns and exact mechanisms of TTX accumulation. Different approaches, including a unified design of experiments and studies of separate organs or tissues, could potentially improve the TTX kinetics understanding. For instance, an in vitro experiment with pufferfish liver tissue slices revealed the involvement of carrier-mediated transport system in TTX uptake [118]. Future researches elucidating the molecular aspects of TTX transport in TTX-bearing animals are needed.
5. Conclusions
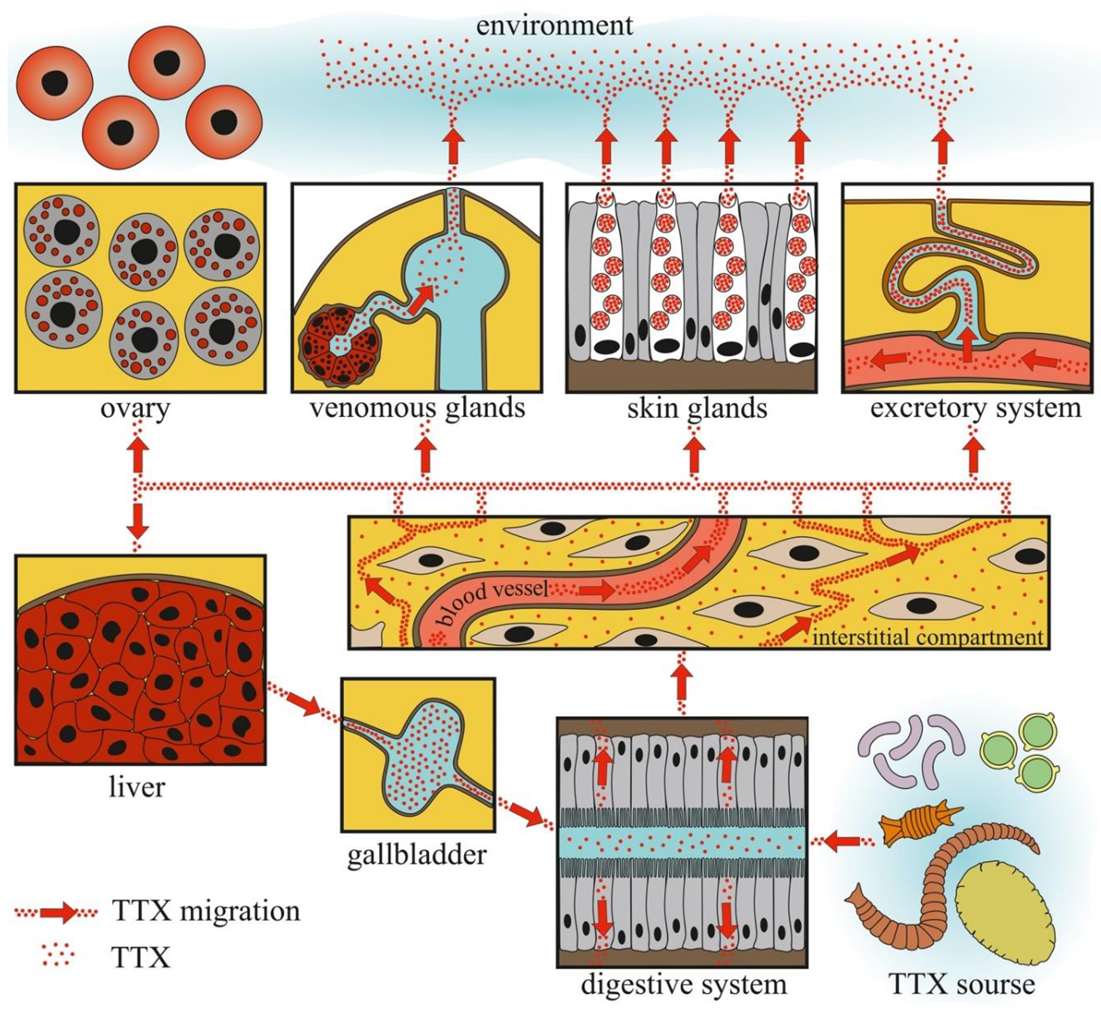
Figure 6 summarizes the currently available data on the localization of TTX in the bodies of TTX-bearing animals in the natural environment and experimental conditions.
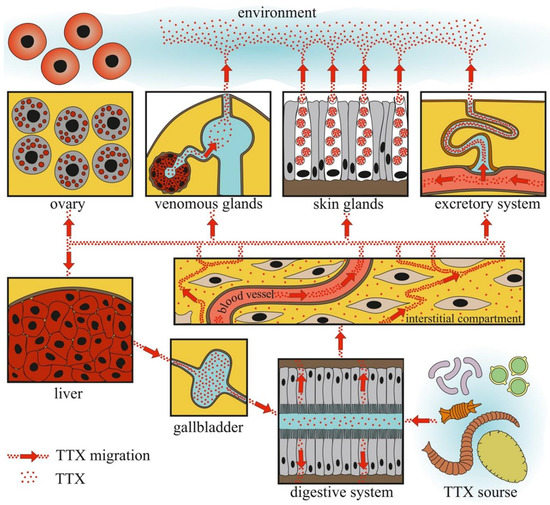
Figure 6.
Schematic illustration of tetrodotoxin (TTX) migration in TTX-bearing animals.
The data obtained indicate that TTX can be absorbed into the body through the gastrointestinal tract. Cases of poisoning and feeding experiments showed that the migration rate of TTX from the intestinal cavity was quite high. The concentration of TTX detected in the intestinal extracts of TTX-bearing animals was minimal, indicating that TTX does not remain in the intestinal epithelium for a long time. High TTX concentrations in the intestines of the pufferfish species Lagocephalus and nemerteans may be related to the consumption of large quantities of TTX-bearing objects and/or slow migration of TTX through the intestines [13,35,151]. From the intestinal cavity, TTX is absorbed by phagocytic enterocytes [155,156].
Furthermore, TTX migration within the body depends on the development and features of the circulatory system. In animals with well-developed circulatory systems, most of the TTX enters the bloodstream and is distributed throughout the body. In most experiments with oral TTX administration to pufferfish, TTX was first detected in the liver and then in other organs and tissues (Table 1). In animals with a simple or absent circulatory system, TTX was mostly detected in organs localized near the anterior intestine. In nemerteans, the anterior parts of the body wall, proboscis, and intestines contained high levels of TTX [151]. In molluscs, the most toxic organs were the digestive and/or salivary glands adjacent to the esophagus and/or stomach (Figure 3, Figure 4 and Figure 5). Relatively high levels of TTX have also been found in organs that penetrate hemal vessels (gills and heart) and lacunae (mantle, leg, gonads, and siphon).
TTX-bearing animals kept on a TTX-free diet can retain TTX in the integument and excrete it with mucus and eggs for a long time. The data obtained suggest the presence of a reservoir for TTX in the animal body. The liver, which contains high concentrations of TTX, can be a toxin reservoir in TTX-bearing vertebrates. The liver, which obtains TTX through the blood, retains a large amount of the toxin and slowly releases the excess through the gallbladder into the gastrointestinal tract, where it is reabsorbed. A similar mechanism of TTX reabsorption has been suggested for the pufferfish T. rubripes [227]. Molluscs probably do not possess a TTX reservoir because the toxin quickly depurates under TTX-free conditions [123]. An experiment with electric stimulation and long-term maintenance in aquaria showed that, in nemerteans, the body wall could serve as a storage compartment for TTX [153].
The organs that release TTX in the environment include the gonads, excretory organs (kidneys or protonephridia), integument, and venom glands. In these organs, unidirectional transport of TTX can be assumed. TTX detection in the protonephridia of marine worms [158,159] and kidneys of pufferfish [218,224,227,228] indicates that the toxin is released through the excretory system. In the gonads, TTX is predominantly associated with egg-yolk granules and is released during yolk resorption during embryonic development [81,196,198,203]. In the glandular cells of the integument and venom glands, TTX was associated with secretory granules (Figure 1, Figure 2, Figure 3, Figure 4 and Figure 6). In the ribbon worm K. alborostrata, the migration of TTX through the organelles of protein synthesis (nuclear membrane, cisterns of the endoplasmic reticulum, and young glandular granules) to mature secretory granules and subsequent excretion into the environment was traced.
Other possible pathways for TTX entry into animals remain unclear. Based on the data obtained from nemerteans and pufferfish, TTX absorption through epithelial cells in the skin can be assumed. In C. cf. simula, the apical part of ciliated skin cells gradually stained for TTX: the distal part of the cell stained brightly, and the intensity gradually decreased toward the proximal part of the cell [156]. Small amounts of TTX have also been detected in the surface epithelial cells of T. alboplumbeus [53]. However, additional studies are needed to elucidate the mechanisms of TTX entry into the cells of the integumentary epithelium.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Katikou, P.; Gokbulut, C.; Kosker, A.R.; Campàs, M.; Ozogul, F. An updated review of tetrodotoxin and its peculiarities. Mar. Drugs 2022, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Kawamura, M. The constituents of the ovaries of globefish. VII Purification of tetrodotoxin by chromatography. J. Pharm. Soc. Jpn. 1952, 6, 771–773. [Google Scholar] [CrossRef]
- Yokoo, A. Study on chemical purification of tetrodotoxin (3)-purification of spheroidine. J. Chem. Soc. Jpn. 1950, 71, 590–592. [Google Scholar]
- Miyazawa, K.; Noguchi, T. Distribution and origin of tetrodotoxin. J. Toxicol.-Toxin Rev. 2001, 20, 11–33. [Google Scholar] [CrossRef]
- Magarlamov, T.Y.; Melnikova, D.I.; Chernyshev, A.V. Tetrodotoxin-producing bacteria: Detection, distribution and migration of the toxin in aquatic systems. Toxins 2017, 9, 166. [Google Scholar] [CrossRef]
- Tani, I. Nihonsan Fugu No Chudokugakuteki Kenkyu (Toxicological Studies on Japanese Puffers); Teikoku Tosho: Tokyo, Japan, 1945. (In Japanese) [Google Scholar]
- Endo, R. Toxicological studies on puffer fishes:—Comparison of toxicities on the various species. J. Toxicol. Sci. 1984, 9, 1–11. [Google Scholar] [CrossRef]
- Khora, S.S.; Isa, J.; Yasumoto, T. Toxicity of puffers from Okinawa, Japan. Nippon Suisan Gakkaishi 1991, 57, 163–167. [Google Scholar] [CrossRef]
- Hwang, D.F.; Kao, C.-Y.; Yang, H.-C.; Jeng, S.-S.; Noguchi, T.; Hashimoto, K. Toxicity of puffer in Taiwan. Nippon Suisan Gakkaishi 1992, 58, 1541–1547. [Google Scholar] [CrossRef]
- Nuñez-Vázquez, E.J.; Yotsu-Yamashita, M.; Sierra-Beltrán, A.P.; Yasumoto, T.; Ochoa, J.L. Toxicities and distribution of tetrodotoxin in the tissues of puffer fish found in the coast of the Baja California Peninsula, Mexico. Toxicon 2000, 38, 729–734. [Google Scholar] [CrossRef]
- Tao, J.; Wei, W.J.; Nan, L.; Lei, L.H.; Hui, H.C.; Fen, G.X.; Jun, L.Y.; Jing, Z.; Rong, J. Development of competitive indirect ELISA for the detection of tetrodotoxin and a survey of the distribution of tetrodotoxin in the tissues of wild puffer fish in the waters of south-east China. Food Addit. Contam.-Part A Chem. Anal. Control. Expo. Risk Assess. 2010, 27, 1589–1597. [Google Scholar] [CrossRef]
- Chulanetra, M.; Sookrung, N.; Srimanote, P.; Indrawattana, N.; Thanongsaksrikul, J.; Sakolvaree, Y.; Chongsa-Nguan, M.; Kurazono, H.; Chaicumpa, W. Toxic marine puffer fish in Thailand seas and tetrodotoxin they contained. Toxins 2011, 3, 1249–1262. [Google Scholar] [CrossRef]
- Thuy, L.V.; Yamamoto, S.; Kawaura, R.; Takemura, N.; Yamaki, K.; Yasumoto, K.; Takada, K.; Watabe, S.; Sato, S. Tissue distribution of tetrodotoxin and its analogs in Lagocephalus pufferfish collected in Vietnam. Fish. Sci. 2020, 86, 1101–1110. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Noguchi, T. Occurrence of a tetrodotoxin-like substance in a goby Gobius criniger. Toxicon 1971, 9, 79–84. [Google Scholar] [CrossRef]
- Noguchi, T.; Hashimoto, Y. Isolation of tetrodotoxin from a goby Gobius criniger. Toxicon 1973, 11, 305–307. [Google Scholar] [CrossRef]
- Itoi, S.; Sato, T.; Takei, M.; Yamada, R.; Ogata, R.; Oyama, H.; Teranishi, S.; Kishiki, A.; Wada, T.; Noguchi, K.; et al. The planocerid flatworm is a main supplier of toxin to tetrodotoxin-bearing fish juveniles. Chemosphere 2020, 249, 126217. [Google Scholar] [CrossRef]
- Ito, M.; Furukawa, R.; Shino, Y.; Sato, M.; Oyama, H.; Okabe, T.; Suo, R.; Sugita, H.; Takatani, T.; Arakawa, O.; et al. Local differences in the toxin amount and composition of tetrodotoxin and related compounds in pufferfish. Toxins 2022, 14, 150. [Google Scholar] [CrossRef]
- Heiser, V.G. Poisonous Fish. In Annual Report of the Bureau of Health for the Philippine Islands; Ulan Press: Washington, DC, USA, 1906; p. 70. [Google Scholar]
- Herre, A.W. Gobies of the Philippines and the China Sea. In Monographs of the Bureau of Science, Manila, Philippine Islands, 23; Bureau of Printing: Hikone, Japan, 1927; p. 352. [Google Scholar]
- Takahashi, D.; Inoko, Y. Unter suchungen über das Fugugift, Centralbl. Meb. Wiss. 1889, 27, 529–530. [Google Scholar]
- Sato, S.; Kawaura, R.; Togashi, K.; Mizusawa, N.; Yasumoto, K.; Takada, K.; Amano, M.; Watabe, S. De novo accumulation of tetrodotoxin and its analogs in pufferfish and newt and dosage-driven accumulation of toxins in newt: Tissue distribution and anatomical localization. J. Mar. Sci. Eng. 2021, 9, 1004. [Google Scholar] [CrossRef]
- Saito, T.; Noguchi, T.; Harada, T.; Murata, O.; Hashimoto, K. Tetrodotoxin as a biological defense agent for puffers. Bull. Jpn. Soc. Sci. Fish. 1985, 51, 1175–1180. [Google Scholar] [CrossRef]
- Fuchi, U.; Narimatsu, H.; Nakama, S.; Kotobuki, H.; Hirakawa, H.; Torishima, Y.; Noguchi, T.; Ohtom, N. Tissue distribution of toxicity in a puferfish, Arothron firmamentum (“Hishifugu”). Food Health 1991, 32, 520–524. [Google Scholar]
- Mahmud, Y.; Okada, K.; Takatani, T.; Kawatsu, K.; Hamano, Y.; Arakawa, O.; Noguchi, T. Intra-tissue distribution of tetrodotoxin in two marine puffers Takifugu vermicularis and Chelonodon patoca. Toxicon 2003, 41, 13–18. [Google Scholar] [CrossRef]
- Jang, J.; Yotsu-Yamashita, M. Distribution of tetrodotoxin, saxitoxin, and their analogs among tissues of the puffer fish Fugu pardalis. Toxicon 2006, 48, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Diener, M.; Christian, B.; Ahmed, M.S.; Luckas, B. Determination of tetrodotoxin and its analogs in the puffer fish Takifugu oblongus from Bangladesh by hydrophilic interaction chromatography and mass-spectrometric detection. Anal. Bioanal. Chem. 2007, 389, 1997–2002. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Takayama, Y.; Tahara, T.; Anraku, K.; Ito, Y.; Akaike, N. Quantitative analysis of toxin extracts from various tissues of wild and cultured puffer fish by an electrophysiological method. Toxicon 2008, 51, 606–614. [Google Scholar] [CrossRef]
- Indumathi, S.M.; Khora, S.S. Toxicity assessment and screening of tetrodotoxin in the oblong blowfish (Takifugu oblongus) from the Tamil Nadu Coast of Bay of Bengal, India. Asian Pac. J. Trop. Med. 2017, 10, 278–284. [Google Scholar] [CrossRef]
- Rambla-Alegre, M.; Reverté, L.; del Río, V.; de la Iglesia, P.; Palacios, O.; Flores, C.; Caixach, J.; Campbell, K.; Elliott, C.T.; Izquierdo-Muñoz, A.; et al. Evaluation of tetrodotoxins in puffer fish caught along the Mediterranean coast of Spain. Toxin profile of Lagocephalus sceleratus. Environ. Res. 2017, 158, 1–6. [Google Scholar] [CrossRef]
- Tatsuno, R.; Umeeda, M.; Miyata, Y.; Ideguchi, R.; Fukuda, T.; Furushita, M.; Ino, Y.; Yoshikawa, H.; Takahashi, H.; Nagashima, Y. Toxicity of Takifugu exascurus collected from the Sea of Kumano. Shokuhin Eiseigaku Zasshi 2021, 62, 28–32. [Google Scholar] [CrossRef]
- Hassoun, A.E.R.; Ujević, I.; Jemaa, S.; Roje-Busatto, R.; Mahfouz, C.; Fakhri, M.; Nazlić, N. Concentrations of tetrodotoxin (TTX) and its analogue 4,9-anhydro TTX in different tissues of the silver-cheeked pufferfish (Lagocephalus sceleratus, Gmelin, 1789) caught in the South-Eastern Mediterranean Sea, Lebanon. Toxins 2022, 14, 123. [Google Scholar] [CrossRef]
- Honda, S.; Ichimaru, S.; Arakawa, O.; Takatani, T.; Noguchi, T.; Ishizaki, S.; Nagashima, Y. Toxicity of puffer fish fins. Shokuhin Eiseigaku Zasshi 2007, 48, 159–162. [Google Scholar] [CrossRef][Green Version]
- Mahmud, Y.; Yamamori, K.; Noguchi, T. Toxicity and tetrodotoxin as the toxic principle of a brackish water puffer, Tetraodon steindachneri, collected from Thailand. J. Food Hyg. Soc. Jpn. 1999, 40, 391–395. [Google Scholar] [CrossRef]
- Saitanu, K.; Laobhripatr, S.; Limpakarnjanarat, K.; Sangwanloy, O.; Sudhasaneya, S.; Anuchatvorakul, B.; Leelasitorn, S. Toxicity of the freshwater puffer fish Tetraodon fangi and T. palembangensis from Thailand. Toxicon 1991, 29, 895–897. [Google Scholar] [CrossRef]
- Katikou, P.; Georgantelis, D.; Sinouris, N.; Petsi, A.; Fotaras, T. First report on toxicity assessment of the Lessepsian migrant pufferfish Lagocephalus sceleratus (Gmelin, 1789) from European waters (Aegean Sea, Greece). Toxicon 2009, 54, 50–55. [Google Scholar] [CrossRef]
- Lin, S.J.; Chai, T.J.; Jeng, S.S.; Hwang, D.F. Toxicity of the puffer Takifugu rubripes cultured in northern Taiwan. Fish. Sci. 1998, 64, 766–770. [Google Scholar] [CrossRef]
- Sato, S.; Komaru, K.; Ogata, T.; Kodama, M. Occurence of tetrodotoxin in cultered pufer. Nippon Suisan Gakkaishi 1990, 56, 1129–1131. [Google Scholar] [CrossRef]
- Noguchi, T.; Arakawa, O.; Takatani, T. Toxicity of pufferfish Takifugu rubripes cultured in netcages at sea or aquaria on land. Comp. Biochem. Physiol.-Part D Genom. Proteom. 2006, 1, 153–157. [Google Scholar] [CrossRef]
- Noguchi, T.; Takatani, T.; Arakawa, O. Toxicity of puffer fish cultured in netcages. Shokuhin Eiseigaku Zasshi 2004, 45, 146–149. [Google Scholar] [CrossRef]
- Nagashima, Y.; Matsumoto, T.; Kadoyama, K.; Ishizaki, S.; Terayama, M. Toxicity and molecular identification of green toadfish Lagocephalus lunaris collected from Kyushu coast, Japan. J. Toxicol. 2011, 2011, 801285. [Google Scholar] [CrossRef][Green Version]
- Ngy, L.; Taniyama, S.; Shibano, K.; Yu, C.F.; Takatani, T.; Arakawa, O. Distribution of tetrodotoxin in pufferfish collected from coastal waters of Sihanouk Ville, Cambodia. J. Food Hyg. Soc. Jpn. 2008, 49, 361–365. [Google Scholar] [CrossRef][Green Version]
- Ikeda, K.; Emoto, Y.; Tatsuno, R.; Wang, J.J.; Ngy, L.; Taniyama, S.; Takatani, T.; Arakawa, O. Maturation-associated changes in toxicity of the pufferfish Takifugu poecilonotus. Toxicon 2010, 55, 289–297. [Google Scholar] [CrossRef]
- Tatsuno, R.; Shikina, M.; Soyano, K.; Ikeda, K.; Takatani, T.; Arakawa, O. Maturation-associated changes in the internal distribution of tetrodotoxin in the female goby Yongeichthys criniger. Toxicon 2013, 63, 64–69. [Google Scholar] [CrossRef]
- Itoi, S.; Ishizuka, K.; Mitsuoka, R.; Takimoto, N.; Yokoyama, N.; Detake, A.; Takayanagi, C.; Yoshikawa, S.; Sugita, H. Seasonal changes in the tetrodotoxin content of the pufferfish Takifugu niphobles. Toxicon 2016, 114, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Kosker, A.R.; Özogul, F.; Durmus, M.; Ucar, Y.; Ayas, D.; Regenstein, J.M.; Özogul, Y. Tetrodotoxin levels in pufferfish (Lagocephalus sceleratus) caught in the Northeastern Mediterranean Sea. Food Chem. 2016, 210, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Kanahara, Y.; Tatsuno, R.; Soyano, K.; Nishihara, G.N.; Urata, C.; Takatani, T.; Arakawa, O. Maturation-associated changes in internal distribution and intra-ovarian microdistribution of tetrodotoxin in the pufferfish Takifugu pardalis. Fish. Sci. 2018, 84, 723–732. [Google Scholar] [CrossRef]
- Kosker, A.R.; Özogul, F.; Durmus, M.; Ucar, Y.; Ayas, D.; Šimat, V.; Özogul, Y. First report on TTX levels of the yellow spotted pufferfish (Torquigener flavimaculosus) in the Mediterranean Sea. Toxicon 2018, 148, 101–106. [Google Scholar] [CrossRef]
- Kosker, A.R.; Özogul, F.; Ayas, D.; Durmus, M.; Ucar, Y.; Regenstein, J.M.; Özogul, Y. Tetrodotoxin levels of three pufferfish species (Lagocephalus sp.) caught in the North-Eastern Mediterranean sea. Chemosphere 2019, 219, 95–99. [Google Scholar] [CrossRef]
- Akbora, H.D.; Kunter, İ.; Erçetin, T.; Elagöz, A.M.; Çiçek, B.A. Determination of tetrodotoxin (TTX) levels in various tissues of the silver cheeked puffer fish (Lagocephalus sceleratus (Gmelin, 1789)) in Northern Cyprus Sea (Eastern Mediterranean). Toxicon 2020, 175, 1–6. [Google Scholar] [CrossRef]
- Kodama, M.; Sato, S.; Ogata, T.; Suzuki, Y.; Kaneko, T.; Aida, K. Tetrodotoxin secreting glands in the skin of puffer fishes. Toxicon 1986, 24, 819–829. [Google Scholar] [CrossRef]
- Tanu, M.B.; Mahmud, Y.; Takatani, T.; Kawatsu, K.; Hamano, Y.; Arakawa, O.; Noguchi, T. Localization of tetrodotoxin in the skin of a brackishwater puffer Tetraodon steindachneri on the basis of immunohistological study. Toxicon 2002, 40, 103–106. [Google Scholar] [CrossRef]
- Mahmud, Y.; Arakawa, O.; Ichinose, A.; Tanu, M.B.; Takatani, T.; Tsuruda, K.; Kawatsu, K.; Hamano, Y.; Noguchi, T. Intracellular visualization of tetrodotoxin (TTX) in the skin of a puffer Tetraodon nigroviridis by immunoenzymatic technique. Toxicon 2003, 41, 605–611. [Google Scholar] [CrossRef]
- Itoi, S.; Yoshikawa, S.; Tatsuno, R.; Suzuki, M.; Asahina, K.; Yamamoto, S.; Takanashi, S.; Takatani, T.; Arakawa, O.; Sakakura, Y.; et al. Difference in the localization of tetrodotoxin between the female and male pufferfish Takifugu niphobles, during spawning. Toxicon 2012, 60, 1000–1004. [Google Scholar] [CrossRef]
- Ikeda, K.; Murakami, Y.; Emoto, Y.; Ngy, L.; Taniyama, S.; Yagi, M.; Takatani, T.; Arakawa, O. Transfer profile of intramuscularly administered tetrodotoxin to non-toxic cultured specimens of the pufferfish Takifugu rubripes. Toxicon 2009, 53, 99–103. [Google Scholar] [CrossRef]
- Okita, K.; Takatani, T.; Nakayasu, J.; Yamazaki, H.; Sakiyama, K.; Ikeda, K.; Arakawa, O.; Sakakura, Y. Comparison of the localization of tetrodotoxin between wild pufferfish Takifugu rubripes juveniles and hatchery-reared juveniles with tetrodotoxin administration. Toxicon 2013, 71, 128–133. [Google Scholar] [CrossRef]
- Tatsuno, R.; Gao, W.; Ibi, K.; Mine, T.; Okita, K.; Nishihara, G.N.; Takatani, T.; Arakawa, O. Profile differences in tetrodotoxin transfer to skin and liver in the pufferfish Takifugu rubripes. Toxicon 2017, 130, 73–78. [Google Scholar] [CrossRef]
- Gao, W.; Yamada, M.; Ohki, R.; Nagashima, Y.; Tatsuno, R.; Ikeda, K.; Kawatsu, K.; Takatani, T.; Arakawa, O. Evaluation of the tetrodotoxin uptake ability of pufferfish Takifugu rubripes tissues according to age using an in vitro tissue slice incubation method. Toxicon 2020, 174, 8–12. [Google Scholar] [CrossRef]
- Henrikson, R.C.; Gedeon Matoltsy, A. The fine structure of teleost epidermis. I. Introduction and filament-containing cells. J. Ultrastruct. Res. 1968, 21, 194–212. [Google Scholar] [CrossRef]
- Nagashima, Y.; Hamada, Y.; Ushio, H.; Nishio, S.; Shimakura, K.; Shiomi, K. Subcellular distribution of tetrodotoxin in puffer fish liver. Toxicon 1999, 37, 1833–1837. [Google Scholar] [CrossRef]
- Matsumura, K. Tetrodotoxin as a pheromone. Nature 1995, 378, 563–564. [Google Scholar] [CrossRef]
- Brown, M.S.; Mosher, H.S. Tarichatoxin: Isolation and purification. Science 1963, 140, 295–296. [Google Scholar] [CrossRef]
- Mosher, H.S.; Fuhrman, F.A.; Buchwald, H.D.; Fischer, H.G. Tarichatoxin—Tetrodotoxin: A potent neurotoxin. Science 1964, 144, 1100–1110. [Google Scholar] [CrossRef]
- Buchwald, D.H.; Durham, L.; Fischer, H.G.; Harada, R.; Mosher, H.S.; Kao, C.Y.; Fuhrman, F.A. Identity of tarichatozin and tetrodotoxin. Science 1964, 143, 474–475. [Google Scholar] [CrossRef]
- Wakely, J.F.; Fuhrman, G.J.; Fuhrman, F.A.; Fischer, H.G.; Mosher, H.S. The occurrence of tetrodotoxin (tarichatoxin) in amphibia and the distribution of the toxin in the organs of newts (Taricha). Toxicon 1966, 3, 195–203. [Google Scholar] [CrossRef]
- Brodie, E.D., Jr.; Hensel, J.L., Jr.; Johnson, J.A. Toxicity of the urodele amphibians Taricha, Notophthalmus, Cynops and Paramesotriton (Salamandridae). Copeia 1974, 1974, 506–511. [Google Scholar] [CrossRef]
- Yotsu, M.; Iorizzi, M.; Yasumoto, T. Distribution of tetrodotoxin, 6-epitetrodotoxin, and 11-deoxytetrodotoxin in newts. Toxicon 1990, 28, 238–241. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Mebs, D.; Kwet, A.; Schneider, M. Tetrodotoxin and its analogue 6-epitetrodotoxin in newts (Triturus spp.; Urodela, Salamandridae) from southern Germany. Toxicon 2007, 50, 306–309. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Toennes, S.W.; Mebs, D. Tetrodotoxin in Asian newts (Salamandridae). Toxicon 2017, 134, 14–17. [Google Scholar] [CrossRef]
- Hanifin, C.T.; Yotsu-Yamashita, M.; Yasumoto, T.; Brodie, E.D., Jr.; Brodie, E.D., III. Toxicity of dangerous prey: Variation of tetrodotoxin levels within and among populations of the newt Taricha granulosa. J. Chem. Ecol. 1999, 25, 2161–2175. [Google Scholar] [CrossRef]
- Mebs, D.; Schneider, J.V.; Schröder, O.; Yotsu-Yamashita, M.; Harley, J.R.; Mogk, L.; Köhler, G. A study on the genetic population structure and the tetrodotoxin content of rough-skinned newts, Taricha granulosa (Salamandridae), from their northern range of distribution. Toxicon 2022, 206, 38–41. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Gilhen, J.; Russell, R.W.; Krysko, K.L.; Melaun, C.; Kurz, A.; Kauferstein, S.; Kordis, D.; Mebs, D. Variability of tetrodotoxin and of its analogues in the red-spotted newt, Notophthalmus viridescens (Amphibia: Urodela: Salamandridae). Toxicon 2012, 59, 257–264. [Google Scholar] [CrossRef]
- Mochida, K.; Kitada, M.; Ikeda, K.; Toda, M.; Takatani, T.; Arakawa, O. Spatial and temporal instability oflocal biotic community mediate a form of aposematic defense in newts, consisting of carotenoid-based coloration and tetrodotoxin. J. Chem. Ecol. 2013, 39, 1186–1192. [Google Scholar] [CrossRef]
- Hanifin, C.T.; Brodie, E.D., III; Brodie, E.D., Jr. Tetrodotoxin levels of the rough-skin newt, Taricha granulosa, inscreas in long-term captivity. Toxicon 2002, 40, 1149–1153. [Google Scholar] [CrossRef]
- Mebs, D.; Arakawa, O.; Yotsu-Yamashita, M. Tissue distribution of tetrodotoxin in the red-spotted newt Notophthalmus viridescens. Toxicon 2010, 55, 1353–1357. [Google Scholar] [CrossRef]
- Tsuruda, K.; Arakawa, O.; Noguchi, K. Toxicity and toxin profile of the newt Cynops pyrrhogaster from western Japan. J. Nat. Toxins 2001, 10, 79–89. [Google Scholar]
- Tsuruda, K.; Arakawa, O.; Kawatsu, K.; Hamano, Y.; Takatani, T.; Noguchi, T. Secretory glands of tetrodotoxin in the skin of the Japanese newt Cynops pyrrhogaster. Toxicon 2002, 40, 131–136. [Google Scholar] [CrossRef]
- Mailho-Fontana, P.L.; Jared, C.; Antoniazzi, M.M.; Sciani, J.M.; Pimenta, D.C.; Stokes, A.N.; Grant, T.; Brodie, E.D.; Brodie, E.D. Variations in tetrodotoxin levels in populations of Taricha granulosa are expressed in the morphology of their cutaneous glands. Sci. Rep. 2019, 9, 18490. [Google Scholar] [CrossRef]
- Spicer, M.M.; Stokes, A.N.; Chapman, T.L.; Brodie, E.D., Jr.; Brodie, E.D., III; Gall, B.G. An investigation into tetrodotoxin (TTX) levels associated with the red dorsal spots in eastern newt (Notophthalmus viridescens) efts and adults. J. Toxicol. 2018, 2018, 1–5. [Google Scholar] [CrossRef]
- Mebs, D.; Yotsu-Yamashita, M.; Seitz, H.M.; Arakawa, O. Tetrodotoxin does not protect red-spotted newts, Notophthalmus viridescens, from intestinal parasites. Toxicon 2012, 60, 66–69. [Google Scholar] [CrossRef]
- Kim, Y.H.; Brown, G.B.; Mosher, H.S.; Fuhrman, F.A. Tetrodotoxin: Occurrence in atelopid frogs of Costa Rica. Science 1975, 189, 151–152. [Google Scholar] [CrossRef]
- Pavelka, L.A.; Kim, Y.H.; Mosher, H.S. Tetrodotoxin and tetrodotoxin-like compounds from the eggs of the costa rican frog, Atelopus chiriquiensis. Toxicon 1977, 15, 135–139. [Google Scholar] [CrossRef]
- Mebs, D.; Schmidt, K. Occurrence of tetrodotoxin in the frog Atelopus oxyrhynchus. Toxicon 1989, 27, 819–822. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Mebs, D.; Yasumoto, T. Tetrodotoxin and its analogues in extracts from the toad Atelopus oxyrhynchus (family: Bufonidae). Toxicon 1992, 30, 1489–1492. [Google Scholar] [CrossRef]
- Daly, J.W.; Gusovsky, F.; Myers, C.W.; Yotsu-Yamashita, M.; Yasumoto, T. First occurrence of tetrodotoxin in a dendrobatid frog (Colostethus inguinalis), with further reports for the bufonid genus Atelopus. Toxicon 1994, 32, 279–285. [Google Scholar] [CrossRef]
- Mebs, D.; Yotsu-Yamashita, M.; Yasumoto, T.; Lötters, S.; Andreas, S. Further report of the occurrence of tetrodotoxin in Atelopus species (Family: Bufonidae)). Toxicon 1995, 33, 246–249. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Tateki, E. First report on toxins in the Panamanian toads Atelopus limosus, A. glyphus and A. certus. Toxicon 2010, 55, 153–156. [Google Scholar] [CrossRef]
- Mebs, D.; Lorentz, M.; Yotsu-Yamashita, M.; Rößler, D.C.; Ernst, R.; Lötters, S. Geographic range expansion of tetrodotoxin in amphibians—First record in Atelopus hoogmoedi from the Guiana Shield. Toxicon 2018, 150, 175–179. [Google Scholar] [CrossRef]
- Pires, O.R.; Sebben, A.; Schwartz, E.F.; Largura, S.W.R.; Bloch, C.; Morales, R.A.V.; Schwartz, C.A. Occurrence of tetrodotoxin and its analogues in the brazilian frog Brachycephalus ephippium (Anura: Brachycephalidae). Toxicon 2002, 40, 761–766. [Google Scholar] [CrossRef]
- Pires, O.R.; Sebben, A.; Schwartz, E.F.; Bloch, C.; Morales, R.A.V.; Schwartz, C.A. The occurrence of 11-oxotetrodotoxin, a rare tetrodotoxin analogue, in the brachycephalidae frog Brachycephalus ephippium. Toxicon 2003, 42, 563–566. [Google Scholar] [CrossRef]
- Pires, O.R.; Sebben, A.; Schwartz, E.F.; Morales, R.A.V.; Bloch, C.; Schwartz, C.A. Further report of the occurrence of tetrodotoxin and new analogues in the Anuran family Brachycephalidae. Toxicon 2005, 45, 73–79. [Google Scholar] [CrossRef]
- Tanu, M.B.; Mahmud, Y.; Tsuruda, K.; Arakawa, O.; Noguchi, T. Occurrence of tetrodotoxin in the skin of a rhacophoridid frog Polypedates sp. from Bangladesh. Toxicon 2001, 39, 937–941. [Google Scholar] [CrossRef]
- Sheumack, D.D.; Howden, M.E.H.; Spence, I.; Quinn, R.J. Maculotoxin: A neurotoxin from the venom glands of the octopus Hapalochlaena maculosa identified as tetrodotoxin. Science 1978, 199, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.F.; Arakawa, O.; Saito, T.; Noguchi, T.; Simidu, U.; Tsukamoto, K.; Shida, Y.; Hashimoto, K. Tetrodotoxin-producing bacteria from the blue-ringed octopus Octopus maculosus. Mar. Biol. 1989, 100, 327–332. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Mebs, D.; Flachsenberger, W. Distribution of tetrodotoxin in the body of the blue-ringed octopus (Hapalochlaena maculosa). Toxicon 2007, 49, 410–412. [Google Scholar] [CrossRef]
- Williams, B.L.; Caldwell, R.L. Intra-organismal distribution of tetrodotoxin in two species of blue-ringed octopuses (Hapalochlaena fasciata and H. lunulata). Toxicon 2009, 54, 345–353. [Google Scholar] [CrossRef]
- Williams, B.L.; Lovenburg, V.; Huffard, C.L.; Caldwell, R.L. Chemical defense in pelagic octopus paralarvae: Tetrodotoxin alone does not protect individual paralarvae of the greater blue-ringed octopus (Hapalochlaena lunulata) from common reef predators. Chemoecology 2011, 21, 131–141. [Google Scholar] [CrossRef]
- Williams, B.L.; Stark, M.R.; Caldwell, R.L. Microdistribution of tetrodotoxin in two species of blue-ringed octopuses (Hapalochlaena lunulata and Hapalochlaena fasciata) detected by fluorescent immunolabeling. Toxicon 2012, 60, 1307–1313. [Google Scholar] [CrossRef]
- Asakawa, M.; Matsumoto, T.; Umezaki, K.; Kaneko, K.; Yu, X.; Gomez-Delan, G.; Tomano, S.; Noguchi, T.; Ohtsuka, S. Toxicity and toxin composition of the greater blue-ringed octopus Hapalochlaena lunulata from ishigaki island, Okinawa prefecture, Japan. Toxins 2019, 11, 245. [Google Scholar] [CrossRef]
- Narita, H.; Noguchi, T.; Maruyama, J.; Ueda, Y.; Hashimoto, K.; Watanabe, Y.; Hida, K. Occurrence of tetrodotoxin in a trumpet shell, “Boshubora” Charonia sauliae. Bull. Jpn. Soc. Sci. Fish. 1981, 47, 935–941. [Google Scholar] [CrossRef]
- Nocuchi, T.; Maruyama, J.; Ueda, Y.; Hashimoto, K.; Harada, T. Occurrence of tetrodotoxin in the japanese ivory shell Babylonia japonica. Bull. Jpn. Soc. Sci. Fish. 1981, 47, 909–913. [Google Scholar] [CrossRef]
- Noguchi, T.; Maruyama, J.; Narita, H.; Kanehisa, H. Occurrence of tetrodotoxin in the gastropod mollusk Tutufa lissostoma (frog shell). Toxicon 1984, 22, 219–226. [Google Scholar] [CrossRef]
- Hort, V.; Arnich, N.; Guérin, T.; Lavison-Bompard, G.; Nicolas, M. First detection of tetrodotoxin in bivalves and gastropods from the French mainland coasts. Toxins 2020, 12, 599. [Google Scholar] [CrossRef]
- Silva, M.; Rodríguez, I.; Barreiro, A.; Kaufmann, M.; Neto, A.I.; Hassouani, M.; Sabour, B.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. Tetrodotoxins occurrence in non-traditional vectors of the north atlantic waters (Portuguese maritime territory, and Morocco coast). Toxins 2019, 11, 306. [Google Scholar] [CrossRef]
- Hwang, D.F.; Tai, R.P.; Chueh, C.H.; Lin, L.C.; Jeng, S.S. Tetrodotoxin and derivatives in several species of the gastropod Naticidae. Toxicon 1991, 29, 1019–1024. [Google Scholar] [CrossRef]
- Hwang, D.F.; Chueh, C.H.; Jeng, S.S. Tetrodotoxin secretion from the lined moon shell Natica lineata in response to external stimulation. Toxicon 1990, 28, 1133–1136. [Google Scholar] [CrossRef]
- Lin, S.J.; Hwang, D.F. Possible source of tetrodotoxin in the starfish Astropecten scoparius. Toxicon 2001, 39, 573–579. [Google Scholar] [CrossRef]
- Silva, M.; Azevedo, J.; Rodriguez, P.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. New gastropod vectors and tetrodotoxin potential expansion in temperate waters of the Atlantic Ocean. Mar. Drugs 2012, 10, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.K.; Narita, H.; Nara, M.; Noguchi, T.; Maruyama, J.; Hashimoto, K. Occurrence of tetrodotoxin in a gastropod mollusk, “Araregai” Niotha clathrata. Bull. Jpn. Soc. Sci. Fish. 1984, 50, 2099–2102. [Google Scholar] [CrossRef]
- Narita, H.; Noguchi, T.; Maruyama, J.; Nara, M.; Hashimoto, K. Occurrence of a tetrodotoxin-associated substance in a gastropod, “Hanamushirogai” Zeuxis siquijore. Bull. Jpn. Soc. Sci. Fish. 1984, 50, 85–88. [Google Scholar] [CrossRef]
- Hwang, D.F.; Lin, L.C.; Jeng, S.S. Occurrence of a new toxin and tetrodotoxin in two species of the gastropod mollusk Nassariidae. Toxicon 1992, 30, 41–46. [Google Scholar] [CrossRef]
- Hwang, D.F.; Shiu, Y.C.; Hwang, P.A.; Lu, Y.H. Tetrodotoxin in gastropods (snails) implicated in food poisoning in Northern Taiwan. J. Food Prot. 2002, 65, 1341–1344. [Google Scholar] [CrossRef]
- Taniyama, S.; Takatani, T.; Sorimachi, T.; Sagara, T.; Kubo, H.; Oshiro, N.; Ono, K.; Xiao, N.; Tachibana, K.; Arakawa, O. Toxicity and toxin profile of scavenging and carnivorous gastropods from the coastal waters of okinawa prefecture, Japan. J. Food Hyg. Soc. Japan 2013, 54, 49–55. [Google Scholar] [CrossRef]
- Hwang, P.A.; Tsai, Y.H.; Deng, J.F.; Cheng, C.A.; Ho, P.H.; Hwang, D.F. Identification of tetrodotoxin in a marine gastropod (Nassarius glans) responsible for human morbidity and mortality in Taiwan. J. Food Prot. 2005, 68, 1696–1701. [Google Scholar] [CrossRef]
- Jen, H.C.; Lin, S.J.; Huang, Y.W.; Liao, I.C.; Arakawa, O.; Hwang, D.F. Occurrence of tetrodotoxin and paralytic shellfish poisons in a gastropod implicated in food poisoning in southern Taiwan. Food Addit. Contam. 2007, 24, 902–909. [Google Scholar] [CrossRef]
- Huang, H.N.; Lin, J.; Lin, H.L. Identification and quantification of tetrodotoxin in the marine gastropod Nassarius by LC-MS. Toxicon 2008, 51, 774–779. [Google Scholar] [CrossRef]
- Wang, X.J.; Yu, R.C.; Luo, X.; Zhou, M.J.; Lin, X.T. Toxin-screening and identification of bacteria isolated from highly toxic marine gastropod Nassarius semiplicatus. Toxicon 2008, 52, 55–61. [Google Scholar] [CrossRef]
- Luo, X.; Yu, R.C.; Wang, X.J.; Zhou, M.J. Toxin composition and toxicity dynamics of marine gastropod Nassarius spp. collected from lianyungang, China. Food Addit. Contam.-Part A Chem. Anal. Control. Expo. Risk Assess. 2012, 29, 117–127. [Google Scholar] [CrossRef]
- Matsumoto, T.; Nagashima, Y.; Kusuhara, H.; Sugiyama, Y.; Ishizaki, S.; Shimakura, K.; Shiomi, K. Involvement of carrier-mediated transport system in uptake of tetrodotoxin into liver tissue slices of puffer fish Takifugu rubripes. Toxicon 2007, 50, 173–179. [Google Scholar] [CrossRef]
- Bacchiocchi, S.; Campacci, D.; Siracusa, M.; Dubbini, A.; Leoni, F.; Tavoloni, T.; Accoroni, S.; Gorbi, S.; Giuliani, M.E.; Stramenga, A.; et al. Tetrodotoxins (Ttxs) and vibrio alginolyticus in mussels from central adriatic sea (Italy): Are they closely related? Mar. Drugs 2021, 19, 304. [Google Scholar] [CrossRef]
- Hwang, D.F.; Chueh, C.H.; Jeng, S.S. Occurrence of tetrodotoxin in the gastropod mollusk Natica lineata (lined moon shell). Toxicon 1990, 28, 21–27. [Google Scholar] [CrossRef]
- Hwang, D.F.; Lin, L.C.; Jeng, S.S. Variation and secretion of toxins in gastropod mollusc Niotha clathrata. Toxicon 1992, 30, 1189–1194. [Google Scholar] [CrossRef]
- McNabb, P.; Selwood, A.I.; Munday, R.; Wood, S.A.; Taylor, D.I.; MacKenzie, L.A.; van Ginkel, R.; Rhodes, L.L.; Cornelisen, C.; Heasman, K.; et al. Detection of tetrodotoxin from the grey side-gilled sea slug—Pleurobranchaea maculata, and associated dog neurotoxicosis on beaches adjacent to the Hauraki Gulf, Auckland, New Zealand. Toxicon 2010, 56, 466–473. [Google Scholar] [CrossRef]
- Wood, S.A.; Casas, M.; Taylor, D.I.; McNabb, P.; Salvitti, L.; Ogilvie, S.; Cary, S.C. Depuration of tetrodotoxin and changes in bacterial communities in Pleurobranchea maculata adults and egg vasses vaintained in captivity. J. Chem. Ecol. 2012, 38, 1342–1350. [Google Scholar] [CrossRef]
- Wood, S.A.; Taylor, D.I.; McNabb, P.; Walker, J.; Adamson, J.; Cary, S.C. Tetrodotoxin concentrations in Pleurobranchaea maculata: Temporal, spatial and individual variability from New Zealand Populations. Mar. Drugs 2012, 10, 163–176. [Google Scholar] [CrossRef]
- Salvitti, L.R.; Wood, S.A.; Winsor, L.; Cary, S.C. Intracellular immunohistochemical detection of tetrodotoxin in Pleurobranchaea maculata (Gastropoda) and Stylochoplana sp. (Turbellaria). Mar. Drugs 2015, 13, 765–769. [Google Scholar] [CrossRef]
- McNabb, P.S.; Taylor, D.I.; Ogilvie, S.C.; Wilkinson, L.; Anderson, A.; Hamon, D.; Wood, S.A.; Peake, B.M. First detection of tetrodotoxin in the bivalve Paphies australis by liquid chromatography coupled to triple quadrupole mass spectrometry with and without precolumn reaction. J. AOAC Int. 2014, 97, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Biessy, L.; Smith, K.F.; Harwood, D.T.; Boundy, M.J.; Hawes, I.; Wood, S.A. Spatial variability and depuration of tetrodotoxin in the bivalve Paphies australis from New Zealand. Toxicon X 2019, 2, 100008. [Google Scholar] [CrossRef] [PubMed]
- Biessy, L.; Smith, K.F.; Boundy, M.J.; Webb, S.C.; Hawes, I.; Wood, S.A. Distribution of tetrodotoxin in the New Zealand Clam, Paphies Australis, established using immunohistochemistry and liquid chromatography-tandem quadrupole mass spectrometry. Toxins 2018, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.D.; Powell, A.; Schofield, A.; Lees, D.N.; Baker-Austin, C. Detection of the pufferfish toxin tetrodotoxin in European bivalves, England, 2013 to 2014. Eurosurveillance 2015, 20, 21009. [Google Scholar] [CrossRef]
- Bordin, P.; Dall’Ara, S.; Tartaglione, L.; Antonelli, P.; Calfapietra, A.; Varriale, F.; Guiatti, D.; Milandri, A.; Dell’Aversano, C.; Arcangeli, G.; et al. First occurrence of tetrodotoxins in bivalve mollusks from Northern Adriatic Sea (Italy). Food Control 2021, 120, 107510. [Google Scholar] [CrossRef]
- Dhanji-Rapkova, M.; Turner, A.D.; Baker-Austin, C.; Huggett, J.F.; Ritchie, J.M. Distribution of tetrodotoxin in pacific oysters (Crassostrea gigas). Mar. Drugs 2021, 19, 84. [Google Scholar] [CrossRef]
- Turner, A.D.; Dhanji-Rapkova, M.; Coates, L.; Bickerstaff, L.; Milligan, S.; O’Neill, A.; Faulkner, D.; McEneny, H.; Baker-Austin, C.; Lees, D.N.; et al. Detection of tetrodotoxin shellfish poisoning (TSP) toxins and causative factors in bivalve molluscs from the UK. Mar. Drugs 2017, 15, 277. [Google Scholar] [CrossRef]
- Gerssen, A.; Bovee, T.H.F.; Klijnstra, M.D.; Poelman, M.; Portier, L.; Hoogenboom, R.L.A.P. First report on the occurrence of tetrodotoxins in bivalve mollusks in the Netherlands. Toxins 2018, 10, 450. [Google Scholar] [CrossRef]
- Dell’Aversano, C.; Tartaglione, L.; Polito, G.; Dean, K.; Giacobbe, M.; Casabianca, S.; Capellacci, S.; Penna, A.; Turner, A.D. First detection of tetrodotoxin and high levels of paralytic shellfish poisoning toxins in shellfish from Sicily (Italy) by three different analytical methods. Chemosphere 2019, 215, 881–892. [Google Scholar] [CrossRef]
- Noguchi, T.; Maruyama, J.; Hashimoto, K.; Narita, H. Tetrodotoxin in the starfish Astropecten polyacanthus, in association with toxification of a trumpet shell, “Boshubora” Charonia sauliae. Nippon Suisan Gakkaishi 1982, 48, 1173–1177. [Google Scholar] [CrossRef]
- Maruyama, J.; Noguchi, T.; Jeon, J.K.; Harada, T.; Hashimoto, K. Occurrence of tetrodotoxin in the starfish Astropecten latespinosus. Experientia 1984, 40, 1395–1396. [Google Scholar] [CrossRef]
- Maruyama, J.; Noguchi, T.; Narita, H.; Nara, M.; Jeon, J.K.; Otsuka, M.; Hashimoto, K. Occurrence of tetrodotoxin in a starfish, Astropecten scoparius. Agric. Biol. Chem. 1985, 49, 3069–3070. [Google Scholar] [CrossRef]
- Kadota, N.; Narita, H.; Murakami, R.; Noguchi, T. The toxicity of starfishes, Astropecten genus, inhabiting the coast of Toyama Bay. Food Hyg. Saf. Sci. 2008, 49, 422–427. [Google Scholar] [CrossRef]
- Khor, S.; Wood, S.A.; Salvitti, L.; Taylor, D.I.; Adamson, J.; McNabb, P.; Cary, S.C. Investigating diet as the source of tetrodotoxin in Pleurobranchaea maculata. Mar. Drugs 2014, 12, 1–16. [Google Scholar] [CrossRef]
- Lin, S.J.; Tsai, Y.H.; Lin, H.P.; Hwang, D.F. Paralytic toxins in Taiwanese starfish Astropecten scoparius. Toxicon 1998, 36, 799–803. [Google Scholar] [CrossRef]
- Miyazawa, K.; Higashiyama, M.; Ito, K.; Noguchi, T.; Arakawa, O.; Shida, Y.; Hashimoto, K. Tetrodotoxin in two species of ribbon worm (Nemertini), Lineus fuscoviridis and Tubulanus punctatus. Toxicon 1988, 26, 867–874. [Google Scholar] [CrossRef]
- Kem, W.R. Purification and characterization of a new family of polypeptide neurotoxins from the heteronemertine Cerebratulus lacteus (Leidy). J. Biol. Chem. 1976, 251, 4184–4192. [Google Scholar] [CrossRef]
- Ali, A.E.; Arakawa, O.; Noguchi, T.; Miyazawa, K.; Shida, Y.; Hashimoto, K. Tetrodotoxin and related substances in a ribbon worm Cephalothrix linearis (Nemertean). Toxicon 1990, 28, 1083–1093. [Google Scholar] [CrossRef]
- Noguchi, T.; Ali, A.E.; Arakawa, O.; Miyazawa, K.; Kanoh, S.; Shida, Y.; Nishio, S.; Hashimoto, K. Tetrodonic acid-like substance; a possible precursor of tetrodotoxin. Toxicon 1991, 29, 845–855. [Google Scholar] [CrossRef]
- Asakawa, M.; Toyoshima, T.; Shida, Y.; Noguchi, T.; Miyazawa, K. Paralytic toxins in a ribbon worm Cephalothrix species (Nemertean) adherent to cultured oysters in Hiroshima Bay, Hiroshima Prefecture, Japan. Toxicon 2000, 38, 763–773. [Google Scholar] [CrossRef]
- Asakawa, M.; Toyoshima, T.; Ito, K.; Bessho, K.; Yamaguchi, C.; Tsunetsugu, S.; Shida, Y.; Kajihara, H.; Mawatari, S.F.; Noguchi, T.; et al. Paralytic toxicity in the ribbon worm Cephalothrix species (Nemertea) in Hiroshima Bay, Hiroshima Prefecture, Japan and the isolation of tetrodotoxin as a main component of its toxins. Toxicon 2003, 41, 747–753. [Google Scholar] [CrossRef]
- Asakawa, M.; Ito, K.; Kajihara, H. Highly toxic ribbon worm Cephalothrix simula containing tetrodotoxin in Hiroshima Bay, Hiroshima Prefecture, Japan. Toxins 2013, 5, 376–395. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.D.; Fenwick, D.; Powell, A.; Dhanji-Rapkova, M.; Ford, C.; Hatfield, R.G.; Santos, A.; Martinez-Urtaza, J.; Bean, T.P.; Baker-Austin, C.; et al. New invasive nemertean species (Cephalothrix simula) in england with high levels of tetrodotoxin and a microbiome linked to toxin metabolism. Mar. Drugs 2018, 16, 452. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.; McEvoy, E.G.; Gibson, R. The production of tetrodotoxin-like substances by nemertean worms in conjunction with bacteria. J. Exp. Mar. Biol. Ecol. 2003, 288, 51–63. [Google Scholar] [CrossRef]
- Vlasenko, A.E.; Velansky, P.V.; Chernyshev, A.V.; Kuznetsov, V.G.; Magarlamov, T.Y. Tetrodotoxin and its analogues profile in nemertean species from the sea of Japan. Toxicon 2018, 156, 48–51. [Google Scholar] [CrossRef]
- Vlasenko, A.E.; Magarlamov, T.Y. Tetrodotoxin and its analogues in Cephalothrix cf. simula (Nemertea: Palaeonemertea) from the Sea of Japan (Peter the Great Gulf): Intrabody distribution and secretions. Toxins 2020, 12, 745. [Google Scholar] [CrossRef]
- Magarlamov, T.Y.; Shokur, O.A.; Chernyshev, A.V. Distribution of tetrodotoxin in the ribbon worm Lineus alborostratus (Takakura, 1898) (nemertea): Immunoelectron and immunofluorescence studies. Toxicon 2016, 112, 29–34. [Google Scholar] [CrossRef]
- Vlasenko, A.E.; Kuznetsov, V.G.; Malykin, G.V.; Pereverzeva, A.O.; Velansky, P.V.; Yakovlev, K.V.; Magarlamov, T.Y. Tetrodotoxins secretion and voltage-gated sodium channel adaptation in the ribbon worm Kulikovia alborostrata (Takakura, 1898) (Nemertea). Toxins 2021, 13, 606. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Min, S.K.; Yeon, S.J.; Hwang, J.H.; Hong, J.S.; Shin, H.S. Assessment of neuronal cell-based cytotoxicity of neurotoxins from an estuarine nemertean in the han river estuary. J. Microbiol. Biotechnol. 2017, 27, 725–730. [Google Scholar] [CrossRef]
- Tanu, M.B.; Mahmud, Y.; Arakawa, O.; Takatani, T.; Kajihara, H.; Kawatsu, K.; Hamano, Y.; Asakawa, M.; Miyazawa, K.; Noguchi, T. Immunoenzymatic visualization of tetrodotoxin (TTX) in Cephalothrix species (Nemertea: Anopla: Palaeonemertea: Cephalotrichidae) and Planocera reticulata (Platyhelminthes: Turbellaria: Polycladida: Planoceridae). Toxicon 2004, 44, 515–520. [Google Scholar] [CrossRef]
- Malykin, G.V.; Chernyshev, A.V.; Magarlamov, T.Y. Intrabody tetrodotoxin distribution and possible hypothesis for its migration in ribbon worms Cephalothrix cf. simula (palaeonemertea, nemertea). Mar. Drugs 2021, 19, 494. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.E.; Schwartz, M. Immunohistological Visualization of Tetrodotoxin in Micrura Verrili and Dushia Atra (Phylum Nemertea). In Proceedings of the National Conferences for Undergraduate Research (NCUR), Salisbury, MD, USA, 10–12 April 2008. [Google Scholar]
- Arndt, W. Polycladen und maricole tricladen als giftträge. In Memórias e Estudios do Museu Zoológico da Universidade de Coimbra; Universidade de Coimbra: Coimbra, Portugal, 1943; Volume 148, pp. 1–15. [Google Scholar]
- Miyazawa, K.; Jeon, J.K.; Maruyama, J.; Noguchi, T.; Ito, K.; Hashimoto, K. Occurrence of tetrodotoxin in the flatworm Planocera multitentaculata. Toxicon 1986, 24, 645–650. [Google Scholar] [CrossRef]
- Miyazawa, K.; Jeon, J.K.; Noguchi, T.; Ito, K.; Hashimoto, K. Distribution of tetrodotoxin in the tissues of the flatworm Planocera multitentaculata (Platyhelminthes). Toxicon 1987, 25, 975–980. [Google Scholar] [CrossRef]
- Kashitani, M.; Okabe, T.; Oyama, H.; Noguchi, K.; Yamazaki, H.; Suo, R.; Mori, T.; Sugita, H.; Itoi, S. Taxonomic distribution of tetrodotoxin in acotylean flatworms (Polycladida: Platyhelminthes). Mar. Biotechnol. 2020, 22, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.K.; Miyazawa, K.; Narita, H.; Nara, M.; Noguchi, T.; Ito, K.; Matsubara, S.; Hashimoto, K. Occurrence of Paralytic Toxicity in Marine Flatworms. Nippon Suisan Gakkaishi 1986, 52, 1065–1069. [Google Scholar] [CrossRef]
- Ritson-Williams, R.; Yotsu-Yamashita, M.; Paul, V.J. Ecological functions of tetrodotoxin in a deadly polyclad flatworm. Proc. Natl. Acad. Sci. USA 2006, 103, 3176–3179. [Google Scholar] [CrossRef]
- Salvitti, L.R.; Wood, S.A.; Taylor, D.I.; McNabb, P.; Cary, S.C. First identification of tetrodotoxin (TTX) in the flatworm Stylochoplana sp.; A source of TTX for the sea slug Pleurobranchaea maculata. Toxicon 2015, 95, 23–29. [Google Scholar] [CrossRef]
- Suo, R.; Kashitani, M.; Oyama, H.; Adachi, M.; Nakahigashi, R.; Sakakibara, R.; Nishikawa, T.; Sugita, H.; Itoi, S. First Detection of tetrodotoxins in the cotylean rlatworm Prosthiostomum trilineatum. Mar. Drugs 2021, 19, 40. [Google Scholar] [CrossRef]
- Stokes, A.N.; Ducey, P.K.; Neuman-Lee, L.; Hanifin, C.T.; French, S.S.; Pfrender, M.E.; Brodie, E.D., III; Brodie, E.D., Jr. Confirmation and distribution of tetrodotoxin for the first time in terrestrial invertebrates: Two terrestrial flatworm species (Bipalium adventitium and Bipalium kewense). PLoS ONE 2014, 9, e100718. [Google Scholar] [CrossRef]
- Yamada, R.; Tsunashima, T.; Takei, M.; Sato, T.; Wajima, Y.; Kawase, M.; Oshikiri, S.; Kajitani, Y.; Kosoba, K.; Ueda, H.; et al. Seasonal changes in the tetrodotoxin content of the flatworm Planocera multitentaculata. Mar. Drugs 2017, 15, 56. [Google Scholar] [CrossRef]
- Yasumoto, T.; Nagai, H.; Yasumura, D.; Michishita, T.; Endo, A.; Yotsu, M.; Kotaki, Y. Interspecies distribution and possible origin of tetrodotoxin. Ann. N. Y. Acad. Sci. 1986, 479, 44–51. [Google Scholar] [CrossRef]
- Thuesen, E.V.; Kogure, K.; Hashimoto, K.; Nemoto, T. Poison arrowworms: A tetrodotoxin venom in the marine phylum Chaetognatha. J. Exp. Mar. Biol. Ecol. 1988, 116, 249–256. [Google Scholar] [CrossRef]
- Noguchi, T.; Uzu, A.; Koyama, K.; Maruyama, J.; Nagashima, Y.; Hashimoto, K. Occurrence of tetrodotoxin as the major toxin in a xanthid crab Atergatis floridus. Nippon Suisan Gakkaishi 1983, 49, 1887–1892. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Hwang, D.F.; Chai, T.J.; Jeng, S.S. Occurrence of tetrodotoxin and paralytic shellfish poison in the Taiwanese crab Lophozozymus pictor. Toxicon 1995, 33, 1669–1673. [Google Scholar] [CrossRef]
- Hwang, D.F.; Tsai, Y.-H.; Chai, T.; Jeng, S.-S. Occurrence of tetrodotoxin and paralytic shellfish poison in Taiwan crab Zosimus aeneus. Fish. Sci. 1996, 62, 500–501. [Google Scholar] [CrossRef]
- Tsai, Y.; Hwang, D.F.; Chai, T.J.; Jeng, S.S. Occurance of paralytic toxin in Taiwanese crab Atergatopsis germaini. Toxicon 1996, 34, 467–474. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Hwang, D.F.; Chai, T.J.; Jeng, S.S. Toxicity and toxic components of two xanthid crabs, Atergatis floridus and Demania reynaudi, in Taiwan. Toxicon 1997, 35, 1327–1335. [Google Scholar] [CrossRef]
- Sagara, T.; Taniyama, S.; Takatani, T.; Nishibori, N.; Nishio, S.; Noguchi, T.; Arakawa, O. Toxicity and toxin profiles of xanthid crabs collected around Nakanoshima, the Tokara islands, Japan. Shokuhin Eiseigaku Zasshi 2009, 50, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, M.; Gomez-Delan, G.; Tsuruda, S.; Shimomura, M.; Shida, Y.; Taniyama, S.; Barte-Quilantang, M.; Shindo, J. Toxicity assessment of the xanthid crab Demania cultripes from Cebu Island, Philippines. J. Toxicol. 2010, 2010, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Kohama, T.; Ui, K.; Watabe, S. Distribution of tetrodotoxin in the xanthid crab (Atergatis floridus) collected in the coastal waters of Kanagawa and Wakayama Prefectures. Comp. Biochem. Physiol.-Part D Genom. Proteom. 2006, 1 Pt D1, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Kungsuwan, A.; Noguchi, T.; Hashimoto, K.; Nagashima, Y.; Shida, Y.; Suvapeepan, S.; Suwansakornku, P. Tetrodotoxin in the horseshoe crab Carcinoscorpius rotundicauda inhabiting Thailand. Nippon Suisan Gakkaishi 1987, 53, 261–266. [Google Scholar] [CrossRef]
- Ikeda, K.; Venmathi Maran, B.A.; Honda, S.; Ohtsuka, S.; Arakawa, O.; Takatani, T.; Asakawa, M.; Boxshall, G.A. Accumulation of tetrodotoxin (TTX) in Pseudocaligus fugu, a parasitic copepod from panther puffer Takifugu pardalis, but without vertical transmission-Using an immunoenzymatic technique. Toxicon 2006, 48, 116–122. [Google Scholar] [CrossRef]
- Ito, K.; Okabe, S.; Asakawa, M.; Bessho, K.; Taniyama, S.; Shida, Y.; Ohtsuka, S. Detection of tetrodotoxin (TTX) from two copepods infecting the grass puffer Takifugu niphobles: TTX attracting the parasites? Toxicon 2006, 48, 620–626. [Google Scholar] [CrossRef]
- Kodama, T.; Ikeda, K.; Arakawa, O.; Kondo, Y.; Asakawa, M.; Kawatsu, K.; Ohtsuka, S. Evidence of accumulation of tetrodotoxin (TTX) in tissues and body parts of ectoparasitic copepods via their feeding on mucus of TTX-bearing pufferfish. Toxicon 2021, 204, 37–43. [Google Scholar] [CrossRef]
- Koyama, K.; Noguchi, T.; Uzu, A.; Hashimoto, K. Resistibility of toxic and nontoxic crabs against paralytic shellfish poison and tetrodotoxin. Nippon Suisan Gakkaishi 1983, 49, 485–489. [Google Scholar] [CrossRef]
- Saito, T.; Maruyama, J.; Kanoh, S.; Jeon, J.K.; Noguchi, T.; Harada, T.; Murata, O.; Hashimoto, K. Toxicity of the cultured pufferfish Fugu rubripes rubripes along with their resistibility against tetrodotoxin. Nippon Suisan Gakkaishi 1984, 50, 1573–1575. [Google Scholar] [CrossRef]
- Daigo, K.; Arakawa, O.; Noguchi, T.; Uzu, A.; Hashimoto, K. Resistibility of two xanthid crab Zoslmus aeneus and Daira perlata against paralytic shellfish poison and tetrodotoxin. Nippon Suisan Gakkaishi 1987, 53, 881–884. [Google Scholar] [CrossRef]
- Shiomi, K.; Yamaguchi, S.; Kikuchi, T.; Yamamori, K.; Matsui, T. Occurrence of tetrodotoxin-binding high molecular weight substances in the body fluid of shore crab (Hemigrapsus sanguineus). Toxicon 1992, 30, 1529–1537. [Google Scholar] [CrossRef]
- Hwang, P.A.; Tsai, Y.H.; Lin, H.P.; Hwang, D.F. Tetrodotoxin-binding proteins isolated from five species of toxic gastropods. Food Chem. 2007, 103, 1153–1158. [Google Scholar] [CrossRef]
- Matsui, T.; Yamamori, K.; Furukawa, K.; Kono, M. Purification and some properties of a tetrodotoxin binding protein from the blood plasma of kusafugu, Takifugu niphobles. Toxicon 2000, 38, 463–468. [Google Scholar] [CrossRef]
- Yotsu-Yamashita, M.; Sugimoto, A.; Terakawa, T.; Shoji, Y.; Miyazawa, T.; Yasumoto, T. Purification, characterization, and cDNA cloning of a novel soluble saxitoxin and tetrodotoxin binding protein from plasma of the puffer fish, Fugu pardalis. Eur. J. Biochem. 2001, 268, 5937–5946. [Google Scholar] [CrossRef]
- Matsumoto, T.; Tanuma, D.; Tsutsumi, K.; Jeon, J.K.; Ishizaki, S.; Nagashima, Y. Plasma protein binding of tetrodotoxin in the marine puffer fish Takifugu rubripes. Toxicon 2010, 55, 415–420. [Google Scholar] [CrossRef]
- Tsuda, K.; Kawamura, M. The constituents of the ovaries of globefish. I. The isolation of meso-inositol and scillitol from the ovaries. J. Pharm. Soc. Jpn. 1950, 70, 70–73. [Google Scholar] [CrossRef][Green Version]
- Noguchi, T.; Arakawa, O. Tetrodotoxin—Distribution and accumulation in aquatic organisms, and cases of human intoxication. Mar. Drugs 2008, 6, 220–242. [Google Scholar] [CrossRef]
- Matsumura, K. Production of tetrodotoxin in puffer fish embryos. Environ. Toxicol. Pharmacol. 1998, 6, 217–219. [Google Scholar] [CrossRef]
- Itoi, S.; Yoshikawa, S.; Asahina, K.; Suzuki, M.; Ishizuka, K.; Takimoto, N.; Mitsuoka, R.; Yokoyama, N.; Detake, A.; Takayanagi, C.; et al. Larval pufferfish protected by maternal tetrodotoxin. Toxicon 2014, 78, 35–40. [Google Scholar] [CrossRef]
- Itoi, S.; Suzuki, M.; Asahina, K.; Sawayama, E.; Nishikubo, J.; Oyama, H.; Takei, M.; Shiibashi, N.; Takatani, T.; Arakawa, O.; et al. Role of maternal tetrodotoxin in survival of larval pufferfish. Toxicon 2018, 148, 95–100. [Google Scholar] [CrossRef]
- Nagashima, Y.; Mataki, I.; Toyoda, M.; Nakajima, H.; Tsumoto, K.; Shimakura, K.; Shiomi, K. Change in tetrodotoxin content of the puffer fish Takifugu rubripes during seed production from fertilized eggs to juveniles. J. Food Hyg. Soc. Jpn. 2010, 51, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Twitty, V.C.; Johnson, H. Motor inhibition in Amblystoma produced by Triturus transplantants. Science 1934, 80, 78–79. [Google Scholar] [CrossRef] [PubMed]
- Twitty, V.C.; Elliott, H.A. The relative growth of the amphibian eye, studied by means of transplantation. J. Exp. Zool. 1934, 68, 247–291. [Google Scholar] [CrossRef]
- Twitty, V.C. Experiments on the phenomenon of paralysis produced by a toxin occurring in triturus embryos. J. Exp. Zool. Part A 1937, 76, 67–104. [Google Scholar] [CrossRef]
- Bucciarelli, G.M.; Li, A.; Kats, L.B.; Green, D.B. Quantifying tetrodotoxin levels in the california newt using a non-destructive sampling method. Toxicon 2014, 80, 87–93. [Google Scholar] [CrossRef]
- Gall, B.G.; Stokes, A.N.; French, S.S.; Brodie, E.D., III; Brodie, E.D., Jr. Female newts (Taricha granulosa) produce tetrodotoxin laden eggs after long term captivity. Toxicon 2012, 60, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Gall, B.G.; Stokes, A.N.; Pett, J.J.; Spivey, K.L.; French, S.S.; Brodie, E.D., III; Bredie, E.D., Jr. Tetrodotoxin concentrations within a clutch and across embryonic development in eggs of the rough-skinned newts (Taricha granulosa). Toxicon 2014, 90, 249–254. [Google Scholar] [CrossRef]
- Hanifin, C.T.; Brodie, E.D., III; Brodie, E.D., Jr. Tetrodotoxin levels in eggs of the rough-skin newt, Taricha granulosa, ara correlated with femail tosicity. J. Chem. Ecol. 2003, 29, 1729–1739. [Google Scholar] [CrossRef]
- Gall, B.G.; Stokes, A.N.; French, S.S.; Schlepphorst, E.A.; Brodie, E.D., Jr.; Brodie, E.D., III. Tetrodotoxin levels in larval and metamorphosed newts (Taricha granulosa) and palatability to predatory dragonflies. Toxicon 2011, 57, 978–983. [Google Scholar] [CrossRef]
- Mebs, D.; Yotsu-Yamashita, M. Acquiring toxicity of a newt, Cynops orientalis. Toxicon 2021, 198, 32–35. [Google Scholar] [CrossRef]
- Zimmer, R.K.; Schar, D.W.; Ferrer, R.P.; Krug, P.J.; Kats, L.B.; Michel, W.C. The scent of danger: Tetrodotoxin (TTX) as an olfactory cue of reduced predation risk. Ecol. Monogr. 2006, 76, 585–600. [Google Scholar] [CrossRef]
- Kudo, Y.; Chiba, C.; Konoki, K.; Cho, Y.; Yotsu-Yamashita, M. Confirmation of the absence of tetrodotoxin and its analogues in the juveniles of the Japanese fire-bellied newt, Cynops pyrrhogaster, captive-reared from eggs in the laboratory using HILIC-LC-MS. Toxicon 2015, 101, 101–105. [Google Scholar] [CrossRef]
- Sheumack, D.D.; Howden, M.E.H.; Spencer, I. Occurrence of a tetrodotoxin-like compound in the eggs of the venomous blue-ringed octopus (Hapalochlaena maculosa). Toxicon 1984, 22, 811–812. [Google Scholar] [CrossRef]
- Williams, B.L.; Hanifin, C.T.; Brodie, E.D., Jr.; Caldwell, R.L. Ontogeny of tetrodotoxin levels in blue-ringed octopuses: Maternal investment and apparent independent production in offspring of Hapalochlaena lunulata. J. Chem. Ecol. 2011, 37, 10–17. [Google Scholar] [CrossRef]
- Yamate, Y.; Takatani, T.; Takegaki, T. Levels and distribution of tetrodotoxin in the blue-lined octopus Hapalochlaena fasciata in Japan, with special reference to within-body allocation. J. Molluscan Stud. 2021, 87, eyaa042. [Google Scholar] [CrossRef]
- Itoi, S.; Ueda, H.; Yamada, R.; Takei, M.; Sato, T.; Oshikiri, S.; Wajima, Y.; Ogata, R.; Oyama, H.; Shitto, T.; et al. Including planocerid flatworms in the diet effectively toxifies the pufferfish, Takifugu niphobles. Sci. Rep. 2018, 8, 12302. [Google Scholar] [CrossRef]
- Okabe, T.; Oyama, H.; Kashitani, M.; Ishimaru, Y.; Suo, R.; Sugita, H.; Itoi, S. Toxic flatworm egg plates serve as a possible source of tetrodotoxin for pufferfish. Toxins 2019, 11, 402. [Google Scholar] [CrossRef]
- Okabe, T.; Saito, R.; Yamamoto, K.; Watanabe, R.; Kaneko, Y.; Yanaoka, M.; Furukoshi, S.; Yasukawa, S.; Ito, M.; Oyama, H.; et al. The role of toxic planocerid flatworm larvae on tetrodotoxin accumulation in marine bivalves. Aquat. Toxicol. 2021, 237, 105908. [Google Scholar] [CrossRef]
- Tanu, M.B.; Noguchi, T. Tetrodotoxin as a toxic principle in the horseshoe crab Carcinoscorpius rotundicauda collected from Bangladesh. J. Food Hyg. Soc. Jpn. 1999, 40, 426–430. [Google Scholar] [CrossRef]
- Ngy, L.; Yu, C.F.; Takatani, T.; Arakawa, O. Toxicity assessment for the horseshoe crab Carcinoscorpius rotundicauda collected from Cambodia. Toxicon 2007, 49, 843–847. [Google Scholar] [CrossRef]
- Dao, H.V.; Takata, Y.; Sato, S.; Fukuyo, Y.; Kodama, M. Frequent occurrence of the tetrodotoxin-bearing horseshoe crab Carcinoscorpius rotundicauda in Vietnam. Fish. Sci. 2009, 75, 435–438. [Google Scholar] [CrossRef]
- Huang, H.N.; Zheng, R.J.; Liu, L.L.; Zheng, M.; Li, Z.J. Determination of tetrodotoxin in Carcinoscorpius rotundicauda (horseshoe crab) by high-performance liquid chromatography-tandem mass spectrometry. Anal. Lett. 2016, 49, 2377–2383. [Google Scholar] [CrossRef]
- Zheng, R.; Guan, Q.; Zheng, M.; Huang, Z.; Huang, H.; Fu, W.; Lin, S.; Yang, Y. Toxin and toxicity identification of mangrove horseshoe crab Carcinoscorpius rotundicauda collected from South China. Toxicon 2019, 161, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Hamada, S.; Konosu, S. Difference in accumulation of puffer fish toxin and crystalline tetrodotoxin in the puffer fish, Fugu rubripes rubripes. Bull. Jpn. Soc. Sci. Fish. 1981, 47, 535–537. [Google Scholar] [CrossRef]
- Matsui, T.; Taketsugu, S.; Sato, H.; Yamamori, K.; Kodama, K.; Ishii, A.; Hirose, H.; Shimizu, C. Toxification of cultured puffer fish by the administration of tetrodotoxin producing bacteria. Nippon Suisan Gakkaishi 1990, 56, 705. [Google Scholar] [CrossRef]
- Honda, S.; Arakawa, O.; Takatani, T.; Tachibana, K.; Yagi, M. Toxification of cultured puffer fish Takifugu rubripes by feeding on tetrodotoxin-containing diet. Nippon Suisan Gakkaishi 2005, 71, 815–820. [Google Scholar] [CrossRef]
- Tatsuno, R.; Shikina, M.; Shirai, Y.; Wang, J.; Soyano, K.; Nishihara, G.N.; Takatani, T.; Arakawa, O. Change in the transfer profile of orally administered tetrodotoxin to non-toxic cultured pufferfish Takifugu rubripes depending of its development stage. Toxicon 2013, 65, 76–80. [Google Scholar] [CrossRef]
- Itoi, S.; Kozaki, A.; Komori, K.; Tsunashima, T.; Noguchi, S.; Kawane, M.; Sugita, H. Toxic Takifugu pardalis eggs found in Takifugu niphobles gut: Implications for TTX accumulation in the pufferfish. Toxicon 2015, 108, 141–146. [Google Scholar] [CrossRef]
- Amano, M.; Amiya, N.; Takaoka, M.; Sato, H.; Takatani, T.; Arakawa, O.; Sakakura, Y. Tetrodotoxin functions as a stress relieving substance in juvenile tiger puffer Takifugu rubripes. Toxicon 2019, 171, 54–61. [Google Scholar] [CrossRef]
- Watabe, S.; Sata, Y.; Nakaya, M.; Nogawa, N.; Oohashi, K.; Noguchi, T.; Morikawa, N.; Hashimoto, K. Distribution of tritiated tetrodotoxin administred intraperitoneally to puggerfish. Toxicon 1987, 25, 1283–1289. [Google Scholar] [CrossRef]
- Matsumoto, T.; Ishizaki, S.; Nagashima, Y. Differential gene expression profile in the liver of the marine puffer fish Takifugu rubripes induced by intramuscular administration of tetrodotoxin. Toxicon 2011, 57, 304–310. [Google Scholar] [CrossRef]
- Matsumoto, T.; Feroudj, H.; Kikuchi, R.; Kawana, Y.; Kondo, H.; Hirono, I.; Mochizuki, T.; Nagashima, Y.; Kaneko, G.; Ushio, H.; et al. DNA microarray analysis on the genes differentially expressed in the liver of the pufferfish, Takifugu rubripes, following an intramuscular administration of tetrodotoxin. Microarrays 2014, 3, 226–244. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kiriake, A.; Ishizaki, S.; Watabe, S.; Nagashima, Y. Biliary excretion of tetrodotoxin in the cultured pufferfish Takifugu rubripes juvenile after intramuscular administration. Toxicon 2015, 93, 98–102. [Google Scholar] [CrossRef]
- Matsumoto, T.; Nagashima, Y.; Kusuhara, H.; Ishizaki, S.; Shimakura, K.; Shiomi, K. Evaluation of hepatic uptake clearance of tetrodotoxin in the puffer fish Takifugu rubripes. Toxicon 2008, 52, 369–374. [Google Scholar] [CrossRef]
- Matsumoto, T.; Nagashima, Y.; Kusuhara, H.; Ishizaki, S.; Shimakura, K.; Shiomi, K. Pharmacokinetics of tetrodotoxin in puffer fish Takifugu rubripes by a single administration technique. Toxicon 2008, 51, 1051–1059. [Google Scholar] [CrossRef]
- Wang, J.; Araki, T.; Tatsuno, R.; Nina, S.; Ikeda, K.; Hamasaki, M.; Sakakura, Y.; Takatani, T.; Arakawa, O. Transfer profile of intramuscularly administered tetrodotoxin to artificial hybrid specimens of pufferfish, Takifugu rubripes and Takifugu niphobles. Toxicon 2011, 58, 565–569. [Google Scholar] [CrossRef]
- Wang, J.; Araki, T.; Tatsuno, R.; Nina, S.; Ikeda, K.; Takatani, T.; Arakawa, O. Transfer profile of orally and intramuscularly administered tetrodotoxin to artificial hybrid specimens of the pufferfish Takifugu rubripes and Takifugu porphyreus. J. Food Hyg. Soc. Jpn. 2012, 53, 33–38. [Google Scholar] [CrossRef][Green Version]
- Yamamori, K.; Kono, M.; Furukawa, K.; Matsui, T. The toxification of juvenile cultured kusafugu Takifugu niphobles by oral administration of crystalline tetrodotoxin. J. Food Hyg. Soc. Jpn. 2004, 45, 73–75. [Google Scholar] [CrossRef]
- Kono, M.; Matsui, T.; Furukawa, K.; Yotsu-Yamashita, M.; Yamamori, K. Accumulation of tetrodotoxin and 4,9-anhydrotetrodotoxin in cultured juvenile kusafugu Fugu niphobles by dietary administration of natural toxic komonfugu Fugu poecilonotus liver. Toxicon 2008, 51, 1269–1273. [Google Scholar] [CrossRef]
- Kono, M.; Matsui, T.; Furukawa, K.; Takase, T.; Yamamori, K.; Kaneda, H.; Aoki, D.; Jang, J.H.; Yotsu-Yamashita, M. Examination of transformation among tetrodotoxin and its analogs in the living cultured juvenile puffer fish, kusafugu, Fugu niphobles by intramuscular administration. Toxicon 2008, 52, 714–720. [Google Scholar] [CrossRef]
- Gao, W.; Kanahara, Y.; Yamada, M.; Tatsuno, R.; Yoshikawa, H.; Doi, H.; Takatani, T.; Arakawa, O. Contrasting toxin selectivity between the marine pufferfish Takifugu pardalis and the freshwater pufferfish Pao suvattii. Toxins 2019, 11, 470. [Google Scholar] [CrossRef]
- Zhang, X.; Zong, J.; Chen, S.; Li, M.; Lu, Y.; Wang, R.; Xu, H. Accumulation and elimination of tetrodotoxin in the pufferfish Takifugu obscurus by dietary administration of the wild toxic gastropod Nassarius semiplicata. Toxins 2020, 12, 278. [Google Scholar] [CrossRef]
- Kudo, Y.; Chiba, C.; Konoki, K.; Cho, Y.; Yotsu-Yamashita, M. Dietary administration of tetrodotoxin and its putative biosynthetic intermediates to the captive-reared non-toxic japanese fire-bellied newt, Cynops pyrrhogaster. Toxicon 2017, 137, 78–82. [Google Scholar] [CrossRef]
- Narita, H.; Nara, M.; Baba, K.; Ohgami, H.; Ai, T.K.; Noguchi, T.; Hashimoto, K. Effect of feeding a trumpet shell, Charonia sauliae with toxic starfish. Food Hyg. Saf. Sci. 1984, 25, 251–255. [Google Scholar] [CrossRef]
- Tatsuno, R.; Sorimachi, T.; Taniyama, S.; Oshiro, N.; Kubo, H.; Takatani, T.; Arakawa, O. Accumulation of tetrodotoxin from diet in two species of scavenging marine snails. Food Hyg. Saf. Sci. 2014, 55, 152–156. [Google Scholar] [CrossRef][Green Version]
- Biessy, L.; Smith, K.F.; Wood, S.A.; Tidy, A.; van Ginkel, R.; Bowater, J.R.D.; Hawes, I. A microencapsulation method for delivering tetrodotoxin to bivalves to investigate uptake and accumulation. Mar. Drugs 2021, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, Y.; Toyoda, M.; Hasobe, M.; Shimakura, K.; Shiomi, K. In vitro accumulation of tetrodotoxin in pufferfish liver tissue slices. Toxicon 2003, 41, 569–574. [Google Scholar] [CrossRef]
- Nagashima, Y.; Ohta, A.; Yin, X.; Ishizaki, S.; Matsumoto, T.; Doi, H.; Ishibashi, T. Difference in uptake of tetrodotoxin and saxitoxins into liver tissue slices among pufferfish, boxfish and porcupinefish. Mar. Drugs 2018, 16, 17. [Google Scholar] [CrossRef]
- Matsumoto, T.; Nagashima, Y.; Takayama, K.; Shimakura, K.; Shiomi, K. Difference between tetrodotoxin and saxitoxins in accumulation in puffer fish Takifugu rubripes liver tissue slices. Fish Physiol. Biochem. 2005, 31, 95–100. [Google Scholar] [CrossRef]
- Kiriake, A.; Ohta, A.; Suga, E.; Matsumoto, T.; Ishizaki, S.; Nagashima, Y. Comparison of tetrodotoxin uptake and gene expression in the liver between juvenile and adult tiger pufferfish, Takifugu rubripes. Toxicon 2016, 111, 6–12. [Google Scholar] [CrossRef]
- Shimizu, Y.; Kobayashi, M. Apparent lack of tetrodotoxin biosynthesis in captured Taricha torosa and Taricha granulosa. Chem. Pharm. Bull. 1983, 31, 3625–3631. [Google Scholar] [CrossRef]
- Gall, B.G.; Stokes, A.N.; Brodie, E.D., 3rd; Brodie, E.D., Jr. Tetrodotoxin levels in lab-reared rough-skinned newts (Taricha granulosa) after 3 years and comparison to wild-caught juveniles. Toxicon 2022, 213, 7–12. [Google Scholar] [CrossRef]
- Daly, J.W.; Padgett, W.L.; Saunders, R.L.; Cover, J.F., Jr. Absence of tetrodotoxins in a captive-raised riparian frog, Atelopus varius. Toxicon 1997, 35, 705–709. [Google Scholar] [CrossRef]
- Biessy, L.; Boundy, M.J.; Smith, K.F.; Harwood, D.T.; Hawes, I.; Wood, S.A. Tetrodotoxin in marine bivalves and edible gastropods: A mini-review. Chemosphere 2019, 236, 124404. [Google Scholar] [CrossRef]
- Brodie, E.D., Jr. Investigations on the skin toxin of the red-spotted newt, Notophthalmus viridescens viridescens. Am. Midl. Nat. 1968, 80, 276–280. [Google Scholar] [CrossRef]
- Williams, B.L.; Brodie, E.D., Jr.; Brodie, E.D., III. A resistant predator and its toxic prey: Persistence of newt toxin leads to poisonous (not venomous) snakes. J. Chem. Ecol. 2004, 30, 1901–1919. [Google Scholar] [CrossRef]
- Gall, B.G.; Brodie, I.D.; Brodie, J.D. Survival and growth of the caddisfly Limnephilus flavastellus after predation on toxic eggs of the rough-skinned newt (Taricha granulosa). Can. J. Zool. 2011, 89, 483–489. [Google Scholar] [CrossRef]
- Gall, B.G.; Stokes, A.N.; French, S.S.; Brodie, E.D., III.; Brodie, E.D., Jr. Predatory caddisfly larvae sequester tetrodotoxin from their prey, eggs of the rough-skinned newt (Taricha granulosa). J. Chem. Ecol. 2012, 38, 1351–1357. [Google Scholar] [CrossRef]
- Jal, S.; Khora, S.S. An overview on the origin and production of tetrodotoxin, a potent neurotoxin. J. Appl. Microbiol. 2015, 119, 907–916. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).