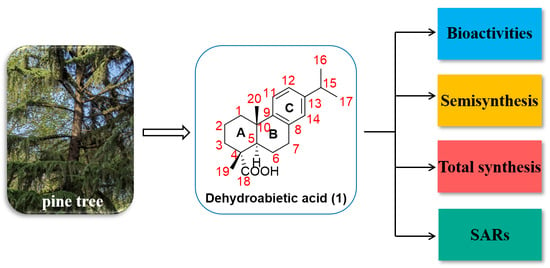
Recent Advances on Biological Activities and Structural Modifications of Dehydroabietic Acid
Abstract
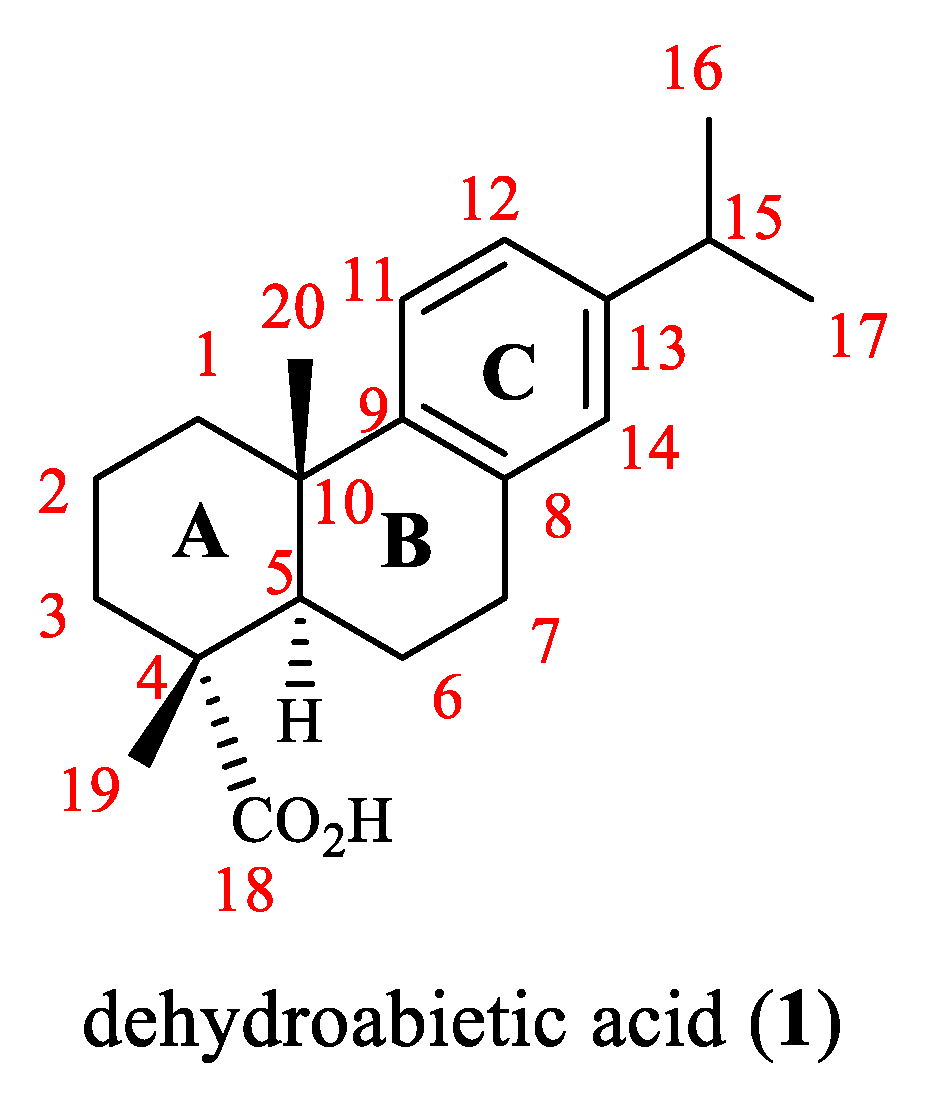
:1. Introduction
2. Bioactivities of Dehydroabietic Acid and Its Derivatives
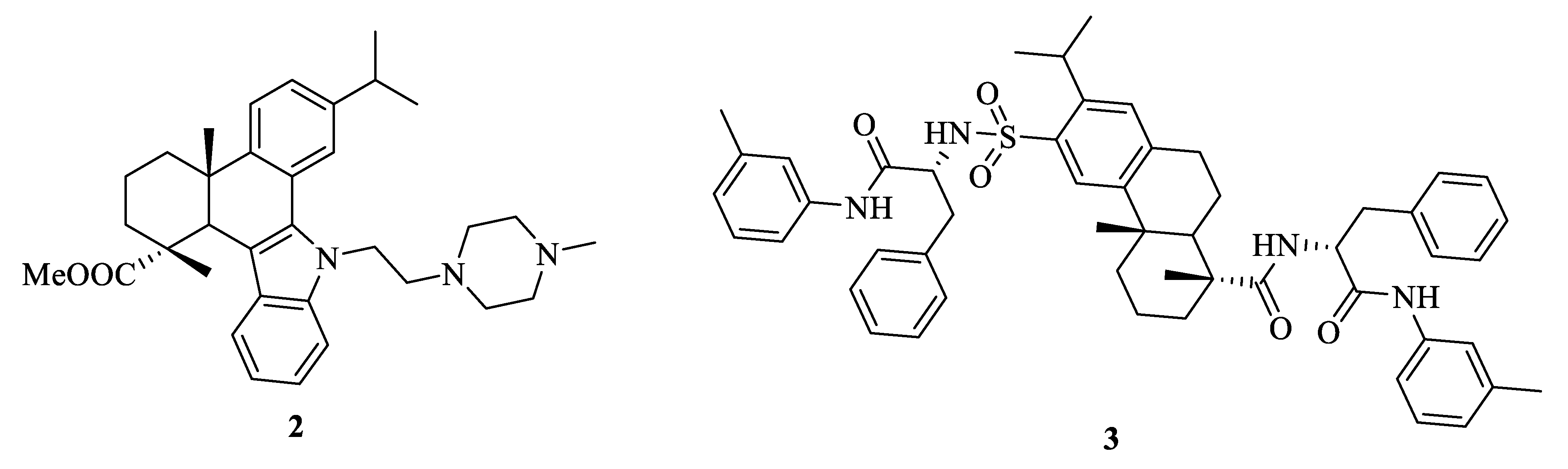
2.1. Antitumor Activity
2.2. Anti-Inflammatory Activity
2.3. Antibacterial and Antifungal Activities
2.4. Insecticidal Activity
2.5. Antiprotozoal Activity
2.6. Other Activities
3. Structural Modification of Dehydroabietic Acid and Its Derivatives
3.1. Structural Modification at C-18 of Dehydroabietic Acid
3.2. Structural Modification at B Ring of Dehydroabietic Acid
3.3. Structural Modification at C Ring of Dehydroabietic Acid
3.4. Biotransformation of Dehydroabietic Acid
4. Total Synthesis of Dehydroabietic Acid
5. Structure-Activity Relationships of Dehydroabietic Acid and Its Derivatives
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AP-1 | Activating protein-1 |
| 5-Fu | 5-Fluorouracil |
| G6Pase | Glucose-6-Phosphatase |
| GS | Glycogen Synthase |
| IC50 | Half-inhibitory concentration |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MIC | Minimum inhibitory concentration |
| NF-κB | Nuclear factor-κB |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NO | Nitric oxide |
| PARP | Poly (ADP-ribose) polymerase |
| PPA | Polyphosphoric acid |
| PPAR | Peroxisome proliferator-activated receptors |
| ROS | Reactive oxygen species |
| TAK1 | Transforming growth factor β-activated kinase 1 |
| TNF-α | Tumor necrosis factor-α |
References
- Zhang, Y.Y.; Xu, H. Recent progress in the chemistry and biology of limonoids. RSC Adv. 2017, 7, 35191–35220. [Google Scholar] [CrossRef]
- Li, Q.; Huang, X.; Li, S.; Ma, J.; Lv, M.; Xu, H. Semisynthesis of esters of fraxinellone C4/10-oxime and their pesticidal activities. J. Agric. Food Chem. 2016, 64, 5472–5478. [Google Scholar] [CrossRef]
- Nagini, S.; Nivetha, R.; Palrasu, M.; Mishra, R. Nimbolide, a neem limonoid, is a promising candidate for the anticancer drug arsenal. J. Med. Chem. 2021, 64, 3560–3577. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.B.; Li, T.; Shan, X.; Lu, R.; Hao, M.; Lv, M.; Sun, Z.; Xu, H. High value-added use of citrus industrial wastes in agriculture: Semisynthesis and anti-tobacco mosaic virus/insecticidal activities of ester derivatives of limonin modified in the B ring. J. Agric. Food Chem. 2020, 68, 12241–12251. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.B.; Lv, M.; Ma, Q.; Zhang, Y.; Xu, H. High value-added application of natural products in crop protection: Semisynthesis and acaricidal activity of limonoid-type derivatives and investigation of their biocompatible O/W nanoemulsions as agronanopesticide candidates. J. Agric. Food Chem. 2021, 69, 14488–14500. [Google Scholar] [CrossRef]
- Sun, Y.; Yin, Y.; Sun, Y.; Li, Q.; Cui, L.; Xu, W.; Kong, L.; Luo, J. Aglatestine A, a rearranged limonoid with a 3/6/6 tricarbocyclic framework from the fruits of Aglaia edulis. J. Org. Chem. 2021, 86, 11263–11268. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.C.; Wang, M.; Pan, Y.M.; Yao, G.Y.; Wang, H.S.; Tian, X.Y.; Qin, J.K.; Zhang, Y. Synthesis and antitumor activities of novel thiourea α-aminophosphonates from dehydroabietic acid. Eur. J. Med. Chem. 2013, 69, 508–520. [Google Scholar] [CrossRef]
- Xu, H.; Liu, L.; Fan, X.; Zhang, G.; Li, Y.; Jiang, B. Identification of a diverse synthetic abietane diterpenoid library for anticancer activity. Bioorganic Med. Chem. Lett. 2017, 27, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.A.; Perez-Guaita, D.; Correa-Royero, J.; Zapata, B.; Agudelo, L.; Mesa-Arango, A.; Betancur-Galvis, L. Synthesis and biological evaluation of dehydroabietic acid derivatives. Eur. J. Med. Chem. 2010, 45, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Han, C.R.; Song, Z.Q.; Shang, S.B. Research progress on abietic acid, dehydroabietic acid and their bioactive derivatives. Chem. Ind. Eng. Prog. 2007, 26, 490–495. [Google Scholar] [CrossRef]
- Costa, M.S.; Rego, A.; Ramos, V.; Afonso, T.B.; Freitas, S.; Preto, M.; Lopes, V.; Vasconcelos, V.; Magalhaes, C.; Leao, P.N. The conifer biomarkers dehydroabietic and abietic acids are widespread in Cyanobacteria. Sci. Rep. 2016, 6, 23436. [Google Scholar] [CrossRef]
- Tagat, J.R.; Nazareno, D.V.; Puar, M.S.; McCombie, S.W.; Ganguly, A.K. Synthesis and anti-herpes activity of some A-ring functionalized dehydroabietane derivatives. Bioorganic Med. Chem. Lett. 1994, 4, 1101–1104. [Google Scholar] [CrossRef]
- Kim, W.-J.; Kang, H.-G.; Kim, S.-J. Dehydroabietic acid inhibits the gastric cancer cell growth via induced apoptosis and cell cycle arrest. Mol. Cell. Toxicol. 2021, 17, 133–139. [Google Scholar] [CrossRef]
- Jokinen, J.J.; Sipponen, A. Refined spruce resin to treat chronic wounds: Rebirth of an old folkloristic therapy. Adv. Wound Care 2016, 5, 198–207. [Google Scholar] [CrossRef]
- Gonzalez, M.A. Aromatic abietane diterpenoids: Their biological activity and synthesis. Nat. Prod. Rep. 2015, 32, 684–704. [Google Scholar] [CrossRef]
- Sepulveda, B.; Astudillo, L.; Rodriguez, J.A.; Yanez, T.; Theoduloz, C.; Schmeda-Hirschmann, G. Gastroprotective and cytotoxic effect of dehydroabietic acid derivatives. Pharmacol. Res. 2005, 52, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Tolmacheva, I.A.; Taranfin, A.V.; Boteva, A.A.; Anikina, L.V.; Vikharev, Y.B.; Grishko, V.V.; Tolstikov, A.G. Synthesis and biological activity of nitrogen-containing derivatives of methyl dehydroabietate. Pharm. Chem. J. 2006, 40, 489–493. [Google Scholar] [CrossRef]
- Duan, W.G.; Li, X.R.; Mo, Q.J.; Huang, J.X.; Cen, B.; Xu, X.T.; Lei, F.H. Synthesis and herbicidal activity of 5-dehydroabietyl-1,3,4-oxadiazole derivatives. Holzforschung 2011, 65, 191–197. [Google Scholar] [CrossRef]
- Helfenstein, A.; Vahermo, M.; Nawrot, D.A.; Demirci, F.; Iscan, G.; Krogerus, S.; Yli-Kauhaluoma, J.; Moreira, V.M.; Tammela, P. Antibacterial profiling of abietane-type diterpenoids. Bioorganic Med. Chem. 2017, 25, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.S.; Isman, M.B.; Yi, F.; Wong, A. Diterpene resin acids: Major active principles in tall oil against Variegated cutworm, Peridroma saucia (Lepidoptera, Noctuidae). J. Chem. Ecol. 1993, 19, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.A.; Madilao, L.L.; White, R.; Yanchuk, A.; King, J.; Bohlmann, J. Terpenoid metabolite profiling in Sitka spruce identifies association of dehydroabietic acid, (+)-3-carene, and terpinolene with resistance against white pine weevil. Botany 2010, 88, 810–820. [Google Scholar] [CrossRef]
- Oh, H.W.; Yun, C.S.; Jeon, J.H.; Kim, J.A.; Park, D.S.; Ryu, H.W.; Oh, S.R.; Song, H.H.; Shin, Y.; Jung, C.S.; et al. Conifer diterpene resin acids disrupt juvenile hormone-mediated endocrine regulation in the Indian meal moth Plodia interpunctella. J. Chem. Ecol. 2017, 43, 703–711. [Google Scholar] [CrossRef]
- Rao, X.; Song, Z.; Han, Z.; Jiang, Z. Synthesis and insect attractant activity of fluorine-containing Pinus diterpenic amides and imines. Nat. Prod. Res. 2009, 23, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Xin, C.; Zhang, Y.; Bao, M.; Yu, C.; Hou, K.; Wang, Z. Novel carrier-free, charge-reversal and DNA-affinity nanodrugs for synergistic cascade cancer chemo-chemodynamic therapy. J. Colloid Interface Sci. 2022, 606, 1488–1508. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, J.; Zhao, B.; Bai, Y.; Peng, Z.; Zhou, J.; Wang, C.; Zhao, X.; Han, S.; Zhang, C. One-step synthesis of biomass-based carbon dots for detection of metal ions and cell imaging. Front. Energy Res. 2022, 10, 871617. [Google Scholar] [CrossRef]
- Singh, A.K.; Chandra, R. Pollutants released from the pulp paper industry: Aquatic toxicity and their health hazards. Aquat. Toxicol. 2019, 211, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Pandelides, Z.; Guchardi, J.; Holdway, D. Dehydroabietic acid (DHAA) alters metabolic enzyme activity and the effects of 17β-estradiol in rainbow trout (Oncorhynchus mykiss). Ecotoxicol. Environ. Saf. 2014, 101, 168–176. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.; Roh, H.S.; Song, S.S.; Ryoo, R.; Pang, C.; Baek, K.H.; Kim, K.H. Cytotoxic constituents from the sclerotia of Poria cocos against human lung adenocarcinoma cells by inducing mitochondrial apoptosis. Cells 2018, 7, 116. [Google Scholar] [CrossRef]
- Luo, D.; Ni, Q.; Ji, A.; Gu, W.; Wu, J.; Jiang, C. Dehydroabietic acid derivative QC4 induces gastric cancer cell death via oncosis and apoptosis. Biomed. Res. Int. 2016, 2016, 2581061. [Google Scholar] [CrossRef]
- Huang, R.Z.; Liang, G.B.; Huang, X.C.; Zhang, B.; Zhou, M.M.; Liao, Z.X.; Wang, H.S. Discovery of dehydroabietic acid sulfonamide based derivatives as selective matrix metalloproteinases inactivators that inhibit cell migration and proliferation. Eur. J. Med. Chem. 2017, 138, 979–992. [Google Scholar] [CrossRef]
- Kolsi, L.E.; Leal, A.S.; Yli-Kauhaluoma, J.; Liby, K.T.; Moreira, V.M. Dehydroabietic oximes halt pancreatic cancer cell growth in the G1 phase through induction of p27 and downregulation of cyclin D1. Sci. Rep. 2018, 8, 15923. [Google Scholar] [CrossRef] [PubMed]
- Zaki, H.; Belhassan, A.; Benlyas, M.; Lakhlifi, T.; Bouachrine, M. New dehydroabietic acid (DHA) derivatives with anticancer activity against HepG2 cancer cell lines as a potential drug targeting EGFR kinase domain. CoMFA study and virtual ligand-based screening. J. Biomol. Struct. Dyn. 2021, 39, 2993–3003. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kang, Y.G.; Kim, Y.J.; Lee, T.R.; Yoo, B.C.; Jo, M.; Kim, J.H.; Kim, J.H.; Kim, D.; Cho, J.Y. Dehydroabietic acid suppresses inflammatory response via suppression of Src-, Syk-, and TAK1-mediated pathways. Int. J. Mol. Sci. 2019, 20, 1593. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Hirai, S.; Goto, T.; Kuroyanagi, K.; Lee, J.Y.; Uemura, T.; Ezaki, Y.; Takahashi, N.; Kawada, T. Dehydroabietic acid, a phytochemical, acts as ligand for PPARs in macrophages and adipocytes to regulate inflammation. Biochem. Biophys. Res. Commun. 2008, 369, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Tretyakova, E.V.; Salimova, E.V.; Parfenova, L.V. Synthesis and antimicrobial and antifungal activity of resin acid acetylene derivatives. Russ. J. Bioorganic Chem. 2020, 45, 545–551. [Google Scholar] [CrossRef]
- Chen, H.; Geng, Y.; Wang, S.F.; Gu, W. Syntheses, crystal structures and antibacterial activities of two new methyl 12-alkylamino-13,14-dinitrodeisopropyl-dehydroabietates. Chin. J. Struct. Chem. 2019, 38, 257–262. [Google Scholar] [CrossRef]
- Sakunpak, A.; Sueree, L. Thin-layer chromatography–contact bioautography as a tool for bioassay-guided isolation of anti-Streptococcus mutans compounds from Pinus merkusii heartwood. JPC-J. Planar Chromat. 2018, 31, 355–359. [Google Scholar] [CrossRef]
- Hassan, G.; Forsman, N.; Wan, X.; Keurulainen, L.; Bimbo, L.M.; Stehl, S.; van Charante, F.; Chrubasik, M.; Prakash, A.S.; Johansson, L.-S.; et al. Non-leaching, highly biocompatible nanocellulose surfaces that efficiently resist fouling by bacteria in an artificial dermis model. ACS Appl. Bio Mater. 2020, 3, 4095–4108. [Google Scholar] [CrossRef]
- Berger, M.; Roller, A.; Maulide, N. Synthesis and antimicrobial evaluation of novel analogues of dehydroabietic acid prepared by C-H-Activation. Eur. J. Med. Chem. 2017, 126, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Chaban, M.F.; Antoniou, A.I.; Karagianni, C.; Toumpa, D.; Joray, M.B.; Bocco, J.L.; Sola, C.; Athanassopoulos, C.M.; Carpinella, M.C. Synthesis and structure-activity relationships of novel abietane diterpenoids with activity against Staphylococcus aureus. Future Med. Chem. 2019, 11, 3109–3124. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.L.; Pan, X.Y.; Yang, T.; Zhang, W.M.; Wang, T.Q.; Wang, H.Y.; Lin, H.X.; Yang, C.G.; Cui, Y.M. The synthesis and antistaphylococcal activity of dehydroabietic acid derivatives: Modifications at C-12. Bioorganic Med. Chem. Lett. 2016, 26, 5492–5496. [Google Scholar] [CrossRef]
- Touré, S.; Dusfour, I.; Stien, D.; Eparvier, V. Two new tetrahydrofuran derivatives from the fungus Mucor spp. SNB-VECD11D isolated from the Chrysomelidae Acalymma bivittula. Tetrahedron Lett. 2017, 58, 3727–3729. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Li, J.; Song, Z.Q.; Song, J.; Shang, S.B.; Xiao, G.M.; Wang, Z.D.; Rao, X.P. Turning renewable resources into value-added products: Development of rosin-based insecticide candidates. Ind. Crops Prod. 2015, 76, 660–671. [Google Scholar] [CrossRef]
- Liu, L.; Yan, X.Y.; Gao, Y.Q.; Rao, X.P. Synthesis and antifeedant activities of rosin-based esters against armyworm. Comb. Chem. High Throughput Screen. 2016, 19, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Keeling, C.I.; Henderson, H.M.; Bohlmann, J. Functions of mountain pine beetle cytochromes P450 CYP6DJ1, CYP6BW1 and CYP6BW3 in the oxidation of pine monoterpenes and diterpene resin acids. PLoS ONE 2019, 14, e0216753. [Google Scholar] [CrossRef]
- Goncalves, M.D.; Bortoleti, B.T.S.; Tomiotto-Pellissier, F.; Miranda-Sapla, M.M.; Assolini, J.P.; Carloto, A.C.M.; Carvalho, P.G.C.; Tudisco, E.T.; Urbano, A.; Ambrosio, S.R.; et al. Dehydroabietic acid isolated from Pinus elliottii exerts in vitro antileishmanial action by pro-oxidant effect, inducing ROS production in promastigote and downregulating Nrf2/ferritin expression in amastigote forms of Leishmania amazonensis. Fitoterapia 2018, 128, 224–232. [Google Scholar] [CrossRef]
- Pertino, M.W.; Vega, C.; Rolon, M.; Coronel, C.; Rojas de Arias, A.; Schmeda-Hirschmann, G. Antiprotozoal activity of triazole derivatives of dehydroabietic acid and oleanolic acid. Molecules 2017, 22, 369. [Google Scholar] [CrossRef] [PubMed]
- Vahermo, M.; Krogerus, S.; Nasereddin, A.; Kaiser, M.; Brun, R.; Jaffe, C.L.; Yli-Kauhaluoma, J.; Moreira, V.M. Antiprotozoal activity of dehydroabietic acid derivatives against Leishmania donovani and Trypanosoma cruzi. MedChemComm 2016, 7, 457–463. [Google Scholar] [CrossRef]
- Xie, Z.; Gao, G.; Wang, H.; Li, E.; Yuan, Y.; Xu, J.; Zhang, Z.; Wang, P.; Fu, Y.; Zeng, H.; et al. Dehydroabietic acid alleviates high fat diet-induced insulin resistance and hepatic steatosis through dual activation of PPAR-γ and PPAR-α. Biomed. Pharmacother. 2020, 127, 110155. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Hirai, S.; Goto, T.; Kuroyanagi, K.; Kim, Y.I.; Ohyama, K.; Uemura, T.; Lee, J.Y.; Sakamoto, T.; Ezaki, Y.; et al. Dehydroabietic acid, a diterpene, improves diabetes and hyperlipidemia in obese diabetic KK-Ay mice. Biofactors 2009, 35, 442–448. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Mubarak, M.S. Anti-obesity effect of plant diterpenes and their derivatives: A review. Phytother. Res. 2020, 34, 1216–1225. [Google Scholar] [CrossRef]
- Gao, G.; Xie, Z.; Li, E.W.; Yuan, Y.; Fu, Y.; Wang, P.; Zhang, X.; Qiao, Y.; Xu, J.; Holscher, C.; et al. Dehydroabietic acid improves nonalcoholic fatty liver disease through activating the Keap1/Nrf2-ARE signaling pathway to reduce ferroptosis. J. Nat. Med. 2021, 75, 540–552. [Google Scholar] [CrossRef]
- Park, J.; Kim, W.J.; Kim, W.; Park, C.; Choi, C.Y.; Cho, J.H.; Kim, S.J.; Cheong, H. Antihypertensive effects of dehydroabietic and 4-epi-trans-communic acid isolated from Pinus densiflora. J. Med. Food 2021, 24, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, Y.G.; Lee, J.Y.; Choi, D.H.; Cho, Y.U.; Shin, J.M.; Park, J.S.; Lee, J.H.; Kim, W.G.; Seo, D.B.; et al. The natural phytochemical dehydroabietic acid is an anti-aging reagent that mediates the direct activation of SIRT1. Mol. Cell. Endocrinol. 2015, 412, 216–225. [Google Scholar] [CrossRef]
- Park, N.H.; Kang, Y.G.; Kim, S.H.; Bae, I.H.; Lee, S.H.; Kim, D.Y.; Hwang, J.S.; Kim, Y.J.; Lee, T.R.; Lee, E.S. Dehydroabietic acid induces regeneration of collagen fibers in ultraviolet B-irradiated human dermal fibroblasts and skin equivalents. Ski. Pharmacol. Physiol. 2019, 32, 109–116. [Google Scholar] [CrossRef]
- Liu, X.; Hu, T.; Lin, G.; Wang, X.; Zhu, Y.; Liang, R.; Duan, W.; Wei, M. The synthesis of a DHAD/ZnAlTi-LDH composite with advanced UV blocking and antibacterial activity for skin protection. RSC Adv. 2020, 10, 9786–9790. [Google Scholar] [CrossRef]
- Lee, S.; Choi, E.; Yang, S.M.; Ryoo, R.; Moon, E.; Kim, S.H.; Kim, K.H. Bioactive compounds from sclerotia extract of Poria cocos that control adipocyte and osteoblast differentiation. Bioorganic Chem. 2018, 81, 27–34. [Google Scholar] [CrossRef]
- Nachar, A.; Saleem, A.; Arnason, J.T.; Haddad, P.S. Regulation of liver cell glucose homeostasis by dehydroabietic acid, abietic acid and squalene isolated from balsam fir (Abies balsamea (L.) Mill.) a plant of the Eastern James Bay Cree traditional pharmacopeia. Phytochemistry 2015, 117, 373–379. [Google Scholar] [CrossRef]
- Sacripante, G.G.; Zhou, K.; Farooque, M. Sustainable polyester resins derived from rosins. Macromolecules 2015, 48, 6876–6881. [Google Scholar] [CrossRef]
- Ohwada, T.; Nonomura, T.; Maki, K.; Sakamoto, K.; Ohya, S.; Muraki, K.; Imaizumi, Y. Dehydroabietic acid derivatives as a novel scaffold for large-conductance calcium-activated K+ channel openers. Bioorganic Med. Chem. Lett. 2003, 13, 3971–3974. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.M.; Liu, X.L.; Zhang, W.M.; Lin, H.X.; Ohwada, T.; Ido, K.; Sawada, K. The synthesis and BK channel-opening activity of N-acylaminoalkyloxime derivatives of dehydroabietic acid. Bioorganic Med. Chem. Lett. 2016, 26, 283–287. [Google Scholar] [CrossRef]
- Li, A.L.; Wang, Z.L.; Wang, W.Y.; Liu, Q.S.; Sun, Y.; Wang, S.F.; Gu, W. A novel dehydroabietic acid-based fluorescent probe for detection of Fe3+ and Hg2+ ions and its application in live-cell imaging. Microchem. J. 2021, 160, 105682. [Google Scholar] [CrossRef]
- Cai, X.M.; Mu, T.Q.; Lin, Y.T.; Zhang, X.D.; Tang, Z.G.; Huang, S.L. Syntheses and photophysical properties of natural dehydroabietic acid-based ligands and their zinc complexes. J. Mol. Struct. 2021, 1229, 129793. [Google Scholar] [CrossRef]
- Huang, X.C.; Jin, L.; Wang, M.; Liang, D.; Chen, Z.F.; Zhang, Y.; Pan, Y.M.; Wang, H.S. Design, synthesis and in vitro evaluation of novel dehydroabietic acid derivatives containing a dipeptide moiety as potential anticancer agents. Eur. J. Med. Chem. 2015, 89, 370–385. [Google Scholar] [CrossRef]
- Huang, X.; Huang, R.; Liao, Z.; Pan, Y.; Gou, S.; Wang, H. Synthesis and pharmacological evaluation of dehydroabietic acid thiourea derivatives containing bisphosphonate moiety as an inducer of apoptosis. Eur. J. Med. Chem. 2016, 108, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Qu, H.E.; Huang, X.C.; Pan, Y.M.; Liang, D.; Chen, Z.F.; Wang, H.S.; Zhang, Y. Synthesis and biological evaluation of novel dehydroabietic acid derivatives conjugated with acyl-thiourea peptide moiety as antitumor agents. Int. J. Mol. Sci. 2015, 16, 14571–14593. [Google Scholar] [CrossRef]
- Li, L.Y.; Fei, B.L.; Wang, P.; Kong, L.Y.; Long, J.Y. Discovery of novel dehydroabietic acid derivatives as DNA/BSA binding and anticancer agents. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 246, 118944. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.Y.; Zhang, K.P.; Chen, H.; Miao, T.T.; Wang, S.F.; Gu, W. Synthesis, in vitro antimicrobial, and cytotoxic activities of new 1,3,4-oxadiazin-5(6H)-one derivatives from dehydroabietic acid. J. Chin. Chem. Soc. 2018, 65, 538–547. [Google Scholar] [CrossRef]
- Li, F.Y.; Wang, X.; Duan, W.G.; Lin, G.S. Synthesis and in vitro anticancer activity of novel dehydroabietic acid-based acylhydrazones. Molecules 2017, 22, 1087. [Google Scholar] [CrossRef]
- Wang, X.; Pang, F.H.; Huang, L.; Yang, X.P.; Ma, X.L.; Jiang, C.N.; Li, F.Y.; Lei, F.H. Synthesis and biological evaluation of novel dehydroabietic acid-oxazolidinone hybrids for antitumor properties. Int. J. Mol. Sci. 2018, 19, 3116. [Google Scholar] [CrossRef] [Green Version]
- Li, F.Y.; Huang, L.; Li, Q.; Wang, X.; Ma, X.L.; Jiang, C.N.; Zhou, X.Q.; Duan, W.G.; Lei, F.H. Synthesis and antiproliferative evaluation of novel hybrids of dehydroabietic acid bearing 1,2,3-triazole moiety. Molecules 2019, 24, 4191. [Google Scholar] [CrossRef]
- Huang, L.; Huang, R.; Pang, F.; Li, A.; Huang, G.; Zhou, X.; Li, Q.; Li, F.; Ma, X. Synthesis and biological evaluation of dehydroabietic acid-pyrimidine hybrids as antitumor agents. RSC Adv. 2020, 10, 18008–18015. [Google Scholar] [CrossRef]
- Li, F.; Huang, L.; Zhou, X.; Li, Q.; Ma, X.; Duan, W.; Wang, X. Synthesis and cytotoxicity evaluation of dehydroabietic acid derivatives bearing nitrate moiety. Chin. J. Org. Chem. 2020, 40, 2845–2854. [Google Scholar] [CrossRef]
- Manner, S.; Vahermo, M.; Skogman, M.E.; Krogerus, S.; Vuorela, P.M.; Yli-Kauhaluoma, J.; Fallarero, A.; Moreira, V.M. New derivatives of dehydroabietic acid target planktonic and biofilm bacteria in Staphylococcus aureus and effectively disrupt bacterial membrane integrity. Eur. J. Med. Chem. 2015, 102, 68–79. [Google Scholar] [CrossRef]
- Chen, N.-Y.; Duan, W.-G.; Liu, L.-Z.; Li, F.-Y.; Lu, M.-P.; Liu, B.-M. Synthesis and antifungal activity of dehydroabietic acid-based thiadiazole-phosphonates. Holzforschung 2015, 69, 1069–1075. [Google Scholar] [CrossRef]
- Chen, N.; Duan, W.; Lin, G.; Liu, L.; Zhang, R.; Li, D. Synthesis and antifungal activity of dehydroabietic acid-based 1,3,4-thiadiazole-thiazolidinone compounds. Mol. Divers. 2016, 20, 897–905. [Google Scholar] [CrossRef]
- Mo, Q.J.; Liu, L.Z.; Duan, W.G.; Cen, B.; Lin, G.S.; Chen, N.Y.; Huang, Y.; Liu, B.M. Synthesis and insecticidal activities of N-(5-dehydroabietyl-1,3,4-thiadiazol-2-yl)-N’-substituted thioureas. Chem. Ind. Forest Prod. 2015, 35, 8–16. [Google Scholar] [CrossRef]
- Zhang, W.M.; Yang, T.; Pan, X.Y.; Liu, X.L.; Lin, H.X.; Gao, Z.B.; Yang, C.G.; Cui, Y.M. The synthesis and antistaphylococcal activity of dehydroabietic acid derivatives: Modifications at C12 and C7. Eur. J. Med. Chem. 2017, 127, 917–927. [Google Scholar] [CrossRef]
- Zhang, W.M.; Yao, Y.; Yang, T.; Wang, X.Y.; Zhu, Z.Y.; Xu, W.T.; Lin, H.X.; Gao, Z.B.; Zhou, H.; Yang, C.G.; et al. The synthesis and antistaphylococcal activity of N-sulfonaminoethyloxime derivatives of dehydroabietic acid. Bioorganic Med. Chem. Lett. 2018, 28, 1943–1948. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, T.T. Synthesis and antibacterial activity of C-7 acylhydrazone derivatives of dehydroabietic acid. J. Chem. Res. 2018, 8, 405–407. [Google Scholar] [CrossRef]
- Chen, N.Y.; Xie, Y.L.; Lu, G.D.; Ye, F.; Li, X.Y.; Huang, Y.W.; Huang, M.L.; Chen, T.Y.; Li, C.P. Synthesis and antitumor evaluation of (aryl)methyl-amine derivatives of dehydroabietic acid-based B ring-fused-thiazole as potential PI3K/AKT/mTOR signaling pathway inhibitors. Mol. Divers. 2021, 25, 967–979. [Google Scholar] [CrossRef]
- Chen, H.; Qiao, C.; Miao, T.T.; Li, A.L.; Wang, W.Y.; Gu, W. Synthesis and biological evaluation of novel N-(piperazin-1-yl)alkyl-1H-dibenzo[a,c]carbazole derivatives of dehydroabietic acid as potential MEK inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 1544–1561. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Zhang, G.; Luo, Z.; Li, D.; Ruan, H.; Ruan, B.H.; Su, L.; Xu, H. Identification of a diverse synthetic abietane diterpenoid library and insight into the structure-activity relationships for antibacterial activity. Bioorg. Med. Chem. Lett. 2017, 27, 5382–5386. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Luo, Z.; Zhang, G.; Cao, D.; Li, D.; Ruan, H.; Ruan, B.H.; Su, L.; Xu, H. Click chemistry-based synthesis and anticancer activity evaluation of novel C-14 1,2,3-triazole dehydroabietic acid hybrids. Eur. J. Med. Chem. 2017, 138, 1042–1052. [Google Scholar] [CrossRef]
- Ahonen, T.J.; Savinainen, J.R.; Yli-Kauhaluoma, J.; Kalso, E.; Laitinen, J.T.; Moreira, V.M. Discovery of 12-thiazole abietanes as selective inhibitors of the human metabolic serine hydrolase hABHD16A. ACS Med. Chem. Lett. 2018, 9, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Miao, T.T.; Hua, D.W.; Jin, X.Y.; Tao, X.B.; Huang, C.B.; Wang, S.F. Synthesis and in vitro cytotoxic evaluation of new 1H-benzo[d]imidazole derivatives of dehydroabietic acid. Bioorganic Med. Chem. Lett. 2017, 27, 1296–1300. [Google Scholar] [CrossRef]
- Gu, W.; Wang, S.; Jin, X.; Zhang, Y.; Hua, D.; Miao, T.; Tao, X.; Wang, S. Synthesis and evaluation of new quinoxaline derivatives of dehydroabietic acid as potential antitumor agents. Molecules 2017, 22, 1154. [Google Scholar] [CrossRef]
- Li, A.L.; Yang, Y.Q.; Wang, W.Y.; Liu, Q.S.; Sun, Y.; Gu, W. Synthesis, cytotoxicity and apoptosis-inducing activity of novel 1H-benzo[d]imidazole derivatives of dehydroabietic acid. J. Chin. Chem. Soc. 2020, 67, 1668–1678. [Google Scholar] [CrossRef]
- Miao, T.-T.; Tao, X.-B.; Li, D.-D.; Chen, H.; Jin, X.-Y.; Geng, Y.; Wang, S.-F.; Gu, W. Synthesis and biological evaluation of 2-aryl-benzimidazole derivatives of dehydroabietic acid as novel tubulin polymerization inhibitors. RSC Adv. 2018, 8, 17511–17526. [Google Scholar] [CrossRef] [PubMed]
- Jun, L.; Ming, L.Y.; Qun, S.L.; Qun, W.A. Synthesis and antitumor activity evaluation of dehydroabietic acid sulfonylureas derivatives. Chin. J. New Drugs 2019, 28, 1778–1783. [Google Scholar]
- Mitsukura, K.; Imoto, T.; Nagaoka, H.; Yoshida, T.; Nagasawa, T. Regio- and stereo-selective hydroxylation of abietic acid derivatives by Mucor circinelloides and Mortierella isabellina. Biotechnol. Lett. 2005, 27, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Rico-Martínez, M.; Medina, F.G.; Marrero, J.G.; Osegueda-Robles, S. Biotransformation of diterpenes. RSC Adv. 2014, 4, 10627–10647. [Google Scholar] [CrossRef]
- van Beek, T.A.; Claassen, F.W.; Dorado, J.; Godejohann, M.; Sierra-Alvarez, R.; Wijnberg, J.B.P.A. Fungal biotransformation products of dehydroabietic acid. J. Nat. Prod. 2007, 70, 154–159. [Google Scholar] [CrossRef] [PubMed]
- González, M.A. Aromatic abietane diterpenoids: Total syntheses and synthetic studies. Tetrahedron 2015, 71, 1883–1908. [Google Scholar] [CrossRef]
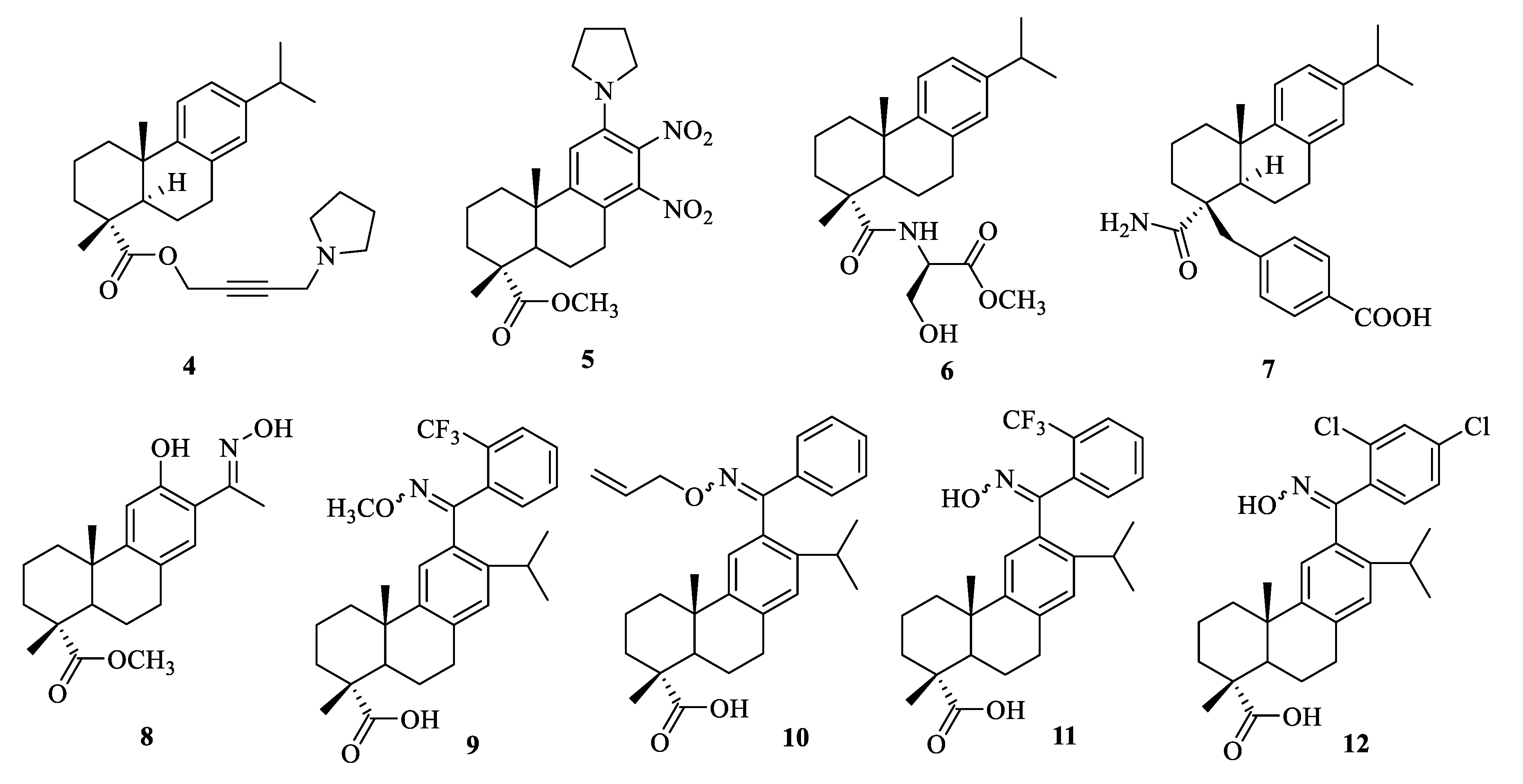
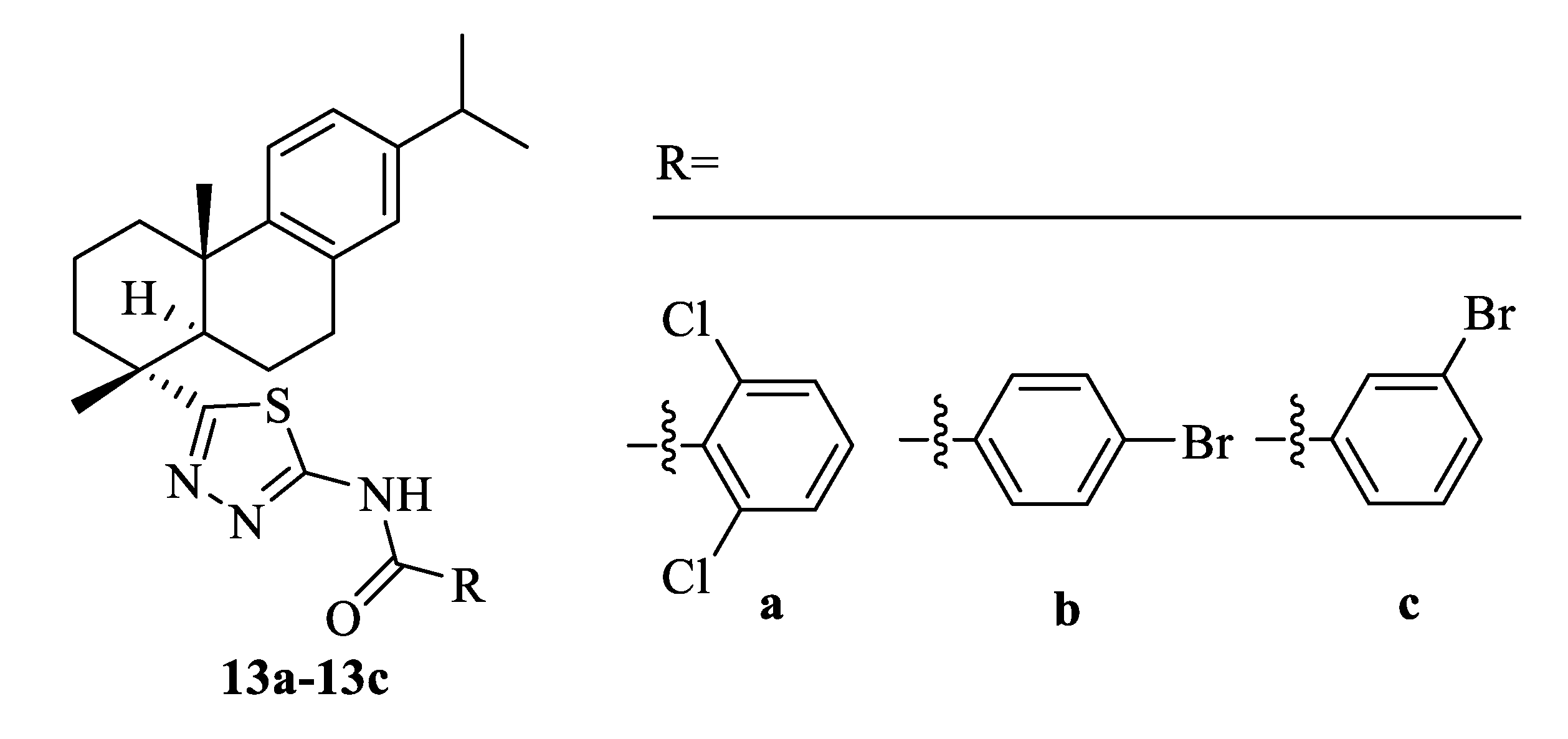
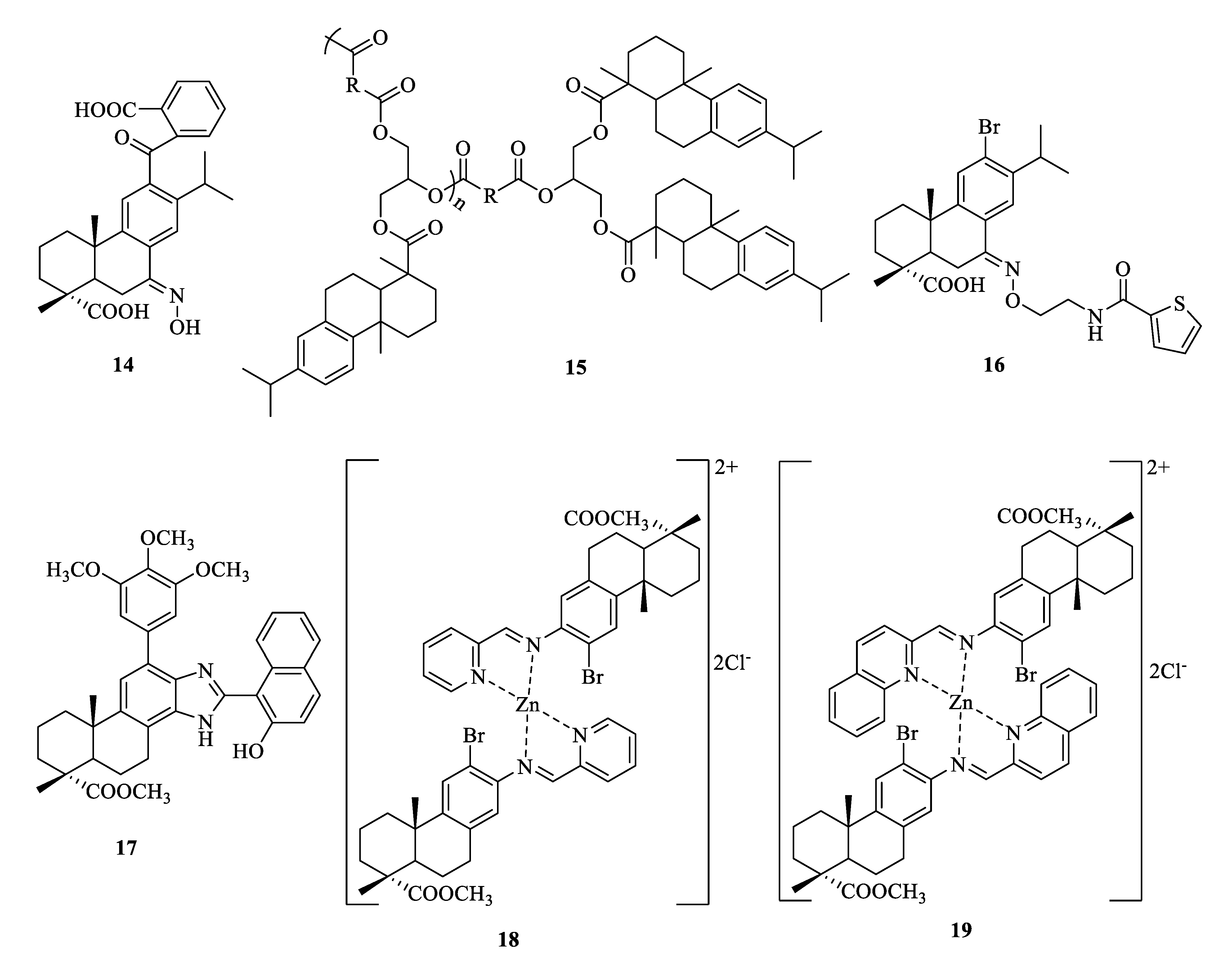
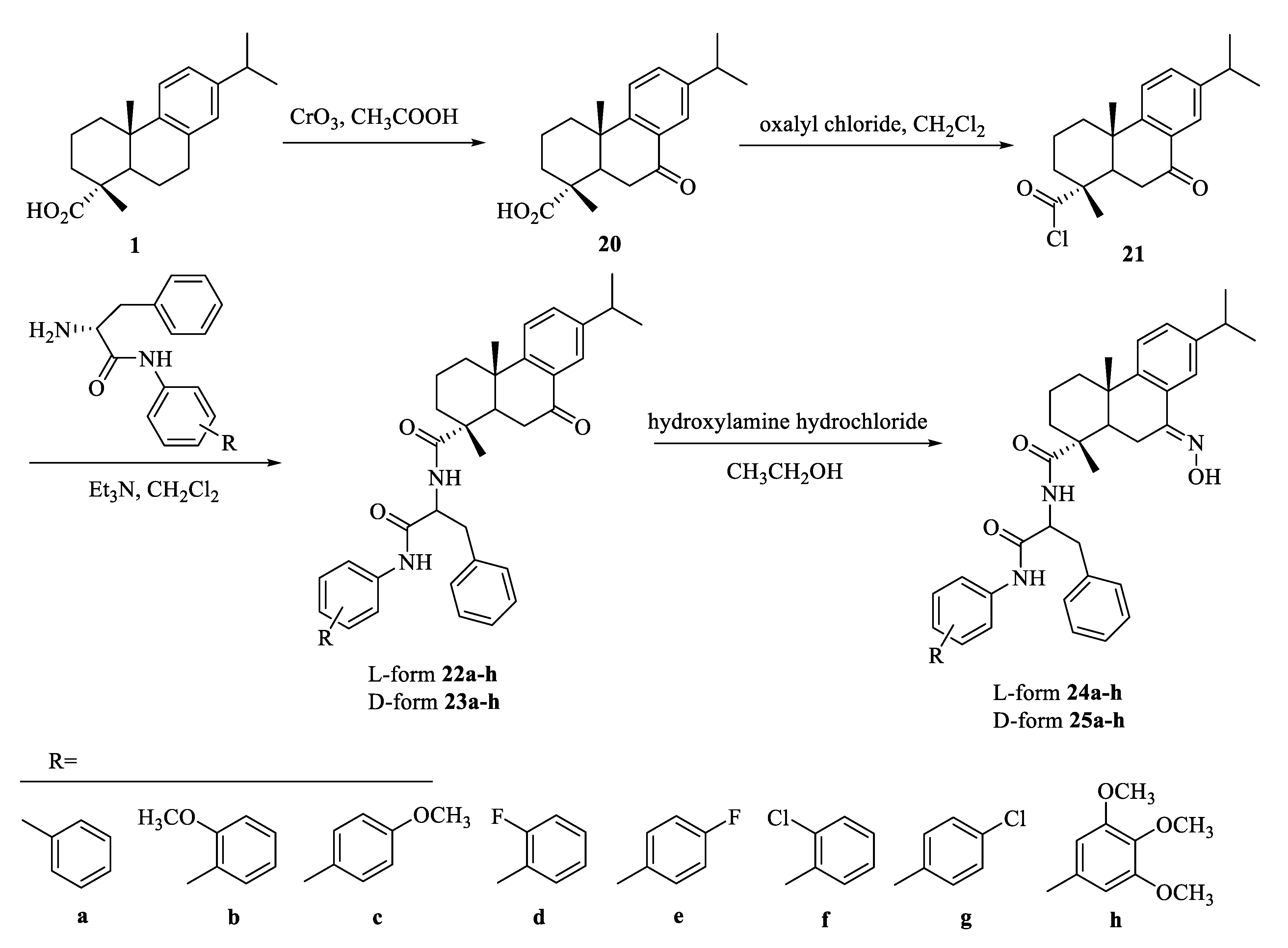
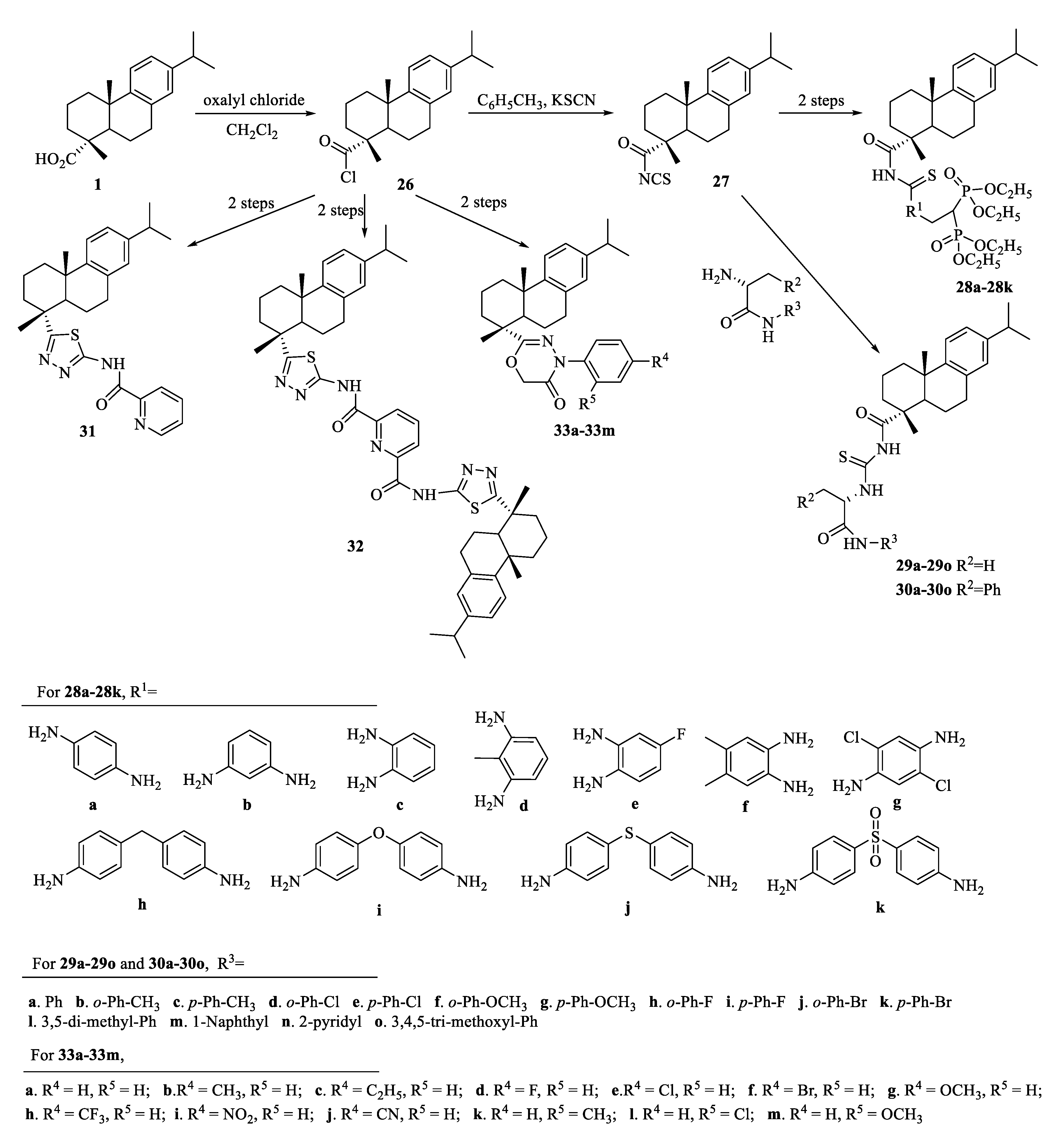
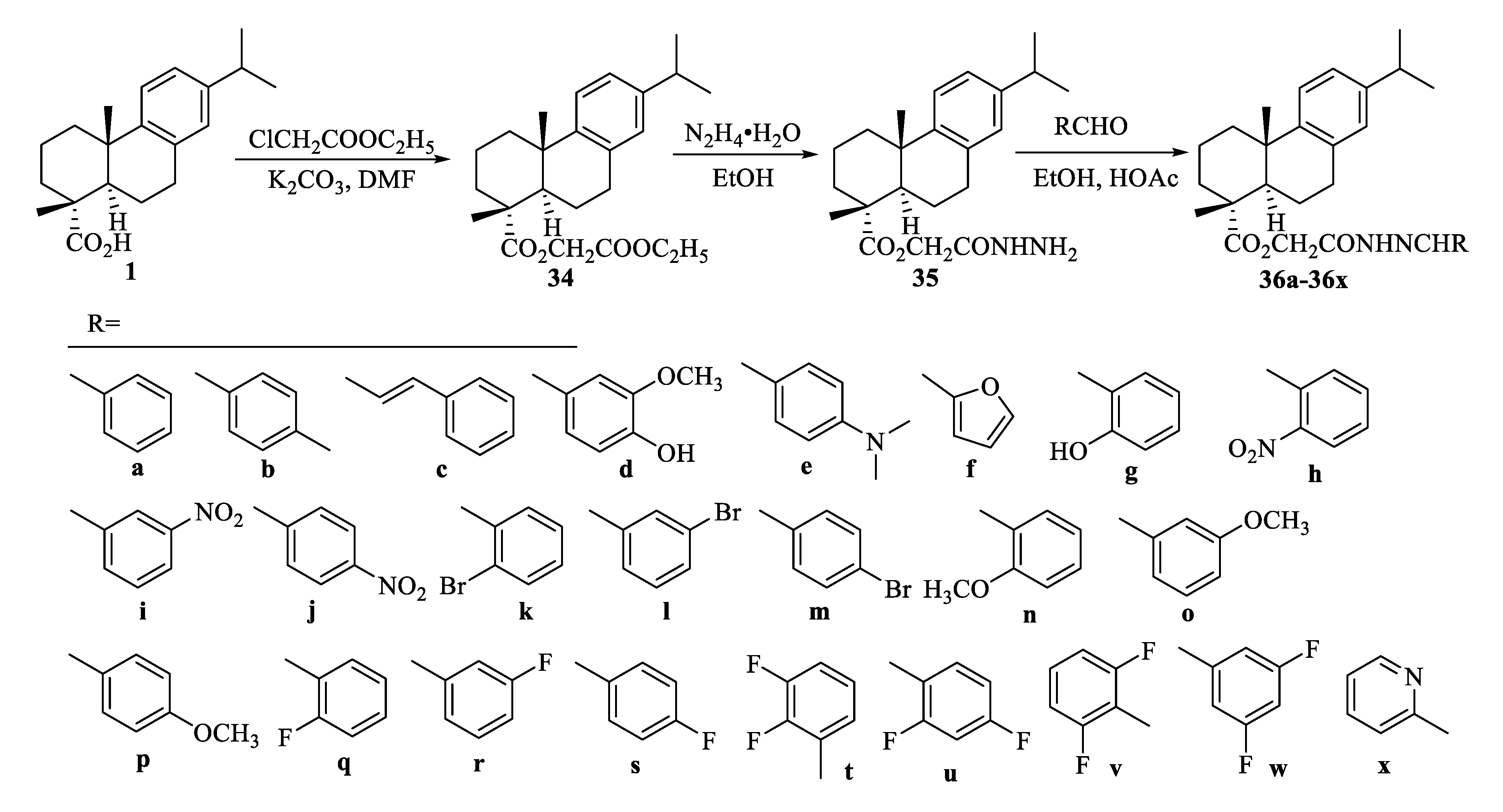

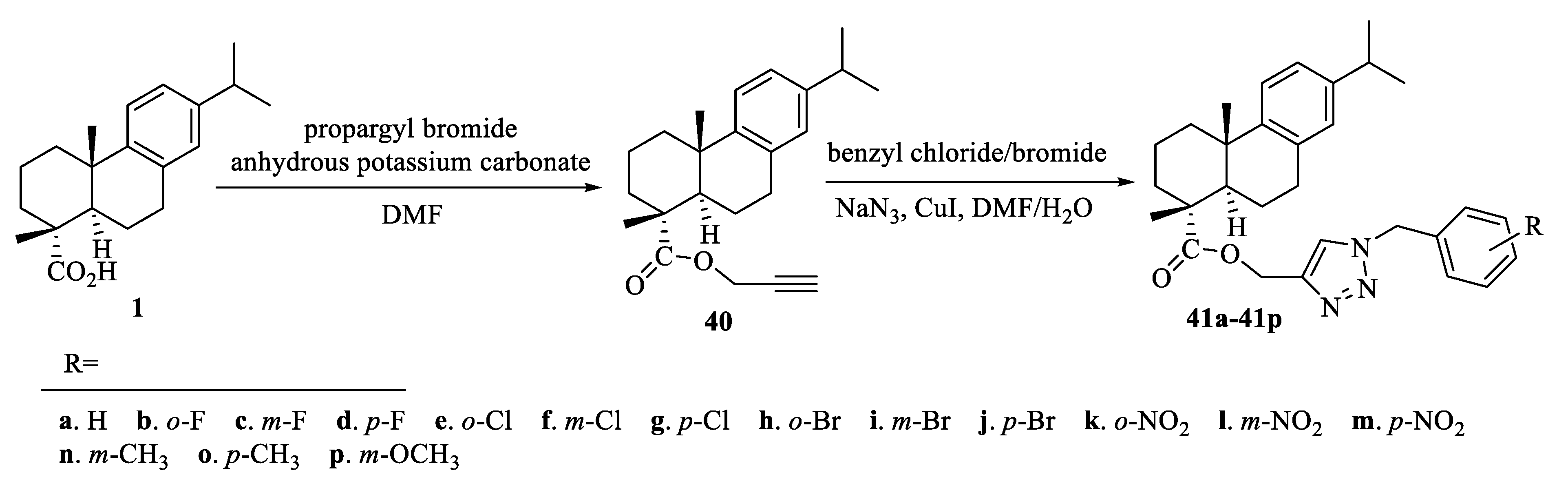
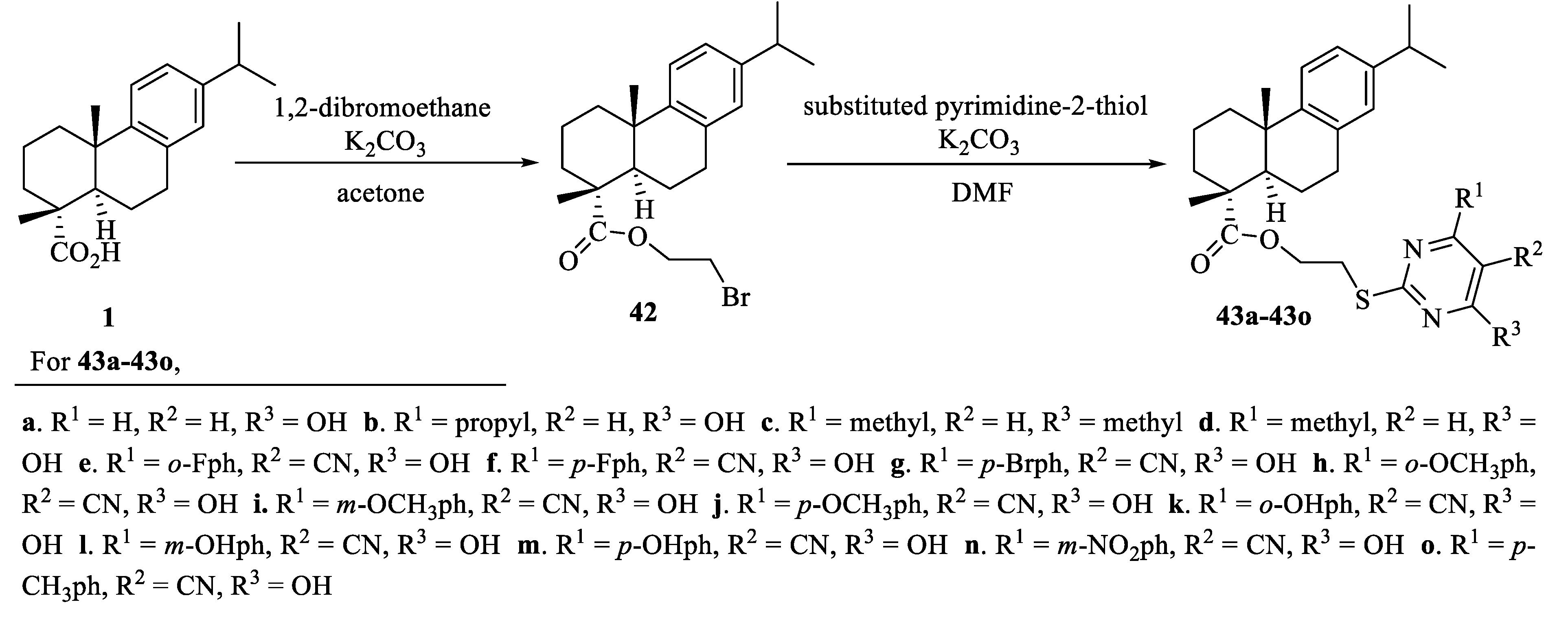


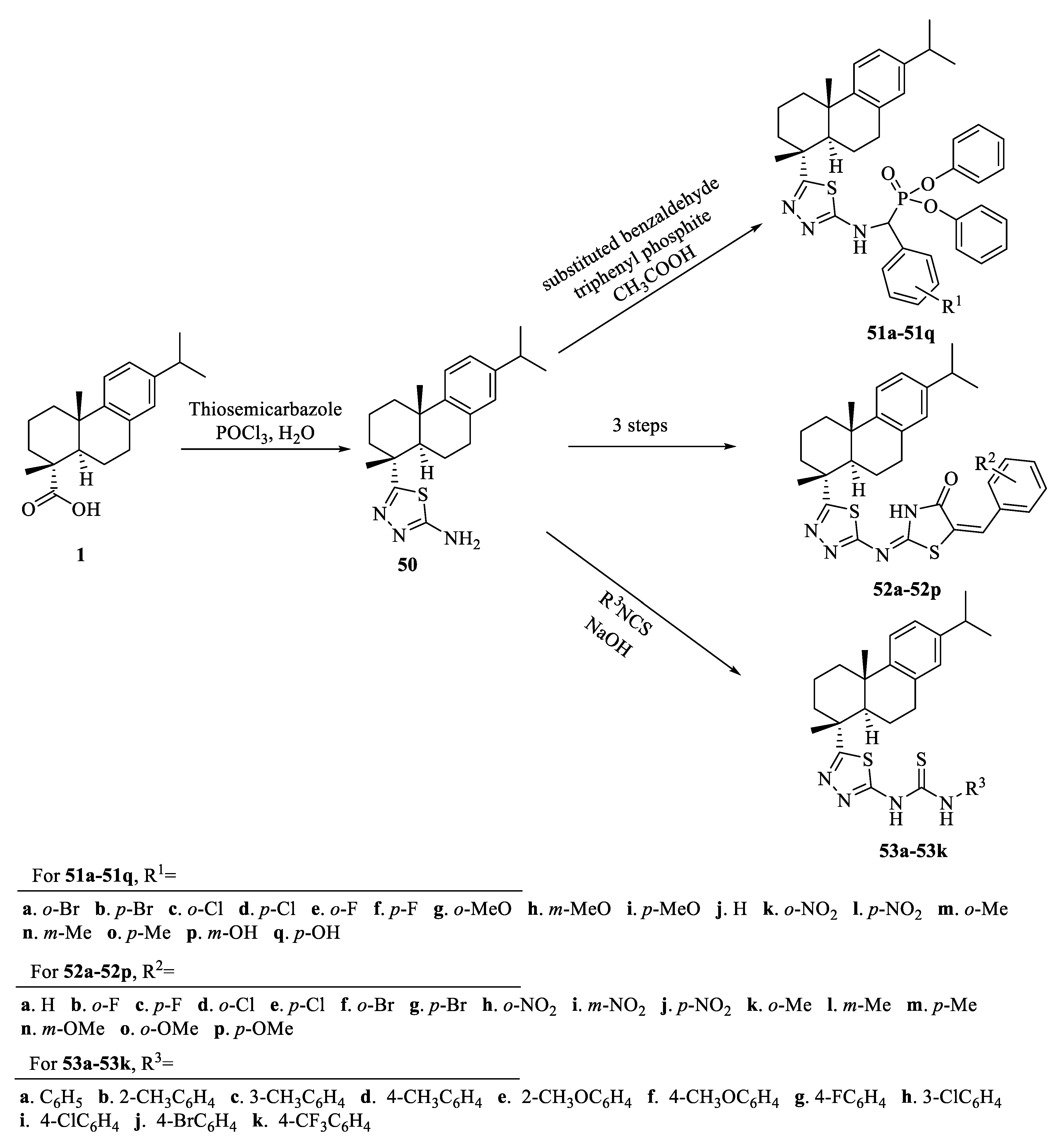

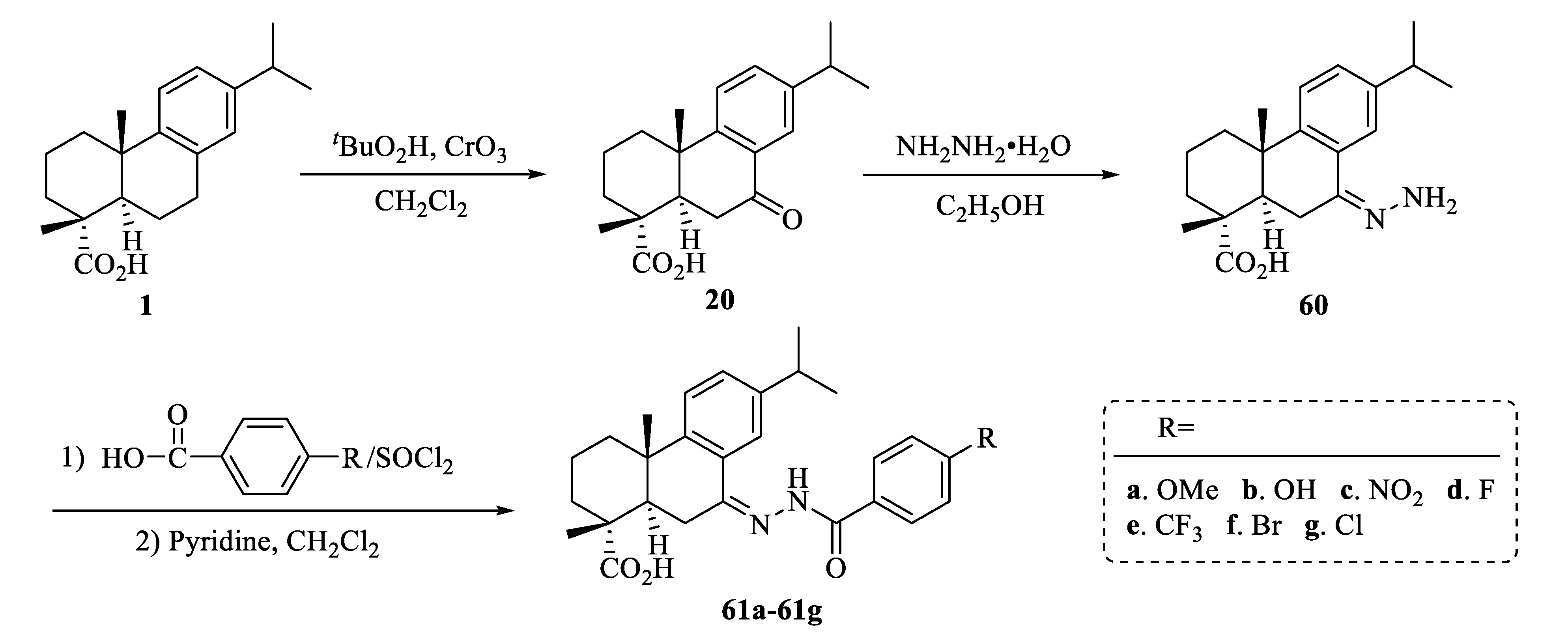
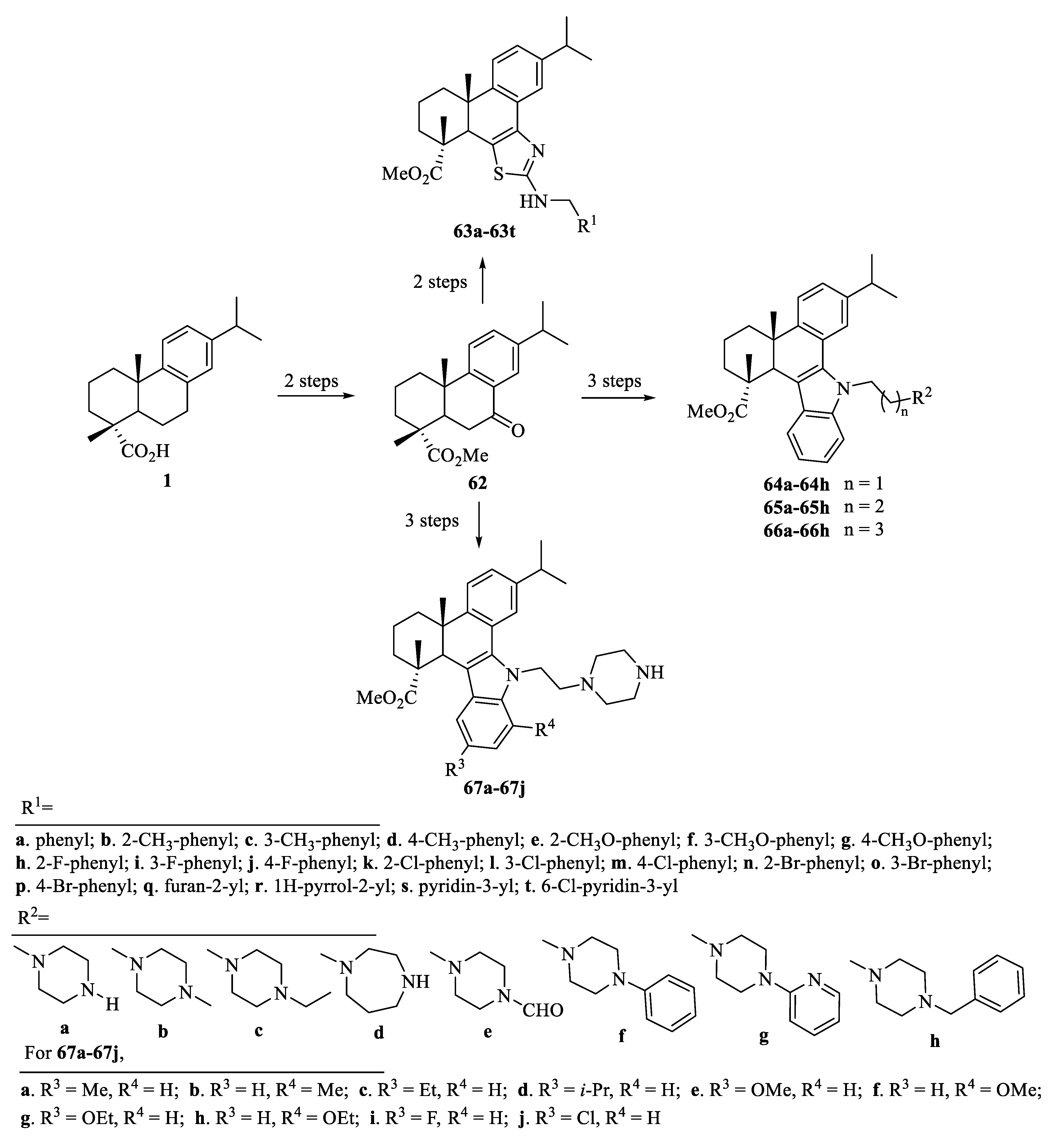
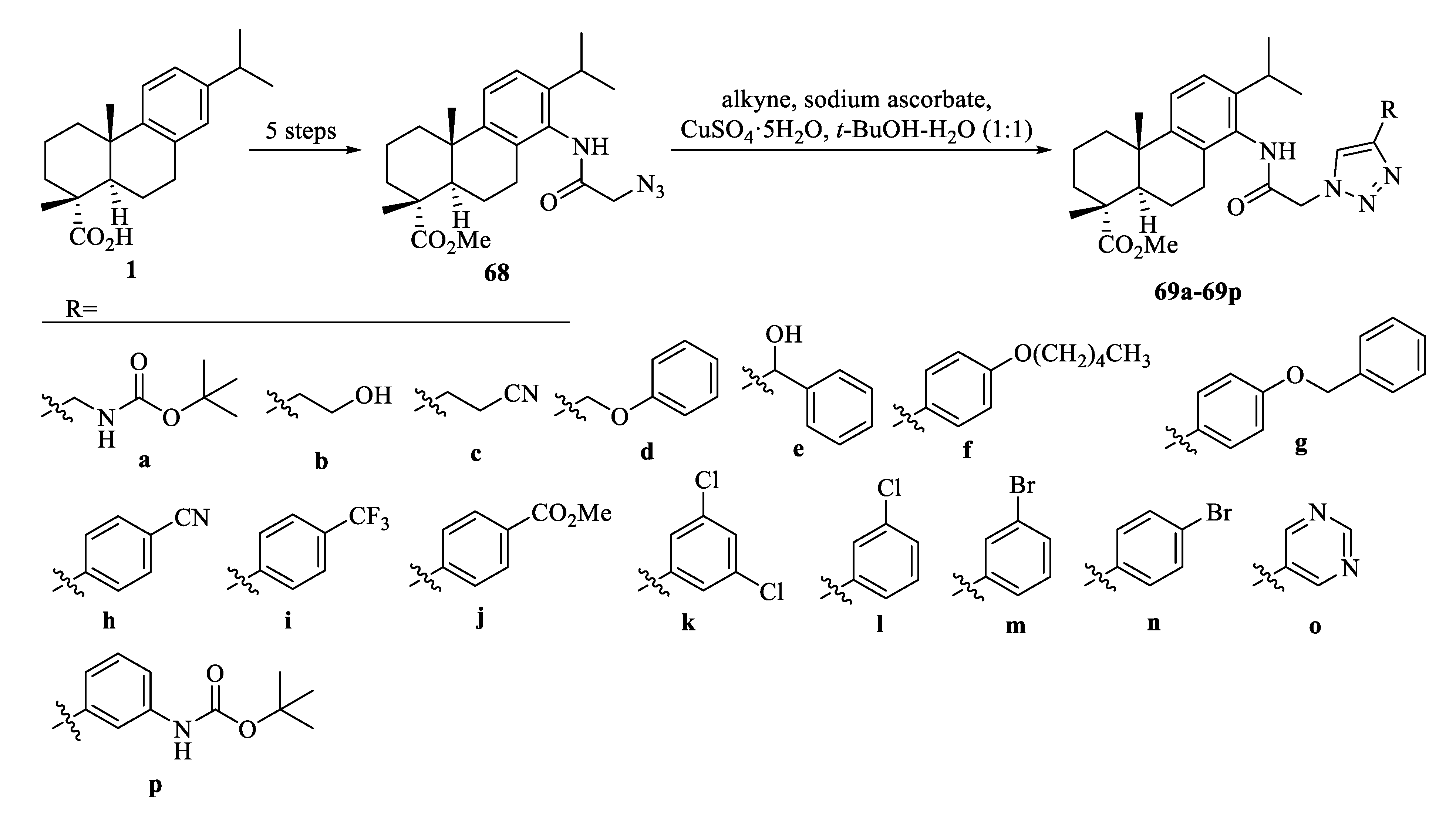
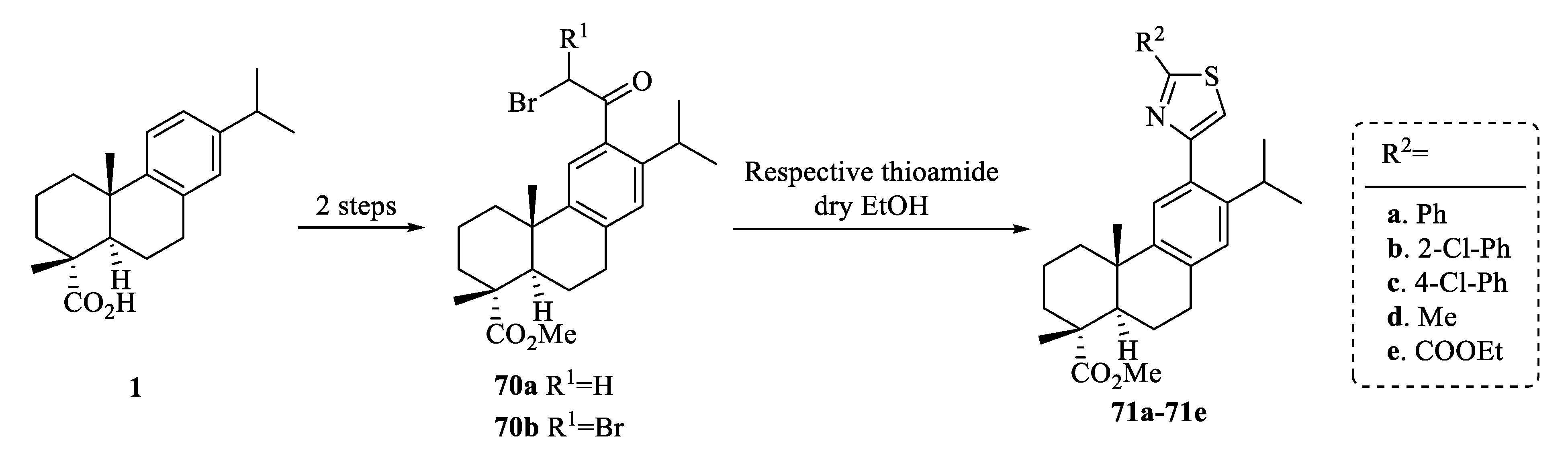


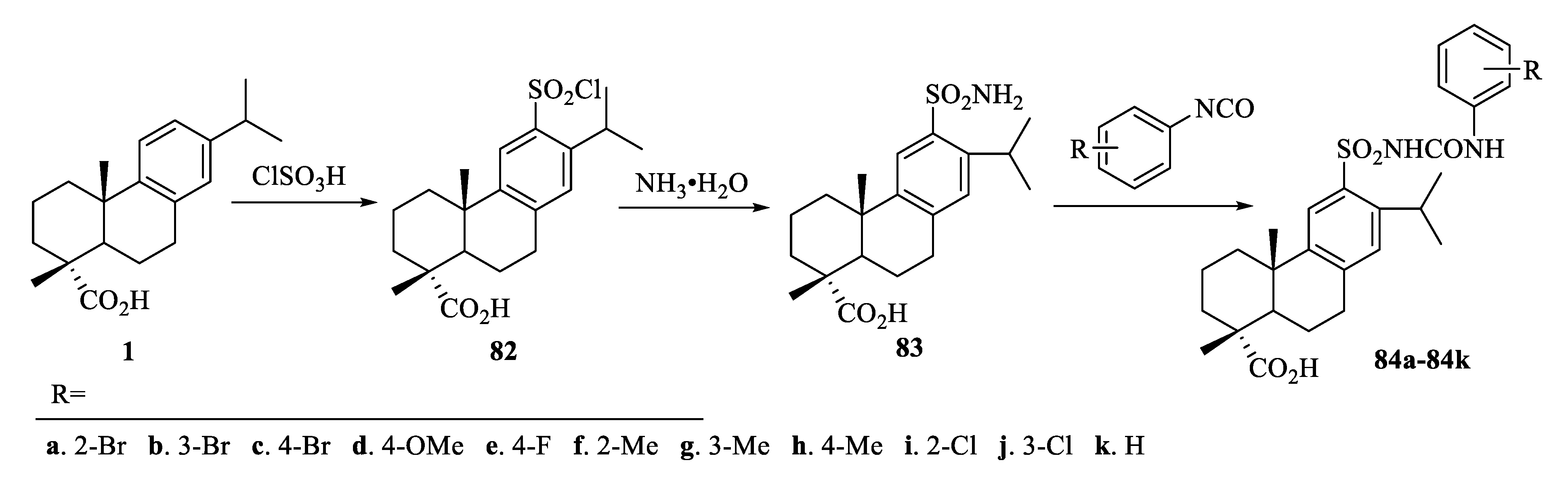
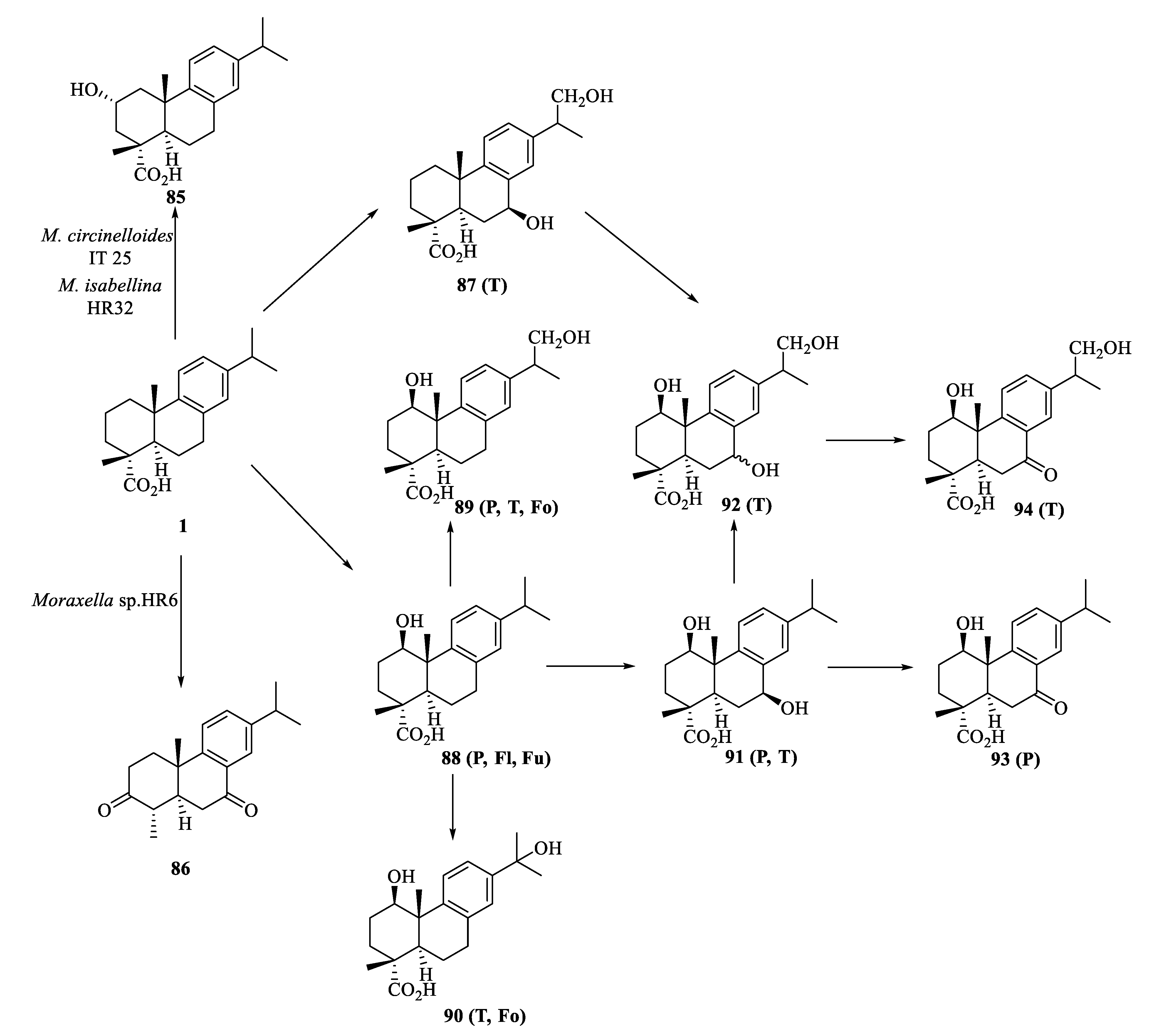

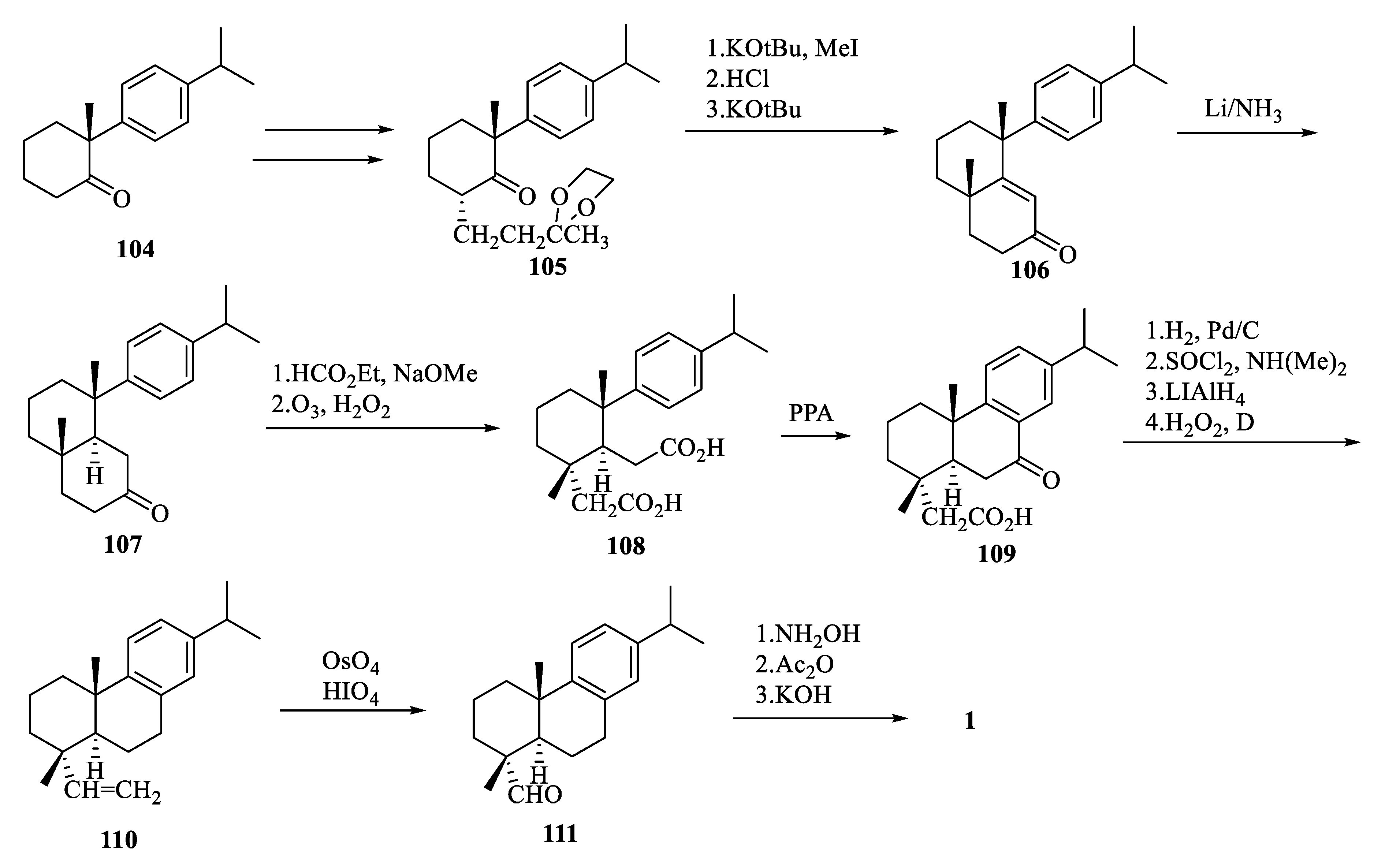

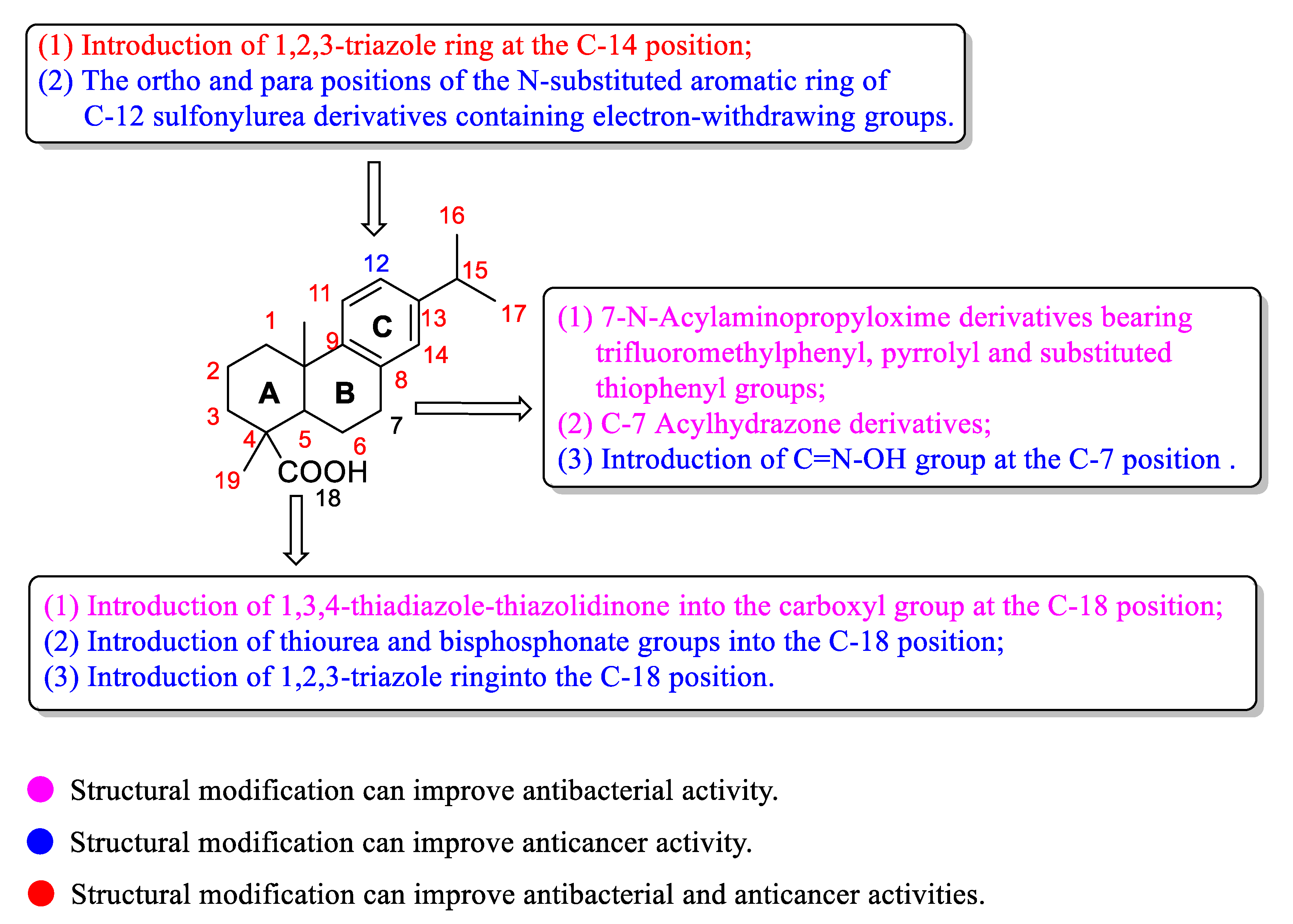
| Compound | Bacteria | MIC Value |
|---|---|---|
| 5 | B. subtilis | 4 μg/mL [36] |
| 5 | S. aureus | 2 μg/mL [36] |
| 7 | methicillin resistant S. aureus | 32 μg/mL [39] |
| 8 | LA-MRSA LGA251(ST425-XI) | 3.9 μg/mL [40] |
| 9 | S. aureus Newman | 0.39–0.78 μg/mL [41] |
| 10 | NRS-70 | 1.25–1.56 μg/mL [41] |
| 11 | S. aureus Newman, NRS-1, NRS-70, NRS-100, NRS-108, and NRS-271 | 1.56–3.13 μg/mL [41] |
| 12 | S. aureus Newman, NRS-70, NRS-108, and NRS-271 | 1.56–3.13 μg/mL [41] |
| Compound | Cancer Cell Line | IC50 Value |
|---|---|---|
| 22f | HeLa | 7.76 ± 0.98 μM [64] |
| 28e | SK-OV-3 | 1.79 ± 0.43 μM [65] |
| 30n | HeLa | 6.58 ± 1.11 μM [66] |
| 36w | HeLa | 2.21 ± 0.04 μM [69] |
| 36w | BEL-7402 | 14.46 ± 0.22 μM [69] |
| 39j | MGC-803 | 3.82 ± 0.18 μM [70] |
| 41c | HepG2 | 5.90 ± 0.41 μM [71] |
| 41k | HepG2 | 6.25 ± 0.37μM [71] |
| 43b | MCF-7 | 7.00 ± 0.96 μM [72] |
| 47n | BEL-7402 | 11.23 ± 0.21 μM [73] |
| 47j | CNE-2 | 8.36 ± 0.14 μM [73] |
| 63r | HepG2 | 24.41 ± 0.26 μM [81] |
| 63s | HepG2 | 22.92 ± 0.24 μM [81] |
| 67g | SMMC-7721 | 1.39 ± 0.13 μM [82] |
| 67g | HepG2 | 0.51 ± 0.09 μM [82] |
| 67g | Hep3B | 0.73 ± 0.08 μM [82] |
| 69p | MDA-MB-231 | 0.7 ± 0.1 μM [84] |
| 74b | SMMC-7721 | 0.36 ± 0.13 μM [86] |
| 74e | HepG2 | 0.12 ± 0.03 μM [86] |
| 77b | SMMC-7721 | 0.72 ± 0.09 μM [87] |
| 79h | MCF-7 | 0.87 ± 0.18 μM [88] |
| 80j | SMMC-7721 | 0.08 ± 0.01 μM [89] |
| 84a | HCT-116 | 1.18 ± 0.52 μM [90] |
| Compound | Bacteria | MIC Value |
|---|---|---|
| 33e | B. subtilis | 1.9 μg/mL [68] |
| 57j | S. aureus NRS-70, NRS-100, NRS-108 and NRS-271 | 1.56–2.5 μg/mL [78] |
| 69o | S. aureus, E. coli and P. fluorescens | 1.6 μg/mL [83] |
| Compound | Human Normal Cell Line | IC50 Value |
|---|---|---|
| 22f | HL-7702 | 53.78 ± 1.4 μM [64] |
| 36w | HL-7702 | 66.08 ± 1.84 μM [69] |
| 39j | LO2 | >100 μM [70] |
| 41c, 41k | HL-7702 | >100 μM [71] |
| 47j | HL-7702, NP69 | >100 μM [73] |
| 47n | NP69 | >100 μM [73] |
| 47n | HL-7702 | 88.18± 0.23 μM [73] |
| 67g | QSG-7701 | 12.52 ± 0.58 μM [80] |
| 74b | LO2 | 3.89 ± 0.29 μM [86] |
| 74e | LO2 | 3.02 ± 0.21 μM [86] |
| 77b | LO2 | 11.09 ± 0.57 μM [87] |
| 79h | LO2 | 42.83 ± 3.18 μM [88] |
| 80j | QSG-7701 | 5.82 ± 0.38 μM [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, M.; Xu, J.; Wen, H.; Du, J.; Zhang, S.; Lv, M.; Xu, H. Recent Advances on Biological Activities and Structural Modifications of Dehydroabietic Acid. Toxins 2022, 14, 632. https://doi.org/10.3390/toxins14090632
Hao M, Xu J, Wen H, Du J, Zhang S, Lv M, Xu H. Recent Advances on Biological Activities and Structural Modifications of Dehydroabietic Acid. Toxins. 2022; 14(9):632. https://doi.org/10.3390/toxins14090632
Chicago/Turabian StyleHao, Meng, Jianwei Xu, Houpeng Wen, Jiawei Du, Shaoyong Zhang, Min Lv, and Hui Xu. 2022. "Recent Advances on Biological Activities and Structural Modifications of Dehydroabietic Acid" Toxins 14, no. 9: 632. https://doi.org/10.3390/toxins14090632
APA StyleHao, M., Xu, J., Wen, H., Du, J., Zhang, S., Lv, M., & Xu, H. (2022). Recent Advances on Biological Activities and Structural Modifications of Dehydroabietic Acid. Toxins, 14(9), 632. https://doi.org/10.3390/toxins14090632