Electronic Nose for the Rapid Detection of Deoxynivalenol in Wheat Using Classification and Regression Trees
Abstract
:1. Introduction
2. Results
2.1. Field Sampling and Deoxynivalenol Contamination
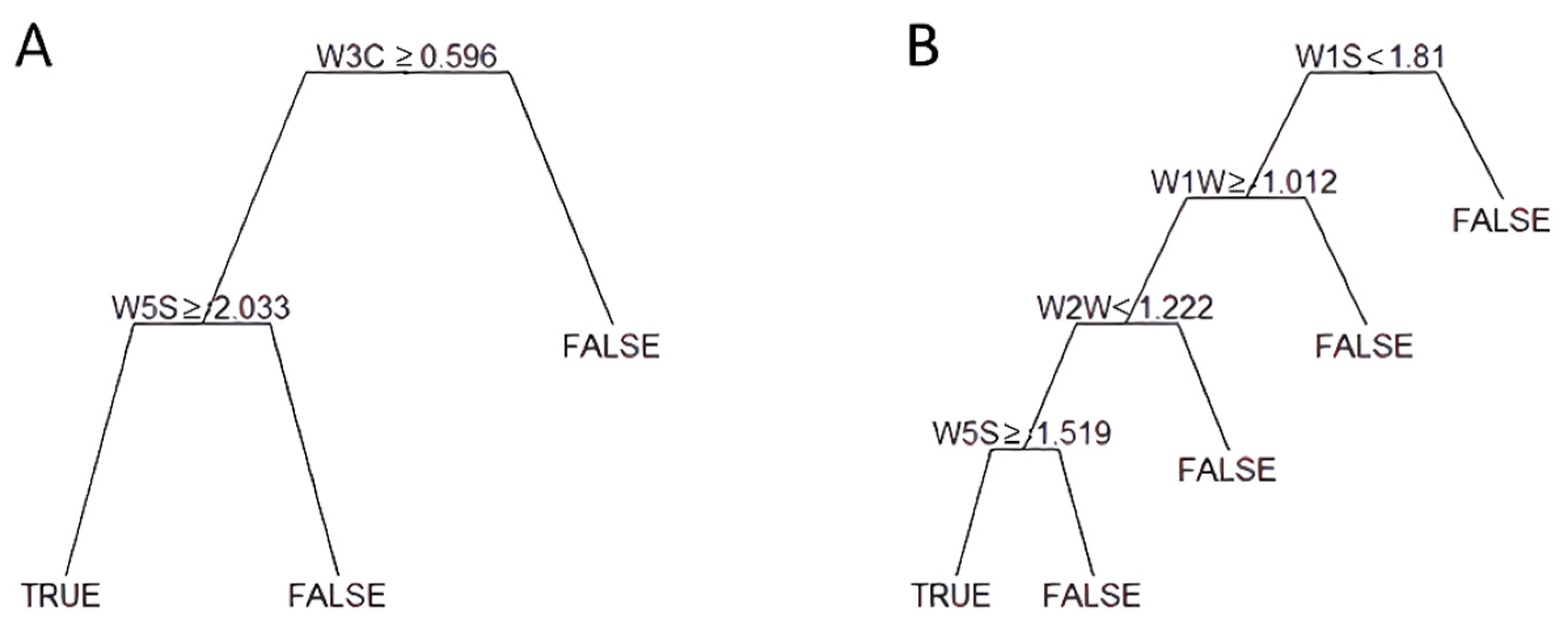
2.2. Data Analysis
2.3. Cross-Validation with Training and Blind Dataset
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Field Sampling and Laboratory Sample Preparation
5.1.1. Samples
5.1.2. e-Nose Analysis
5.2. Mycotoxin Analysis
5.3. Data Analysis
5.3.1. Classification and Regression Trees (CART)
5.3.2. Model Evaluation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Leonard, K.J.; Bushnell, W.R. Breeding wheat for fusarium head blight resistance in europe. In Fusarium Head Blight of Wheat and Barley; American Phytopathological Society (APS Press): St. Paul, MN, USA, 2003; pp. 211–241. [Google Scholar]
- Parry, D.W.; Jenkinson, P.; McLeod, L. Fusarium ear blight (scab) in small grain cereals—A review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Del Ponte, E.M.; Moreira, G.M.; Ward, T.J.; O’Donnell, K.; Nicolli, C.P.; Machado, F.J.; Duffeck, M.R.; Alves, K.S.; Tessmann, D.J.; Waalwijk, C.; et al. Fusarium graminearum species complex: A bibliographic analysis and web-accessible database for global mapping of species and trichothecene toxin chemotypes. Phytopathology 2022, 112, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Polak-śliwińska, M.; Paszczyk, B. Trichothecenes in food and feed, relevance to human and animal health and methods of detection: A systematic review. Molecules 2021, 26, 454. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (ec) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs; Official Journal of European Union: Luxembourg, 2006; pp. 5–24. [Google Scholar]
- Food and Agriculture Organization Corporate Statistical Database (FAOSTAT). Faostat Database Collections. Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 4 February 2022).
- Lattanzio, V.M.T.; Pascale, M.; Visconti, A. Current analytical methods for trichothecene mycotoxins in cereals. TrAC Trends Anal. Chem. 2009, 28, 758–768. [Google Scholar] [CrossRef]
- Ran, R.; Wang, C.; Han, Z.; Wu, A.; Zhang, D.; Shi, J. Determination of deoxynivalenol (DON) and its derivatives: Current status of analytical methods. Food Control 2013, 34, 138–148. [Google Scholar] [CrossRef]
- Berna, A. Metal oxide sensors for electronic noses and their application to food analysis. Sensors 2010, 10, 3882–3910. [Google Scholar] [CrossRef]
- Lippolis, V.; Cervellieri, S.; Damascelli, A.; Pascale, M.; Di Gioia, A.; Longobardi, F.; De Girolamo, A. Rapid prediction of deoxynivalenol contamination in wheat bran by mos-based electronic nose and characterization of the relevant pattern of volatile compounds. J. Sci. Food Agric. 2018, 98, 4955–4962. [Google Scholar] [CrossRef]
- Camardo Leggieri, M.; Mazzoni, M.; Fodil, S.; Moschini, M.; Bertuzzi, T.; Prandini, A.; Battilani, P. An electronic nose supported by an artificial neural network for the rapid detection of aflatoxin b1 and fumonisins in maize. Food Control 2021, 123, 107722. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, D.; Liu, H.; Huang, X.; Deng, J.; Jia, R.; He, X.; Tahir, M.; Lan, Y. Research hotspots and frontiers in agricultural multispectral technology: Bibliometrics and scientometrics analysis of the web of science. Front. Plant Sci. 2022, 13, 955340. [Google Scholar] [CrossRef]
- Chavez, R.A.; Cheng, X.; Herrman, T.J.; Stasiewicz, M.J. Single kernel aflatoxin and fumonisin contamination distribution and spectral classification in commercial corn. Food Control 2022, 131, 108393. [Google Scholar] [CrossRef]
- Wilson, A.D. Advanced methods for teaching electronic-nose technologies to diagnosticians and clinical laboratory technicians. Procedia—Soc. Behav. Sci. 2012, 46, 4544–4554. [Google Scholar] [CrossRef]
- Lippolis, V.; Pascale, M.; Cervellieri, S.; Damascelli, A.; Visconti, A. Screening of deoxynivalenol contamination in durum wheat by mos-based electronic nose and identification of the relevant pattern of volatile compounds. Food Control 2014, 37, 263–271. [Google Scholar] [CrossRef]
- Evans, P.; Persaud, K.C.; McNeish, A.S.; Sneath, R.W.; Hobson, N.; Magan, N. Evaluation of a radial basis function neural network for the determination of wheat quality from electronic nose data. Sens. Actuators B Chem. 2000, 69, 348–358. [Google Scholar] [CrossRef]
- Jia, W.; Liang, G.; Tian, H.; Sun, J.; Wan, C. Electronic nose-based technique for rapid detection and recognition of moldy apples. Sensors 2019, 19, 1526. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Tongson, E.; Unnithan, R.R.; Gonzalez Viejo, C. Early detection of aphid infestation and insect-plant interaction assessment in wheat using a low-cost electronic nose (e-nose), near-infrared spectroscopy and machine learning modeling. Sensors 2021, 21, 5948. [Google Scholar] [CrossRef] [PubMed]
- Femenias, A.; Gatius, F.; Ramos, A.J.; Teixido-Orries, I.; Marín, S. Hyperspectral imaging for the classification of individual cereal kernels according to fungal and mycotoxins contamination: A review. Food Res. Int. 2022, 155, 111102. [Google Scholar] [CrossRef]
- Öner, T.; Thiam, P.; Kos, G.; Krska, R.; Schwenker, F.; Mizaikoff, B. Machine learning algorithms for the automated classification of contaminated maize at regulatory limits via infrared attenuated total reflection spectroscopy. World Mycotoxin J. 2019, 12, 113–122. [Google Scholar] [CrossRef]
- Singh, J.; Mehta, A. Rapid and sensitive detection of mycotoxins by advanced and emerging analytical methods: A review. Food Sci. Nutr. 2020, 8, 2183–2204. [Google Scholar] [CrossRef]
- Ottoboni, M.; Pinotti, L.; Tretola, M.; Giromini, C.; Fusi, E.; Rebucci, R.; Grillo, M.; Tassoni, L.; Foresta, S.; Gastaldello, S.; et al. Combining e-nose and lateral flow immunoassays (lfias) for rapid occurrence/co-occurrence aflatoxin and fumonisin detection in maize. Toxins 2018, 10, 416. [Google Scholar] [CrossRef]
- Bertuzzi, T.; Camardo Leggieri, M.; Battilani, P.; Pietri, A. Co-occurrence of type a and b trichothecenes and zearalenone in wheat grown in northern italy over the years 2009–2011. Food Addit. Contam. Part B Surveill. 2014, 7, 273–281. [Google Scholar] [CrossRef]
- Camardo Leggieri, M.; Bertuzzi, T.; Pietri, A.; Battilani, P. Mycotoxin occurrence in maize produced in northern italy over the years 2009–2011: Focus on the role of crop related factors. Phytopathol. Medit. 2015, 54, 212–221. [Google Scholar]
- Infantino, A.; Aureli, G.; Costa, C.; Taiti, C.; Antonucci, F.; Menesatti, P.; Pallottino, F.; De Felice, S.; D’Egidio, M.G.; Mancuso, S. Potential application of ptr-tofms for the detection of deoxynivalenol (don) in durum wheat. Food Control 2015, 57, 96–104. [Google Scholar] [CrossRef]
- Machungo, C.; Berna, A.Z.; McNevin, D.; Wang, R.; Trowell, S. Comparison of the performance of metal oxide and conducting polymer electronic noses for detection of aflatoxin using artificially contaminated maize. Sens. Actuators B Chem. 2022, 360, 131681. [Google Scholar] [CrossRef]
- Li, H.; Larsen, D.H.; Cao, R.; van de Peppel, A.C.; Tikunov, Y.M.; Marcelis, L.F.M.; Woltering, E.J.; van Kan, J.A.L.; Schouten, R.E. The association between the susceptibility to Botrytis cinerea and the levels of volatile and non-volatile metabolites in red ripe strawberry genotypes. Food Chem. 2022, 393, 133252. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Hanson, M.M.; Tang, F.; Jiang, H.; Chen, M.; Liu, R.; Lin, H.; Chen, Q. Total fungi counts and metabolic dynamics of volatile organic compounds in paddy contaminated by Aspergillus niger during storage employing gas chromatography-ion mobility spectrometry. Food Anal. Methods 2022, 15, 1638–1651. [Google Scholar] [CrossRef]
- Campagnoli, A.; Cheli, F.; Savoini, G.; Crotti, A.; Pastori, A.G.; Dell’Orto, V. Application of an electronic nose to detection of aflatoxins in corn. Vet. Res. Commun. 2009, 33, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Campagnoli, A.; Cheli, F.; Polidori, C.; Zaninelli, M.; Zecca, O.; Savoini, G.; Pinotti, L.; Dell’Orto, V. Use of the electronic nose as a screening tool for the recognition of durum wheat naturally contaminated by deoxynivalenol: A preliminary approach. Sensors 2011, 11, 4899–4916. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (ec) No 401/2006 Laying down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs; Official Journal of the European Union: Luxembourg, 2006; pp. 12–34. [Google Scholar]
- European Commission. Commission Regulation (ec) No 1126/2007 Amending Regulation (ec) no 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs as Reguards fusarium Toxins in Maize and Maize Products; Official Journal of European Union: Luxembourg, 2007; pp. 14–17. [Google Scholar]
- Kuhn, M. Building predictive models in r using the caret package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Hastie, T.; Hastie, T.; Tibshirani, R.; Friedman, J.H. The Elements of Statistical Learning: Data Mining, Inference, and Prediction, 2nd ed.; Springer: New York, NY, USA, 2001. [Google Scholar]
| Year | N # | Mean | StDev | Minimum | Maximum |
|---|---|---|---|---|---|
| 2014 | 52 | 98 | 126.4 | <LOQ * | 615 |
| 2015 | 55 | 205 | 233.9 | <LOQ ** | 1171 |
| 2017 | 57 | 1069 | 2208.4 | 20 | 14,829 |
| 2018 | 50 | 1147 | 2217.9 | 59 | 10,898 |
| Threshold (μg/kg) | Original Dataset | Training Dataset | Blind Dataset | |||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | |
| 1750 | 18 | 196 | 13 | 138 | 5 | 58 |
| 1250 | 20 | 194 | 14 | 136 | 6 | 58 |
| 750 | 34 | 180 | 24 | 126 | 10 | 54 |
| 500 | 49 | 165 | 35 | 116 | 14 | 49 |
| Thresholds (μg/kg) | Observed | Predicted | |
|---|---|---|---|
| Positive (%) | Negative (%) | ||
| 1750 | Positive | 3 | 5 |
| Negative | 3 | 89 | |
| 1250 | Positive | 3 | 5 |
| Negative | 5 | 87 | |
| 750 | Positive | 5 | 2 |
| Negative | 8 | 85 | |
| 500 | Positive | 6 | 2 |
| Negative | 14 | 78 | |
| Threshold (μg/kg) | 1750 | 1250 | 750 | 500 | ||||
|---|---|---|---|---|---|---|---|---|
| Data Set | TD | BD | TD | BD | TD | BD | TD | BD |
| Cross validation method | ||||||||
| * ACC | 0.92 | 0.89 | 0.91 | 0.88 | 0.91 | 0.81 | 0.85 | 0.83 |
| TPR | 0.31 | 0.50 | 0.29 | 0.40 | 0.54 | 0.37 | 0.40 | 0.36 |
| TNR | 0.98 | 0.97 | 0.98 | 0.97 | 0.98 | 0.94 | 0.99 | 0.96 |
| PPV | 0.57 | 0.40 | 0.57 | 0.40 | 0.87 | 0.75 | 0.93 | 0.71 |
| BA | 0.64 | 0.72 | 0.63 | 0.67 | 0.76 | 0.567 | 0.70 | 0.66 |
| Number in Array | Sensor | General Description | Reference |
|---|---|---|---|
| 1 | W1C aromatic | Aromatic compounds | Toluene, 10 ppm |
| 2 | W5S broad range | Broad range sensitivity, react on nitrogen oxides and ozone, very sensitive with negative signal | NO2, 1 ppm |
| 3 | W3C aromatic | Ammonia, used as sensor for aromatic compounds | Benzene, 10 ppm |
| 4 | W6S hydrogen | Mainly hydrogen, selectively (breath gases) | H2, 100 ppb |
| 5 | W5C aromatic-aliphatic | Alkanes, aromatic compounds, less polar compounds | Propane, 1 ppm |
| 6 | W1S broad methane | Sensitive to methane (environment) ca. 10 ppm, broad range, similar to W2S | CH4, 100 ppm |
| 7 | W1W sulphur organic | Reacts on sulphur compounds (H2S 0,1 ppm), otherwise sensitive to many terpenes and sulphur organic compounds, which are important for smell (limonene, pyrazine) | H2S, 1 ppm |
| 8 | W2S broad alcohol | Detects alcohol’s, partially aromatic compounds, broad range | CO, 100 ppm |
| 9 | W2W sulphur-chlorine | Aromatic compounds, sulfur organic compounds | H2S, 1 ppm |
| 10 | W3S methane-aliphatic | Reacts on high concentrations > 100 ppm sometimes very selective (methane) | CH4, 100 ppm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camardo Leggieri, M.; Mazzoni, M.; Bertuzzi, T.; Moschini, M.; Prandini, A.; Battilani, P. Electronic Nose for the Rapid Detection of Deoxynivalenol in Wheat Using Classification and Regression Trees. Toxins 2022, 14, 617. https://doi.org/10.3390/toxins14090617
Camardo Leggieri M, Mazzoni M, Bertuzzi T, Moschini M, Prandini A, Battilani P. Electronic Nose for the Rapid Detection of Deoxynivalenol in Wheat Using Classification and Regression Trees. Toxins. 2022; 14(9):617. https://doi.org/10.3390/toxins14090617
Chicago/Turabian StyleCamardo Leggieri, Marco, Marco Mazzoni, Terenzio Bertuzzi, Maurizio Moschini, Aldo Prandini, and Paola Battilani. 2022. "Electronic Nose for the Rapid Detection of Deoxynivalenol in Wheat Using Classification and Regression Trees" Toxins 14, no. 9: 617. https://doi.org/10.3390/toxins14090617
APA StyleCamardo Leggieri, M., Mazzoni, M., Bertuzzi, T., Moschini, M., Prandini, A., & Battilani, P. (2022). Electronic Nose for the Rapid Detection of Deoxynivalenol in Wheat Using Classification and Regression Trees. Toxins, 14(9), 617. https://doi.org/10.3390/toxins14090617







