Protective Effect of SeMet on Liver Injury Induced by Ochratoxin A in Rabbits
Abstract
:1. Introduction
2. Results
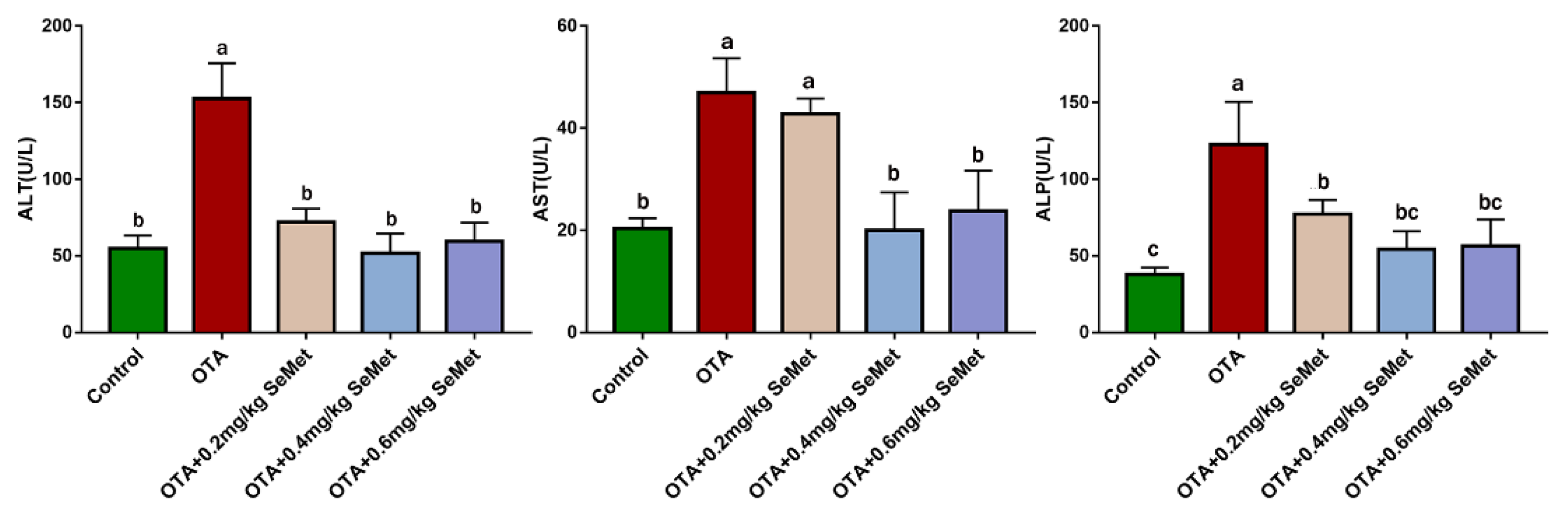
2.1. Plasma Biochemical Analysis
2.2. SeMet Improves OTA-Induced Liver Pathological Changes
2.3. SeMet Suppresses OTA-Induced Liver Oxidative Stress
2.4. SeMet Modulated Nrf2 and HO-1 Expression in OTA-Induced Rabbit Hepatic Toxicity
2.5. SeMet Improves Rabbit Hepatic Inflammation Induced by OTA
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animals and Treatments
5.2. Sample Preparation
5.3. Biochemical Assays
5.4. Histopathological Analysis
5.5. Measurement of ROS
5.6. Measurement of Oxidative Stress Markers
5.7. Quantitative Real-Time PCR Analysis
5.8. Enzyme-Linked Immunosorbent Assay
5.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, Z.; Huang, K.; Luo, Y. Ochratoxin A and ochratoxin-producing fungi on cereal grain in China: A review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 461–470. [Google Scholar]
- Escriva, L.; Font, G.; Manyes, L. In vivo toxicity studies of fusarium mycotoxins in the last decade: A review. Food Chem. Toxicol. 2015, 78, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Liu, F.; Wang, Q.; Selvaraj, J.N.; Xing, F.; Zhao, Y.; Liu, Y. Ochratoxin A Producing Fungi, Biosynthetic Pathway and Regulatory Mechanisms. Toxins 2016, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Escriva, L.; Font, G.; Manyes, L.; Berrada, H. Studies on the Presence of Mycotoxins in Biological Samples: An Overview. Toxins 2017, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Manderville, R.A.; Wetmore, S.D. Mutagenicity of Ochratoxin A: Role for a Carbon-Linked C8-Deoxyguanosine Adduct? J. Agric. Food Chem. 2017, 65, 7097–7105. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; You, S.; Song, G. Ochratoxin A exerts neurotoxicity in human astrocytes through mitochondria-dependent apoptosis and intracellular calcium overload. Toxicol. Lett. 2019, 313, 42–49. [Google Scholar] [CrossRef]
- Heussner, A.H.; Bingle, L.E. Comparative Ochratoxin Toxicity: A Review of the Available Data. Toxins 2015, 7, 4253–4282. [Google Scholar] [CrossRef]
- Yu, Z.; Wu, F.; Tian, J.; Guo, X.; An, R. Protective effects of compound ammonium glycyrrhizin, Larginine, silymarin and glucurolactone against liver damage induced by ochratoxin A in primary chicken hepatocytes. Mol. Med. Rep. 2018, 18, 2551–2560. [Google Scholar]
- Liu, Y.; Wang, H.; Zhang, M.; Wang, J.; Zhang, Z.; Wang, Y.; Sun, Y.; Zhang, Z. Protective effect of selenomethionine on T-2 toxin-induced liver injury in New Zealand rabbits. BMC Vet. Res. 2021, 17, 153. [Google Scholar] [CrossRef]
- Liu, F.; Ichihara, S.; Valentine, W.M.; Itoh, K.; Yamamoto, M.; Sheik Mohideen, S.; Kitoh, J.; Ichihara, G. Increased susceptibility of Nrf2-null mice to 1-bromopropane-induced hepatotoxicity. Toxicol. Sci. 2010, 115, 596–606. [Google Scholar] [CrossRef]
- Limonciel, A.; Jennings, P. A review of the evidence that ochratoxin A is an Nrf2 inhibitor, implications for nephrotoxicity and renal carcinogenicity. Toxins 2014, 6, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Ramyaa, P.; Krishnaswamy, R.; Padma, V.V. Quercetin modulates OTA-induced oxidative stress and redox signalling in HepG2 cells—Up regulation of Nrf2 expression and down regulation of NF-kappaB and COX-2. Biochim. Biophys. Acta 2014, 1840, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.G.; Saraiva, N.; Guerreiro, P.S.; Louro, H.; Silva, M.J.; Miranda, J.P.; Castro, M.; Batinic-Haberle, I.; Fernandes, A.S.; Oliveira, N. Ochratoxin A-induced cytotoxicity, genotoxicity and reactive oxygen species in kidney cells: An integrative approach of complementary endpoints. Food Chem. Toxicol. 2016, 87, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Ertani, A.; Parrasia, S.; Vecchia, F.D. Selenium accumulation and metabolism in algae. Aquat. Toxicol. 2017, 189, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aaseth, J.; Alexander, J.; Bjørklund, G.; Hestad, K.; Dusek, P.; Roos, P.M.; Alehagen, U. Treatment strategies in Alzheimer’s disease: A review with focus on selenium supplementation. Biometals 2016, 29, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Demirci, K.; Nazıroğlu, M.; Övey, I.S.; Balaban, H. Selenium attenuates apoptosis, inflammation and oxidative stress in the blood and brain of aged rats with scopolamine-induced dementia. Metab. Brain Dis. 2017, 32, 321–329. [Google Scholar] [CrossRef]
- Ghorbel, I.; Elwej, A.; Chaabane, M.; Jamoussi, K.; Mnif, H.; Boudawara, T.; Zeghal, N. Selenium Alleviates Oxidative Stress and Lung Damage Induced by Aluminum Chloride in Adult Rats: Biochemical and Histological Approach. Biol. Trace Elem. Res. 2017, 176, 181–191. [Google Scholar] [CrossRef]
- Liu, C.; Zuo, Z.; Zhu, P.; Zheng, Z.; Peng, X.; Fang, J.; Cui, H.; Zhou, Y.; Ouyang, P.; Geng, Y.; et al. Sodium selenite prevents suppression of mucosal humoral response by AFB1 in broiler’s cecal tonsil. Oncotarget 2017, 8, 54215–54226. [Google Scholar] [CrossRef]
- Gan, F.; Hu, Z.; Zhou, Y.; Huang, K. Overexpression and Low Expression of Selenoprotein S Impact Ochratoxin A-Induced Porcine Cytotoxicity and Apoptosis in Vitro. J. Agric. Food Chem. 2017, 65, 6972–6981. [Google Scholar] [CrossRef]
- McKelvey, S.M.; Horgan, K.A.; Murphy, R.A. Chemical form of selenium differentially influences DNA repair pathways following exposure to lead nitrate. J. Trace Elem. Med. Biol. 2015, 29, 151–169. [Google Scholar] [CrossRef]
- Zhang, Y. Research of Sea Buckthorn (Hippophaë rhamnoides Linn.) Fruitoil Extraction and Preventive Effects on Light-Induced Retinal Damage of Pigmented Rabbits. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2016. [Google Scholar]
- Liu, Y.; Yang, Y.; Dong, R.; Zhang, Z.; Jia, F.; Yu, H.; Wang, Y. Protective effect of selenomethionine on intestinal injury induced by T-2 toxin. Res. Vet. Sci. 2020, 132, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, K.; Zou, C.; Tong, C.; Sun, L.; Cao, Z.; Yang, S.; Lyu, Q. Selenium Yeast Alleviates Ochratoxin A-Induced Hepatotoxicity via Modulation of the PI3K/AKT and Nrf2/Keap1 Signaling Pathways in Chickens. Toxins 2020, 12, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, F.; Zhou, Y.; Hu, Z.; Hou, L.; Chen, X.; Xu, S.; Huang, K. GPx1-mediated DNMT1 expression is involved in the blocking effects of selenium on OTA-induced cytotoxicity and DNA damage. Int. J. Biol. Macromol. 2020, 146, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Lausted, C.; Yoo, H.; Yan, X.; Brightman, A.; Chen, J.; Wang, W.; Bu, X.; Hood, L. Quantitative liver-specific protein fingerprint in blood: A signature for hepatotoxicity. Theranostics 2014, 4, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Sherman, K.E. Alanine aminotransferase in clinical practice. A review. Arch. Intern. Med. 1991, 151, 260–265. [Google Scholar] [CrossRef]
- Liu, H.; Zha, X.; Ding, C.; Hu, L.; Li, M.; Yu, Y.; Zhou, W.; Wang, T.; Zhu, L.; Bao, H.; et al. AST/ALT Ratio and Peripheral Artery Disease in a Chinese Hypertensive Population: A Cross-Sectional Study. Angiology 2021, 72, 916–922. [Google Scholar] [CrossRef]
- Poupon, R. Liver alkaline phosphatase: A missing link between choleresis and biliary inflammation. Hepatology 2015, 61, 2080–2090. [Google Scholar] [CrossRef]
- Damiano, S.; Longobardi, C.; Andretta, E.; Prisco, F.; Piegari, G.; Squillacioti, C.; Montagnaro, S.; Pagnini, F.; Badino, P.; Florio, S.; et al. Antioxidative Effects of Curcumin on the Hepatotoxicity Induced by Ochratoxin A in Rats. Antioxidants 2021, 10, 125. [Google Scholar] [CrossRef]
- Aydın, G.; Ōzçelik, N.; Cicek, E.; Soyöz, M. Histopathologic changes in liver and renal tissues induced by Ochratoxin A and melatonin in rats. Hum. Exp. Toxicol. 2003, 22, 383–391. [Google Scholar]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef]
- Hu, Q.; Chen, X.; An, X.; Lyu, X. Effects of selenium-enriched tea on improving non-specific immune function in rats. Food Sci. 2000, 11, 56–58. [Google Scholar]
- Wang, H.; Chen, Y.; Zhai, N.; Chen, X.; Gan, F.; Li, H.; Huang, K. Ochratoxin A-Induced Apoptosis of IPEC-J2 Cells through ROS-Mediated Mitochondrial Permeability Transition Pore Opening Pathway. J. Agric. Food Chem. 2017, 65, 10630–10637. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, G.; Nijmeijer, S.; Maas, R.; Roestenberg, P.; de Groene, E.; Fink-Gremmels, J. The role of oxidative stress in the ochratoxin A-mediated toxicity in proximal tubular cells. Biochim. Biophys. Acta 2002, 1588, 149–158. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Q.; Cai, J.; Wang, Y.; Lai, X.; Qiu, Y.; Huang, Y.; Ke, Q.; Zhang, Y.; Guan, Y.; et al. Nestin regulates cellular redox homeostasis in lung cancer through the Keap1-Nrf2 feedback loop. Nat. Commun. 2019, 10, 5043. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Perez, E.; Ryu, D.; Lee, C.; Lee, H.J. Ochratoxin A Induces Oxidative Stress in HepG2 Cells by Impairing the Gene Expression of Antioxidant Enzymes. Toxins 2021, 13, 271. [Google Scholar] [CrossRef]
- Tang, W.; Jiang, Y.-F.; Ponnusamy, M.; Diallo, M. Role of Nrf2 in chronic liver disease. World J. Gastroenterol. 2014, 20, 13079–13087. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Rajput, S.A.; Sun, L.; Zhang, N.Y.; Khalil, M.M.; Ling, Z.; Chong, L.; Wang, S.; Rajput, I.R.; Bloch, D.M.; Khan, F.A.; et al. Grape Seed Proanthocyanidin Extract Alleviates AflatoxinB1-Induced Immunotoxicity and Oxidative Stress via Modulation of NF-kappaB and Nrf2 Signaling Pathways in Broilers. Toxins 2019, 11, 23. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Bekyarova, G.; Apostolova, M.; Kotzev, I. Melatonin protection against burn-induced hepatic injury by down-regulation of nuclear factor kappa B activation. Int. J. Immunopathol. Pharmacol. 2012, 25, 591–596. [Google Scholar] [CrossRef]
- Liu, C.M.; Ma, J.Q.; Xie, W.R.; Liu, S.S.; Feng, Z.J.; Zheng, G.H.; Wang, A.M. Quercetin protects mouse liver against nickel-induced DNA methylation and inflammation associated with the Nrf2/HO-1 and p38/STAT1/NF-kappaB pathway. Food Chem. Toxicol. 2015, 82, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Cao, Z.; Guo, Y.; Tong, C.; Yang, S.; Long, M.; Li, P.; He, J. Selenium Yeast Alleviates Ochratoxin A-Induced Apoptosis and Oxidative Stress via Modulation of the PI3K/AKT and Nrf2/Keap1 Signaling Pathways in the Kidneys of Chickens. Oxidative Med. Cell. Longev. 2020, 2020, 4048706. [Google Scholar] [CrossRef] [PubMed]
- Cavin, C.; Delatour, T.; Marin-Kuan, M.; Holzhäuser, D.; Higgins, L.; Bezençon, C.; Guignard, G.; Junod, S.; Richoz-Payot, J.; Gremaud, E.; et al. Reduction in antioxidant defenses may contribute to ochratoxin A toxicity and carcinogenicity. Toxicol. Sci. 2007, 96, 30–39. [Google Scholar] [CrossRef]
- Cavin, C.; Delatour, T.; Marin-Kuan, M.; Fenaille, F.; Holzhäuser, D.; Guignard, G.; Bezençon, C.; Piguet, D.; Parisod, V.; Richoz-Payot, J.; et al. Ochratoxin A-mediated DNA and protein damage: Roles of nitrosative and oxidative stresses. Toxicol. Sci. 2009, 110, 84–94. [Google Scholar] [CrossRef] [Green Version]
- Yazici, A.; Aksit, H.; Sari, E.S.; Yay, A.; Erken, H.A.; Aksit, D.; Cakmak, H.; Seyrek, K.; Ermis, S.S. Comparison of pre-treatment and post-treatment use of selenium in retinal ischemia reperfusion injury. Int. J. Ophthalmol. 2015, 8, 263–268. [Google Scholar] [PubMed]
- Guo, Y.; Zhao, Y.; Ren, H.; Han, Z.; Chen, A. Effects of Selenomethionine on Growth Performance, Antioxidant and Immune Performance of Weaned Piglets. China Feed. 2019, 4, 50–54. [Google Scholar]
- Zhang, T.; Yao, C.; Hu, Z.; Li, D.; Tang, R. Protective Effect of Selenium on the Oxidative Damage of Kidney Cells Induced by Sodium Nitrite in Grass Carp (Ctenopharyngodon idellus). Biol. Trace Elem. Res. 2022, 8, 3876–3884. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, R.; Yang, Y.; Xie, H.; Huang, Y.; Chen, X.; Wang, D.; Zhang, Z. Protective Effect of Organic Selenium on Oxidative Damage and Inflammatory Reaction of Rabbit Kidney Induced by T-2 Toxin. Biol. Trace Elem. Res. 2021, 199, 1833–1842. [Google Scholar] [CrossRef]
- Wang, G.; Niu, Z. Research progress on toxicity of trace element selenium. Northwest Pharm. J. 2010, 25, 237–238. [Google Scholar]
- Lv, Q.; Liang, X.; Nong, K.; Gong, Z.; Qin, T.; Qin, X.; Wang, D.; Zhu, Y. Advances in Research on the Toxicological Effects of Selenium. Bull. Environ. Contam. Toxicol. 2021, 106, 715–726. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, S.; Rampal, S. Effect of sub-chronic selenium toxicosis on lipid peroxidation, glutathione redox cycle and antioxidant enzymes in calves. Vet. Hum. Toxicol. 2003, 45, 190–192. [Google Scholar]
- Wu, D.; Lian, X.; Fu, Y.; Xia, C.; Kang, S. Relationship between free radicals and apoptosis in selenium poisoning ducklings. Chin. J. Vet. Sci. 2007, 5, 737–740. [Google Scholar]
- Glauert, H.P. Role of NF-kappaB in hepatocarcinogenesis and its potential inhibition by dietary antioxidants. Curr. Cancer Drug Targets 2012, 12, 1160–1172. [Google Scholar] [PubMed]
- Lauridsen, C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019, 98, 4240–4246. [Google Scholar] [CrossRef] [PubMed]
- Han, B.H.; Lee, Y.J.; Yoon, J.J.; Choi, E.S.; Namgung, S.; Jin, X.J.; Jeong, D.H.; Kang, D.K.; Lee, H.S. Hwangryunhaedoktang exerts anti-inflammation on LPS-induced NO production by suppressing MAPK and NF-κB activation in RAW264.7 macrophages. J. Integr. Med. 2017, 15, 326–336. [Google Scholar] [CrossRef]
- Ju, M.; Liu, B.; He, H.; Gu, Z.; Liu, Y.; Su, Y.; Zhu, D.; Cang, J.; Luo, Z. MicroRNA-27a alleviates LPS-induced acute lung injury in mice via inhibiting inflammation and apoptosis through modulating TLR4/MyD88/NF-kappaB pathway. Cell Cycle 2018, 17, 2001–2018. [Google Scholar] [CrossRef]
- Su, Y.-W.; Chiou, W.-F.; Chao, S.-H.; Lee, M.-H.; Chen, C.-C.; Tsai, Y.-C. Ligustilide prevents LPS-induced iNOS expression in RAW 264.7 macrophages by preventing ROS production and down-regulating the MAPK, NF-kappaB and AP-1 signaling pathways. Int. Immunopharmacol. 2011, 11, 1166–1172. [Google Scholar] [CrossRef]
- Feng, C.; Luo, Y.; Nian, Y.; Liu, D.; Yin, X.; Wu, J.; Di, J.; Zhang, R.; Zhang, J. Diallyl Disulfide Suppresses the Inflammation and Apoptosis Resistance Induced by DCA Through ROS and the NF-kappaB Signaling Pathway in Human Barrett’s Epithelial Cells. Inflammation 2017, 40, 818–831. [Google Scholar] [CrossRef]
- Hou, S.; Jiao, Y.; Yuan, Q.; Zhai, J.; Tian, T.; Sun, K.; Chen, Z.; Wu, Z.; Zhang, J. S100A4 protects mice from high-fat diet-induced obesity and inflammation. Lab. Investig. 2018, 98, 1025–1038. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Brenner, D.A. Mechanisms of Liver Injury. I. TNF-alpha-induced liver injury: Role of IKK, JNK, and ROS pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G583–G589. [Google Scholar] [CrossRef]
- Olteanu, D.; Filip, A.; Mureşan, A.; Nagy, A.; Tabaran, F.; Moldovan, R.; Decea, N.; Catoi, C.; Clichici, S. The effects of chitosan and low dose dexamethasone on extrahepatic cholestasis after bile duct ligation in Wistar rats. Acta Physiol. Hung. 2012, 99, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, M.; Cui, G.; Li, L.; Jiao, D.; Yao, B.; Xu, K.; Chen, Y.; Long, M.; Yang, S.; et al. Astaxanthin Protects OTA-Induced Lung Injury in Mice through the Nrf2/NF-kappaB Pathway. Toxins 2019, 11, 540. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, C.; Grilli, E.; Duvigneau, J.C.; Zannoni, A.; Tugnoli, B.; Gentilini, F.; Bertuzzi, T.; Spinozzi, S.; Camborata, C.; Bacci, M.L.; et al. Cellular stress marker alteration and inflammatory response in pigs fed with an ochratoxin contaminated diet. Res. Vet. Sci. 2014, 97, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Gan, F.; Xue, H.; Huang, Y.; Pan, C.; Huang, K. Selenium alleviates porcine nephrotoxicity of ochratoxin A by improving selenoenzyme expression in vitro. PLoS ONE 2015, 10, e119808. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Lei, C.; Niu, X.; Wei, J.; Zhu, J.; Zhu, Y. Interaction of glutathione peroxidase-1 and selenium in endemic dilated cardiomyopathy. Clin. Chim. Acta 2009, 399, 102–108. [Google Scholar] [CrossRef]
- Cox, A.J.; Lehtinen, A.B.; Xu, J.; Langefeld, C.D.; Freedman, B.I.; Carr, J.J.; Bowden, D.W. Polymorphisms in the Selenoprotein S gene and subclinical cardiovascular disease in the Diabetes Heart Study. Acta Diabetol. 2013, 50, 391–399. [Google Scholar] [CrossRef]
- Talbi, W.; Ghazouani, T.; Braconi, D.; Ben Abdallah, R.; Raboudi, F.; Santucci, A.; Fattouch, S. Effects of selenium on oxidative damage and antioxidant enzymes of eukaryotic cells: Wine Saccharomyces cerevisiae. J. Appl. Microbiol. 2019, 126, 555–566. [Google Scholar] [CrossRef]
- Naito, Y.; Takagi, T.; Higashimura, Y. Heme oxygenase-1 and anti-inflammatory M2 macrophages. Arch. Biochem. Biophys. 2014, 564, 83–88. [Google Scholar] [CrossRef]
- Sass, G.; Barikbin, R.; Tiegs, G. The multiple functions of heme oxygenase-1 in the liver. Z. Gastroenterol. 2012, 50, 34–40. [Google Scholar] [CrossRef]
- Assar, D.; Asa, S.; El-Abasy, M.A.; Elbialy, Z.I.; Shukry, M.; El Latif, A.A.; BinMowyna, M.N.; Althobaiti, N.A.; El-Magd, M.A. Aspergillus awamori attenuates ochratoxin A-induced renal and cardiac injuries in rabbits by activating the Nrf2/HO-1 signaling pathway and downregulating IL1β, TNFα, and iNOS gene expressions. Environ. Sci. Pollut. Res. 2021, 26, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Li, L.; Xu, W.; Wang, M.; Jiao, D.; Yao, B.; Xu, K.; Chen, Y.; Yang, S.; Long, M.; et al. Astaxanthin Protects Ochratoxin A-Induced Oxidative Stress and Apoptosis in the Heart via the Nrf2 Pathway. Oxidative Med. Cell. Longev. 2020, 2020, 7639109. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Feed Ingredients | Percent % | Nutritional Level | Percent % |
|---|---|---|---|
| Corn | 19 | Crude Protein | ≥17 |
| Soya bean meal | 19 | Crude Fiber | ≤20 |
| Wheat bran | 18 | Crude ash | ≤12 |
| PeanutHull | 14 | Calcium | ≤12 |
| Bean straw | 10 | phosphorus | ≥0.55 |
| wormwood rod | 7 | sodium chloride | 0.3–1.2 |
| vinasse | 6 | lysine | ≥0.65 |
| premix | 4 | moisture | ≤12.5 |
| soybean oil | 3 | digestible energy (MJ/kg) | 9.37 |
| Gene | Primer Sequences (5′ to 3′) | Product Length (bp) |
|---|---|---|
| β-actin | CGTGCGGGACATCAAGGAG | 177 |
| AGGAAGGAGGGCTGGAAGAG | ||
| Nrf2 | CTCCATATCCCATTCCCTGTA | 148 |
| TCTGAGCAGCCACTTTATTCT | ||
| HO-1 | CAGGTGACTGCCGAGGGTT | 100 |
| GACCGGGTTCTCCTTGTTGTG | ||
| IL-1β | AAGACGATAAACCTACCCTGC | 121 |
| GACTCAAATTCCAGCTTGTCC | ||
| IL-6 | GAAGACGACCACGATCCAC | 115 |
| GCCCATGAAATTCCGCAAG | ||
| TNF-α | AAGAGTCCCCAAACAACCTCC | 122 |
| CTCCACTTGCGGGTTTGCTAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Xu, J.; Zhang, X.; Wang, J.; Xie, H.; Sun, Y.; Zhang, Q.; Chang, Z.; Liu, Y. Protective Effect of SeMet on Liver Injury Induced by Ochratoxin A in Rabbits. Toxins 2022, 14, 628. https://doi.org/10.3390/toxins14090628
Zhang Z, Xu J, Zhang X, Wang J, Xie H, Sun Y, Zhang Q, Chang Z, Liu Y. Protective Effect of SeMet on Liver Injury Induced by Ochratoxin A in Rabbits. Toxins. 2022; 14(9):628. https://doi.org/10.3390/toxins14090628
Chicago/Turabian StyleZhang, Ziqiang, Jingyi Xu, Xin Zhang, Jiajia Wang, Hui Xie, Yingying Sun, Qianwen Zhang, Zhaoyang Chang, and Yumei Liu. 2022. "Protective Effect of SeMet on Liver Injury Induced by Ochratoxin A in Rabbits" Toxins 14, no. 9: 628. https://doi.org/10.3390/toxins14090628




