Histopathological Changes in the Liver, Heart and Kidneys Following Malayan Pit Viper (Calloselasma rhodostoma) Envenoming and the Neutralising Effects of Hemato Polyvalent Snake Antivenom
Abstract
1. Introduction
2. Results
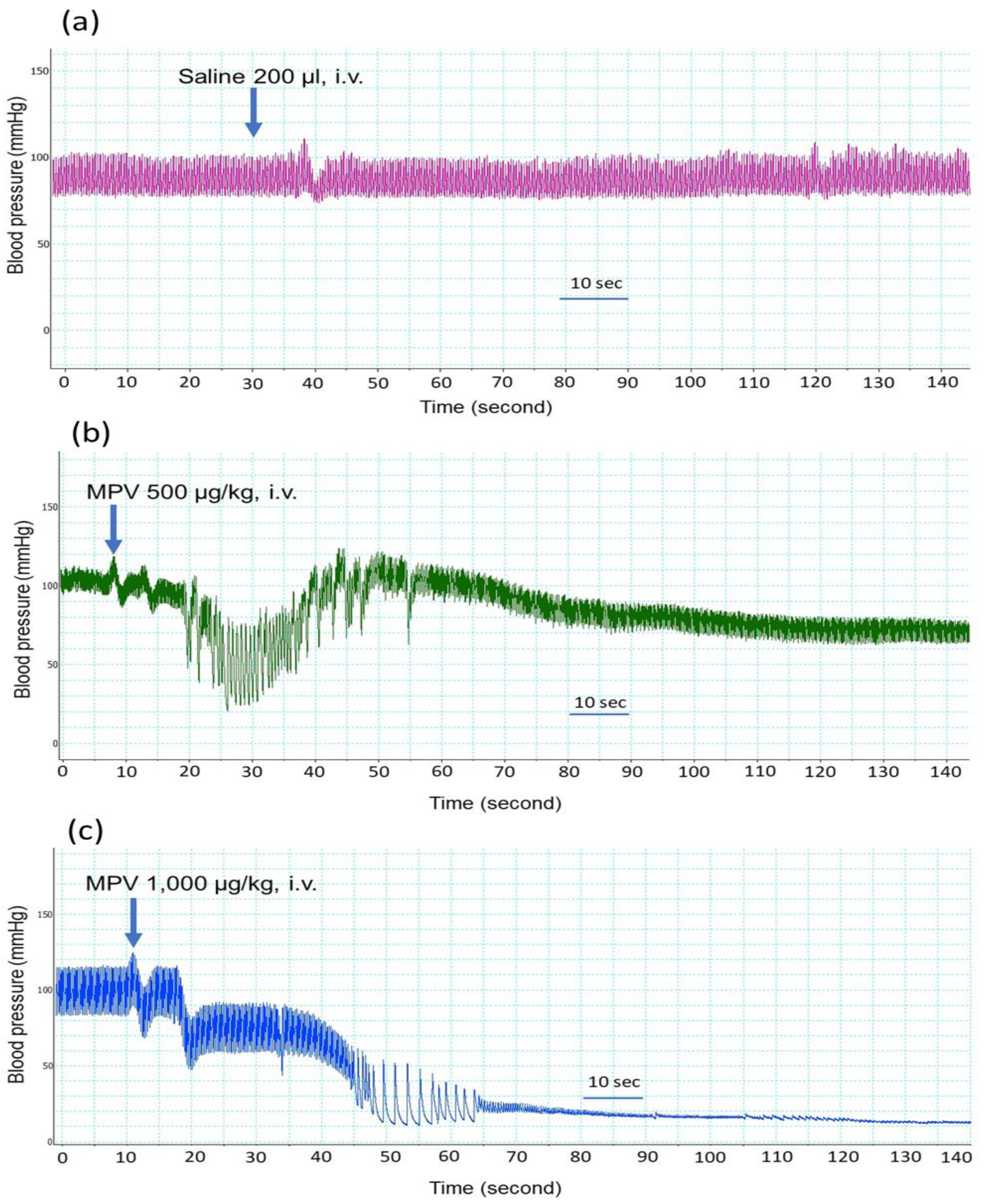
2.1. Effect of the Intravenous Administration of C. rhodostoma Venom on Anaesthetized Rats
2.2. Neutralization of Venom Lethality In Vivo by Hemato Polyvalent Antivenom in Mice
2.3. Evaluation of the Effectiveness of Hemato Polyvalent Antivenom on C. rhodostoma Venom-Induced Morphological Changes in Mice
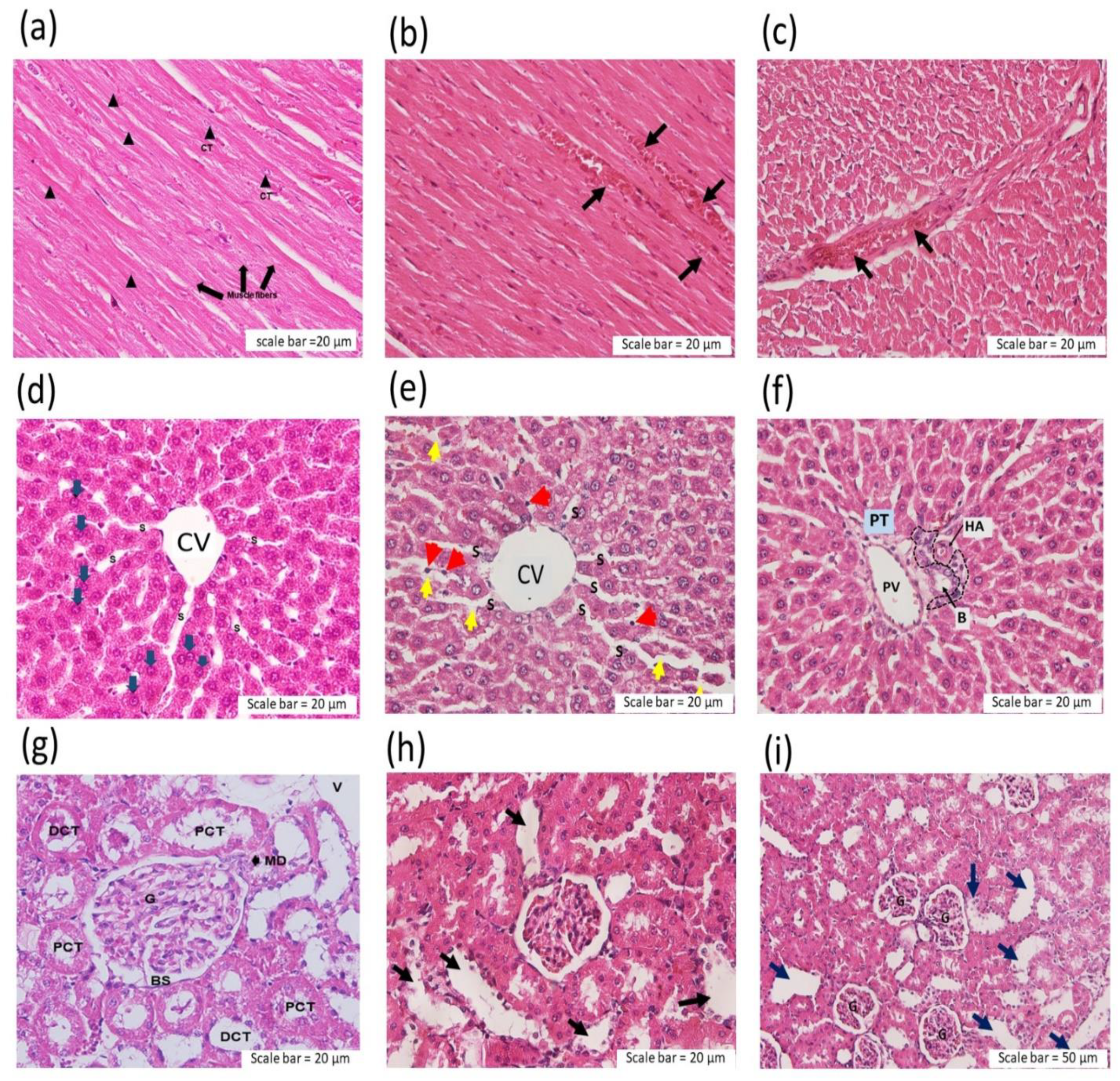
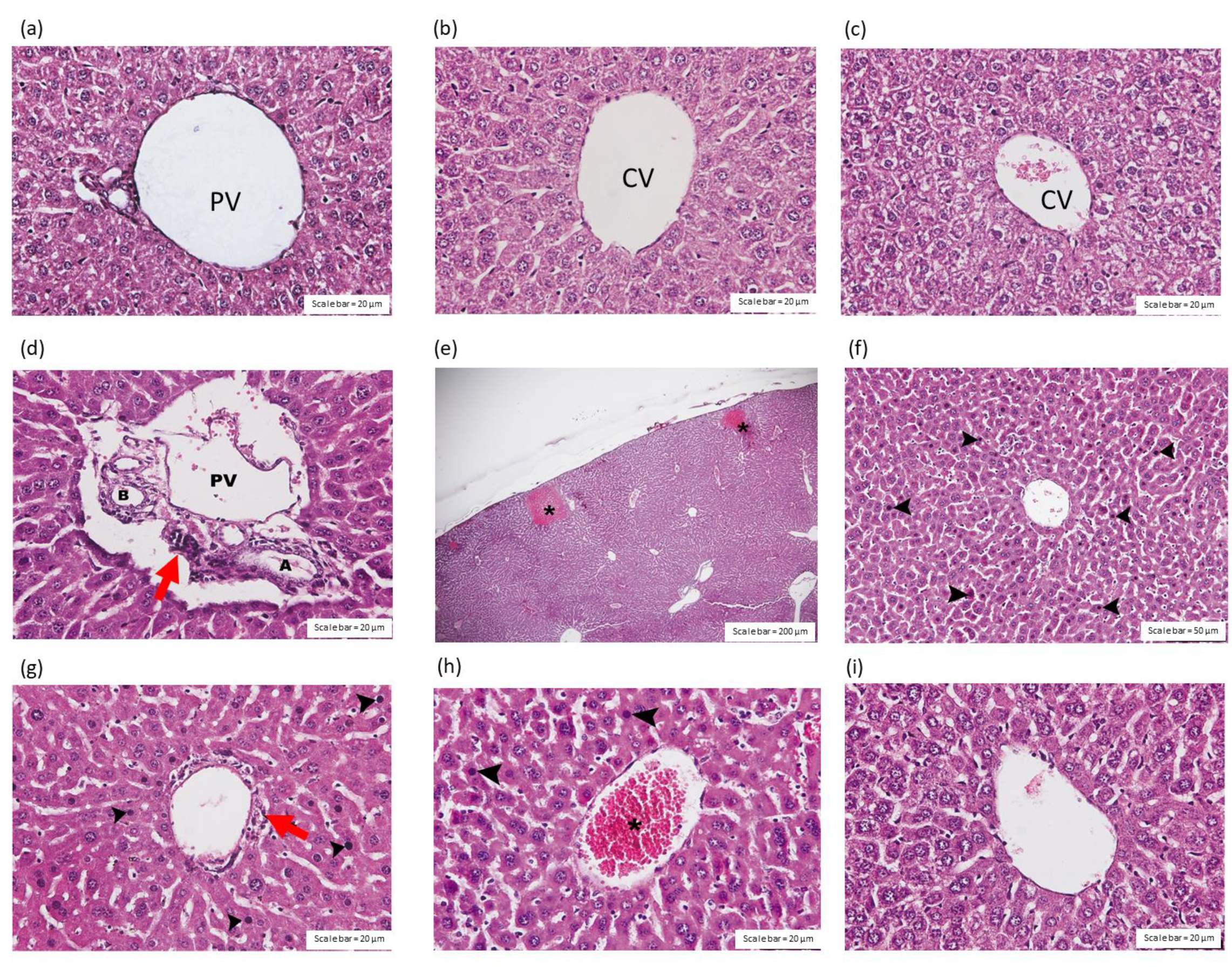
2.3.1. Effect of C. rhodostoma Venom on Morphological Changes in the Liver and the Effect of HPAV on the Histopathology of the Liver
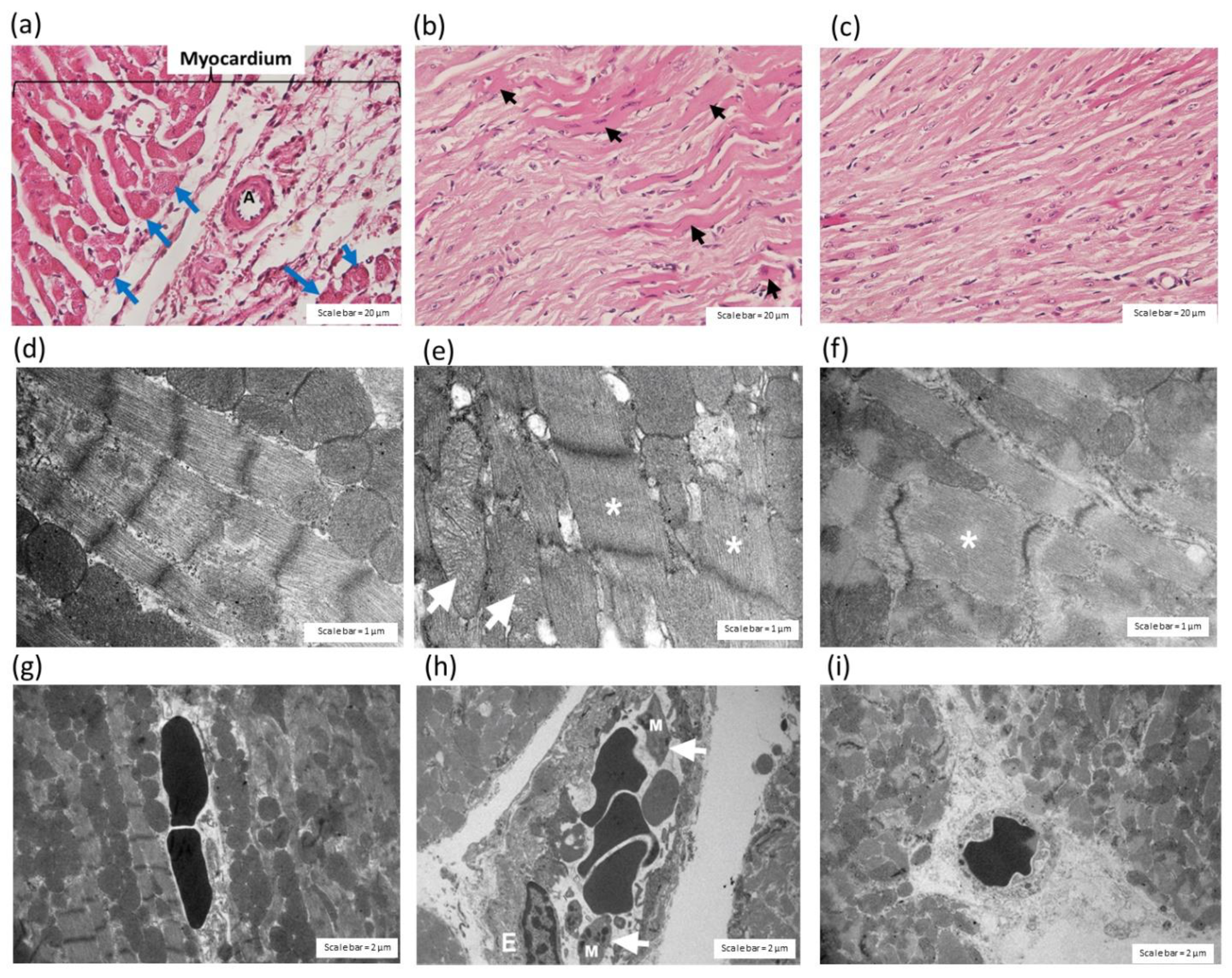
2.3.2. Effect of C. rhodostoma Venom on Morphological Changes in Heart Tissues
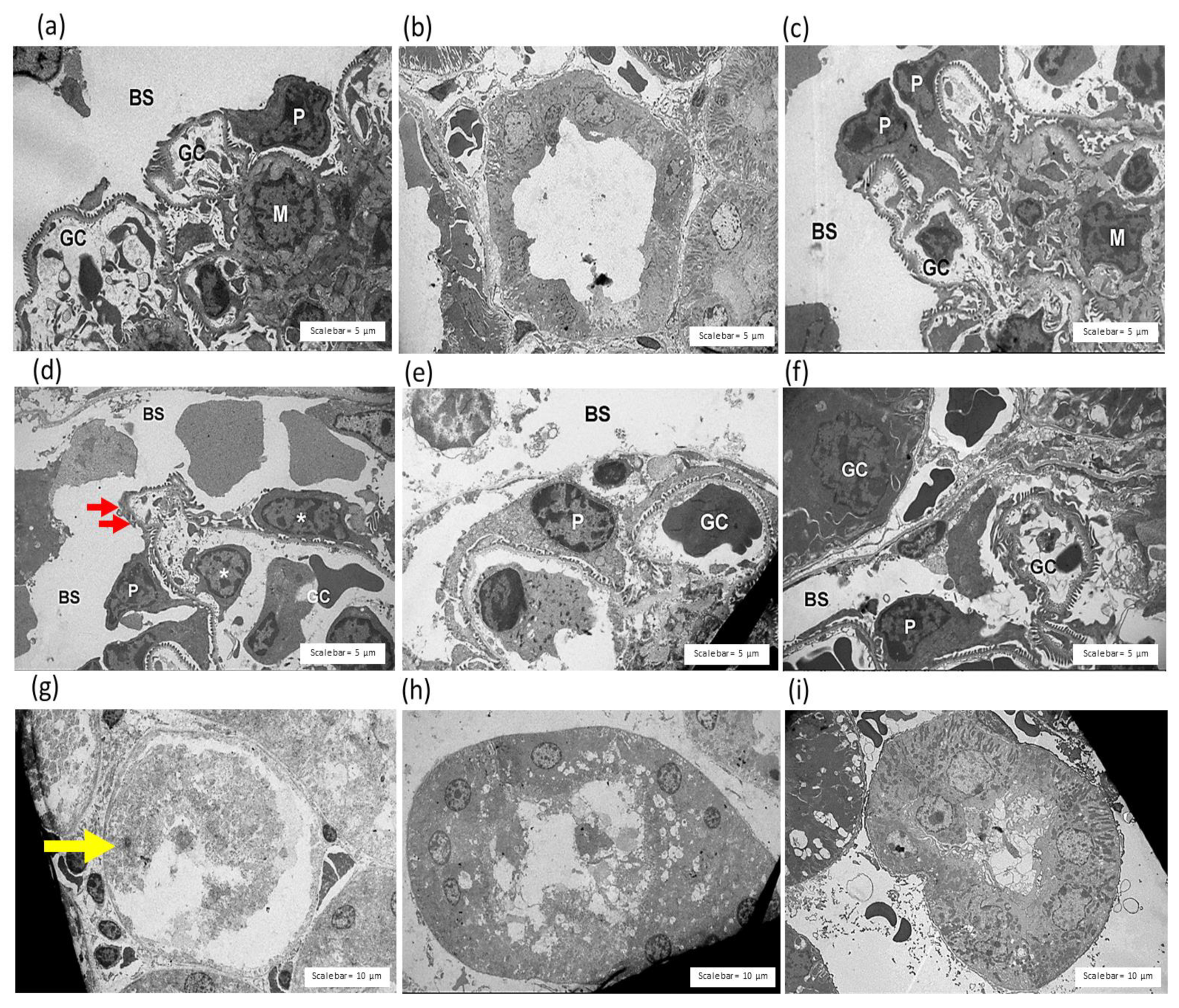
2.3.3. Effect of C. rhodostoma Venom on Morphological Changes in the Kidneys and the Protective Effect of HPAV on the Pathohistology of the Kidneys
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Snake Venoms
5.2. Antivenoms
5.3. Animal Ethics and Care
5.4. Anaesthetized Rat Preparation
5.5. In Vivo Venom Lethality
5.6. Histopathological Studies
5.6.1. Histological Preparation for Haematoxylin and Eosin (H&E) Staining
5.6.2. Histological Preparation for Transmission Electron Microscopy
5.7. Data Analysis and Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Longbottom, J.; Shearer, F.M.; Devine, M.; Alcoba, G.; Chappuis, F.; Weiss, D.J.; Ray, S.E.; Ray, N.; Warrell, D.A.; Ruiz de Castaneda, R.; et al. Vulnerability to snakebite envenoming: A global mapping of hotspots. Lancet 2018, 392, 673–684. [Google Scholar] [CrossRef]
- Gutierrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17079. [Google Scholar] [CrossRef] [PubMed]
- WHO. Venomous snakes of the South-East Asia Region, their venoms and pathophysiology of human envenoming. In Guidelines for the Management of Snake-Bites, 2nd ed.; WHO, Regional Office for South-East Asia: Delhi, India, 2016; Volume 2. [Google Scholar]
- Ismail, A.K. Snakebite and Envenomation Management in Malaysia. In Clinical Toxinology in Asia Pacific and Africa, Toxinology; Gopalakrishnakone, P., Ed.; Springer Science: Dordrecht, The Netherlands, 2015; pp. 72–102. [Google Scholar]
- Tang, E.L.; Tan, C.H.; Fung, S.Y.; Tan, N.H. Venomics of Calloselasma rhodostoma, the Malayan pit viper: A complex toxin arsenal unraveled. J. Proteom. 2016, 148, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Tang, E.L.H.; Tan, N.H.; Fung, S.Y.; Tan, C.H. Comparative proteomes, immunoreactivities and neutralization of procoagulant activities of Calloselasma rhodostoma (Malayan pit viper) venoms from four regions in Southeast Asia. Toxicon 2019, 169, 91–102. [Google Scholar] [CrossRef]
- Bruserud, O. The snake venom rhodocytin from Calloselasma rhodostoma- a clinically important toxin and a useful experimental tool for studies of C-type lectin-like receptor 2 (CLEC-2). Toxins 2013, 5, 665–674. [Google Scholar] [CrossRef]
- Kunalan, S.; Othman, I.; Syed Hassan, S.; Hodgson, W.C. Proteomic Characterization of Two Medically Important Malaysian Snake Venoms, Calloselasma rhodostoma (Malayan Pit Viper) and Ophiophagus hannah (King Cobra). Toxins 2018, 10, 434. [Google Scholar] [CrossRef]
- Wongtongkam, N.; Wilde, H.; Sitthi-Amorn, C.; Ratanabanangkoon, K. A study of 225 Malayan pit viper bites in Thailand. Mil. Med. 2005, 170, 342–348. [Google Scholar] [CrossRef]
- Gutierrez, J.M.; Ponce-Soto, L.A.; Marangoni, S.; Lomonte, B. Systemic and local myotoxicity induced by snake venom group II phospholipases A2: Comparison between crotoxin, crotoxin B and a Lys49 PLA2 homologue. Toxicon 2008, 51, 80–92. [Google Scholar] [CrossRef]
- Gutierrez, J.M.; Ownby, C.L. Skeletal muscle degeneration induced by venom phospholipases A2: Insights into the mechanisms of local and systemic myotoxicity. Toxicon 2003, 42, 915–931. [Google Scholar] [CrossRef]
- Gutierrez, J.M.; Rucavado, A.; Chaves, F.; Diaz, C.; Escalante, T. Experimental pathology of local tissue damage induced by Bothrops asper snake venom. Toxicon 2009, 54, 958–975. [Google Scholar] [CrossRef]
- Sitprija, V. Snakebite nephropathy. Nephrology (Carlton) 2006, 11, 442–448. [Google Scholar] [CrossRef]
- Kasempimolporn, S.; Jitapunkul, S.; Sitprija, V. Moving towards the elimination of rabies in Thailand. J. Med Assoc. Thail. = Chotmaihet Thangphaet 2008, 91, 433–437. [Google Scholar]
- Kraisawat, K.; Promwang, N. Duration after Malayan Pit Viper Bite to Detect Coagulopathy in Songklanagarind Hospital. J. Health Sci. Med. Res. 2020, 38, 93–101. [Google Scholar] [CrossRef]
- Tangtrongchitr, T.; Thumtecho, S.; Janprasert, J.; Sanprasert, K.; Tongpoo, A.; Tanpudsa, Y.; Trakulsrichai, S.; Wananukul, W.; Srisuma, S. Malayan Pit Viper Envenomation and Treatment in Thailand. Ther. Clin. Risk Manag. 2021, 17, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Chaisakul, J.; Rusmili, M.R.A.; Alsolaiss, J.; Albulescu, L.O.; Harrison, R.A.; Othman, I.; Casewell, N.R. In Vitro Immunological Cross-Reactivity of Thai Polyvalent and Monovalent Antivenoms with Asian Viper Venoms. Toxins 2020, 12, 766. [Google Scholar] [CrossRef]
- Leong, P.K.; Tan, C.H.; Sim, S.M.; Fung, S.Y.; Sumana, K.; Sitprija, V.; Tan, N.H. Cross neutralization of common Southeast Asian viperid venoms by a Thai polyvalent snake antivenom (Hemato Polyvalent Snake Antivenom). Acta Trop. 2014, 132, 7–14. [Google Scholar] [CrossRef]
- Chaisakul, J.; Alsolaiss, J.; Charoenpitakchai, M.; Wiwatwarayos, K.; Sookprasert, N.; Harrison, R.A.; Chaiyabutr, N.; Chanhome, L.; Tan, C.H.; Casewell, N.R. Evaluation of the geographical utility of Eastern Russell’s viper (Daboia siamensis) antivenom from Thailand and an assessment of its protective effects against venom-induced nephrotoxicity. PLoS Negl. Trop. Dis. 2019, 13, e0007338. [Google Scholar] [CrossRef]
- WHO. Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins. Geneva. 2010. Available online: https://www.who.int/biologicals/expert_committee/Antivenom_WHO_Guidelines_DJW_DEB_mn_cp.pdf (accessed on 26 February 2019).
- Markland, F.S., Jr.; Swenson, S. Snake venom metalloproteinases. Toxicon 2013, 62, 3–18. [Google Scholar] [CrossRef]
- Chippaux, J.P. Venomous and poisonous animals. II. Viper bites. Med. Trop. Rev. Du Corps De Sante Colonial 2006, 66, 423–428. [Google Scholar]
- Uzair, B.; Atlas, N.; Malik, S.B.; Jamil, N.; Ojuolape, S.T.; Rehman, M.U.; Khan, B.A. Snake Venom as an Effective Tool against Colorectal Cancer. Protein Pept. Lett. 2018, 25, 626–632. [Google Scholar] [CrossRef]
- Kini, R.M. Excitement ahead: Structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon 2003, 42, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Chaisakul, J.; Rusmili, M.R.; Hodgson, W.C.; Hatthachote, P.; Suwan, K.; Inchan, A.; Chanhome, L.; Othman, I.; Chootip, K. A Pharmacological Examination of the Cardiovascular Effects of Malayan Krait (Bungarus candidus) Venoms. Toxins 2017, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Chaisakul, J.; Isbister, G.K.; Konstantakopoulos, N.; Tare, M.; Parkington, H.C.; Hodgson, W.C. In vivo and in vitro cardiovascular effects of Papuan taipan (Oxyuranus scutellatus) venom: Exploring “sudden collapse”. Toxicol. Lett. 2012, 213, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Chaisakul, J.; Isbister, G.K.; Tare, M.; Parkington, H.C.; Hodgson, W.C. Hypotensive and vascular relaxant effects of phospholipase A2 toxins from Papuan taipan (Oxyuranus scutellatus) venom. Eur. J. Pharmacol. 2014, 723, 227–233. [Google Scholar] [CrossRef]
- Chaisakul, J.; Isbister, G.K.; O’Leary, M.A.; Parkington, H.C.; Smith, A.I.; Hodgson, W.C.; Kuruppu, S. Prothrombin activator-like toxin appears to mediate cardiovascular collapse following envenoming by Pseudonaja textilis. Toxicon 2015, 102, 48–54. [Google Scholar] [CrossRef]
- Al-Quraishy, S.; Dkhil, M.A.; Abdel Moneim, A.E. Hepatotoxicity and oxidative stress induced by Naja haje crude venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 42. [Google Scholar] [CrossRef]
- Barraviera, B.; Coelho, K.Y.; Curi, P.R.; Meira, D.A. Liver dysfunction in patients bitten by Crotalus durissus terrificus (Laurenti, 1768) snakes in Botucatu (State of Sao Paulo, Brazil). Rev. Do Inst. De Med. Trop. De Sao Paulo 1995, 37, 63–69. [Google Scholar] [CrossRef]
- Naumann, G.B.; Silva, L.F.; Silva, L.; Faria, G.; Richardson, M.; Evangelista, K.; Kohlhoff, M.; Gontijo, C.M.; Navdaev, A.; de Rezende, F.F.; et al. Cytotoxicity and inhibition of platelet aggregation caused by an l-amino acid oxidase from Bothrops leucurus venom. Biochim. Et Biophys. Acta 2011, 1810, 683–694. [Google Scholar] [CrossRef]
- Kanjanabuch, T.; Sitprija, V. Snakebite nephrotoxicity in Asia. Semin. Nephrol. 2008, 28, 363–372. [Google Scholar] [CrossRef]
- Chaisakul, J.; Khow, O.; Wiwatwarayos, K.; Rusmili, M.R.A.; Prasert, W.; Othman, I.; Abidin, S.A.Z.; Charoenpitakchai, M.; Hodgson, W.C.; Chanhome, L.; et al. A Biochemical and Pharmacological Characterization of Phospholipase A2 and Metalloproteinase Fractions from Eastern Russell’s Viper (Daboia siamensis) Venom: Two Major Components Associated with Acute Kidney Injury. Toxins 2021, 13, 521. [Google Scholar] [CrossRef]
- Finney, D. Probit Analysis, 3rd ed.; Cambridge University: New York, NY, USA, 1971. [Google Scholar]
- Charoenpitakchai, M.; Wiwatwarayos, K.; Jaisupa, N.; Rusmili, M.R.A.; Mangmool, S.; Hodgson, W.C.; Ruangpratheep, C.; Chanhome, L.; Chaisakul, J. Non-neurotoxic activity of Malayan krait (Bungarus candidus) venom from Thailand. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 9. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, J.; Dai, W.; Kloner, R.A. Functional and histological assessment of an experimental model of Takotsubo’s cardiomyopathy. J. Am. Heart Assoc. 2014, 3, e000921. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, D.P.; Ratnayake, R.M.P.; Shirani Ranasinghe, J.G. Histopathological Changes in Brain, Kidney and Liver of Mice following Intramuscular Administration of Krait Venom. Cey. J. Sci. (Bio. Sci.) 2009, 38, 1–10. [Google Scholar] [CrossRef][Green Version]



| Group | Scores | ||
|---|---|---|---|
| Congestion | Inflammatory Infiltration | Necrosis | |
| Saline | 0 | 0 | 0 |
| C. rhodostoma venom (CRV, i.p.) | +++ | ++ | +++ |
| HPAV (i.p.) | 0 | 0 | 0 |
| CRV followed by HPAV 15 min later | + | + | + |
| HPAV followed by CRV 15 min later | + | + | + |
| Group | Heart (Scores) | Description |
|---|---|---|
| Myocardial Damage | ||
| Saline | 0 | No lesions |
| C. rhodostoma venom (CRV, 11.1 mg/kg; i.p.) | 2.5 | Focal lesions extending over a wider area of both ventricles, extensive inflammatory cell infiltration, interstitial oedema, rupture of myofibers |
| HPAV (i.p.) | 0.5 | Slight derangement of muscle fibres, few inflammatory cells and vacuoles |
| CRV followed by HPAV | 1.5 | Focal lesions of the subendocardium of the apical and mid ventricular region with right ventricular involvement |
| HPAV followed by CRV | 1.5 | Focal lesions of the subendocardium of the apical and mid ventricular region with right ventricular involvement |
| Group | Kidney (Scores) | ||
|---|---|---|---|
| Congestion | Inflammatory Infiltration | Tubular Injury | |
| Saline | 0 | 0 | 0 |
| C. rhodostoma venom (CRV, i.p.) | +++ | ++ | +++ |
| HPAV (i.p.) | 0 | 0 | 0 |
| CRV followed by HPAV | + | + | + |
| HPAV followed by CRV | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khimmaktong, W.; Nuanyaem, N.; Lorthong, N.; Hodgson, W.C.; Chaisakul, J. Histopathological Changes in the Liver, Heart and Kidneys Following Malayan Pit Viper (Calloselasma rhodostoma) Envenoming and the Neutralising Effects of Hemato Polyvalent Snake Antivenom. Toxins 2022, 14, 601. https://doi.org/10.3390/toxins14090601
Khimmaktong W, Nuanyaem N, Lorthong N, Hodgson WC, Chaisakul J. Histopathological Changes in the Liver, Heart and Kidneys Following Malayan Pit Viper (Calloselasma rhodostoma) Envenoming and the Neutralising Effects of Hemato Polyvalent Snake Antivenom. Toxins. 2022; 14(9):601. https://doi.org/10.3390/toxins14090601
Chicago/Turabian StyleKhimmaktong, Wipapan, Nazmi Nuanyaem, Nissara Lorthong, Wayne C. Hodgson, and Janeyuth Chaisakul. 2022. "Histopathological Changes in the Liver, Heart and Kidneys Following Malayan Pit Viper (Calloselasma rhodostoma) Envenoming and the Neutralising Effects of Hemato Polyvalent Snake Antivenom" Toxins 14, no. 9: 601. https://doi.org/10.3390/toxins14090601
APA StyleKhimmaktong, W., Nuanyaem, N., Lorthong, N., Hodgson, W. C., & Chaisakul, J. (2022). Histopathological Changes in the Liver, Heart and Kidneys Following Malayan Pit Viper (Calloselasma rhodostoma) Envenoming and the Neutralising Effects of Hemato Polyvalent Snake Antivenom. Toxins, 14(9), 601. https://doi.org/10.3390/toxins14090601






