Abstract
Background: Panton–Valentine Leukocidin sustains a strong cytotoxic activity, targeting immune cells and, consequently, perforating the plasma membrane and inducing cell death. The present study is aimed to examine the individual effect of ascorbic acid and nicotinamide on PVL cytotoxicity ex vivo, as well as their effect on granulocytes viability when treated with PVL. Materials and Methods: The PVL cytotoxicity assay was performed in triplicates using the commercial Cytotoxicity Detection Kit PLUS (LDH). LDH release was measured to determine cell damage and cell viability was measured via flow cytometry. Results and discussion: A clear reduction in PVL cytotoxicity was demonstrated (p < 0.001). Treatment with ascorbic acid at 5 mg/mL has shown a 3-fold reduction in PVL cytotoxicity; likewise, nicotinamide illustrated a 4-fold reduction in PVL cytotoxicity. Moreover, granulocytes’ viability after PVL treatment was maintained when incubated with 5 mg/mL of ascorbic acid and nicotinamide. Conclusions: our findings illustrated that ascorbic acid and nicotinamide exhibit an inhibitory effect on PVL cytotoxicity and promote cell viability, as the cytotoxic effect of the toxin is postulated to be neutralized by antioxidant incubation. Further investigations are needed to assess whether these antioxidants may be viable options in PVL cytotoxicity attenuation in PVL-associated diseases.
Key Contribution:
This study illustrates the anti-toxin and cytoprotective role of ascorbic acid and nicotinamide against PVL.
1. Introduction
Panton–Valentine Leukocidin (PVL) is a bi-component pore-forming staphylococcal exotoxin that has shown remarkable emergence attributable to being commonly harbored by epidemic community-associated staphylococcal strains [1]. It sustains a strong cytotoxic activity, targeting immune cells, mainly neutrophils, and to some extent, monocytes and macrophages, perforating the plasma membrane of these immune cells and inducing cell death [2,3]. PVL has been associated with complications in lung injuries, necrotizing pneumonia, skin infections, osteomyelitis, necrotizing fasciitis, and inflammatory cytokine storms [4,5,6,7].
The two components comprising PVL’s β-barrel configuration are the LukS subunit (slow) and the LukF subunit (fast). These components are released by the bacteria into the microenvironment as un-assembled monomers [8]. Initially, the S-subunit binds to the target cell membrane, which prompts the recruitment of the F-subunit; hence, oligomerization may ensue, resulting in the formation of a lytic hetero-octameric pore-forming complex [8,9,10]. Moreover, the main immune cell receptor that mediates LukS-PV binding and PVL cytotoxicity is the C5a anaphylatoxin chemotactic receptor 1 (C5aR1); also, C5aR2 is targeted but with lesser affinity [11].
The mechanism by which PVL induces cell death is not fully understood; however, it has been elucidated that PVL activity induces potassium ions (K+) efflux and, in turn, calcium ions (Ca2+) influx, as well as cathepsin B (CTSB) maturation [12,13], activating NLRP3 inflammasome and leading to the secretion of pro-inflammatory cytokines, such as IL-1β [12,14]. In turn, IL-1β facilitates the upregulation of numerous proinflammatory components such as TNF, IL-6, Phospholipase A2 (PLA-2), the Intracellular Adhesion Molecule -1 (ICAM-1), and Type-2 Cyclooxygenase (COX-2), among others, thus perpetuating the inflammatory effect of PVL [15]. It is worth noting that cellular stress induced by PVL prompts granulocytes to release additional proinflammatory mediators, including leukotriene B4 (LTB4), IL-8, and histamine [16,17].
Despite the substantial epidemiological association between PVL and necrotizing infections, PVL pathophysiology is yet to be fully elucidated. One major hurdle in investigating PVL cytotoxicity is that it exhibits activity in a species-specific manner, as it shows affinity toward human neutrophils but not murine, macaque, or cow receptors [18]. However, few successful models have been developed. PVL-treated rabbit neutrophils have shown somewhat substantial cytotoxic activity, as have humanized mice models (non-obese diabetic (NOD)/severe combined immune deficiency (SCID)/IL2Rγnull (NSG) mice engrafted with primary human hematopoietic cells) in pneumonia and skin infection models [19,20,21].
Recently, antioxidants have gained notoriety for their antimicrobial and anti-inflammatory effects, whether by upregulating anti-inflammatory genes (e.g., β-carotene and lycopene), downregulating bacterial virulence genes (e.g., cholecalciferol and α-tocopherol), or improving the efficacy of current antimicrobial agents (e.g., ascorbic acid, nicotinamide, and α-tocopherol). Indeed, they may provide a viable option for supportive therapy [22,23,24,25,26,27]. In particular, ascorbic acid and nicotinamide may prove to be outstanding candidates. They are cheap, widely available, water-soluble, and have been used in clinical trials for their antimicrobial, anti-inflammatory, and antioxidant properties; henceforth, they provide a reliable option to be considered in this ex vivo experiment.
The aim of this study is to examine the individual effect of ascorbic acid and nicotinamide on PVL cytotoxicity ex vivo, as well as their effect on granulocyte viability when treated with PVL. To our knowledge, these interactions between PVL and vitamins B3 and C have never been investigated before. Nevertheless, we believe that antioxidants can, in fact, enhance the clinical picture of many ailments, with due future research.
2. Results and Discussion
Ascorbic acid, commonly known as vitamin C, is an essential water-soluble micronutrient that is involved in maintaining homeostatic body function, as well as in the absorption of iron, the biosynthesis of carnitine, catecholamines and collagen, and in preventing the development of dental caries [28,29,30]. It is found in fresh vegetables and fruits such as broccoli, tomatoes, strawberries, kiwi, mangoes, citrus fruits, and leafy vegetables [31]. Vitamin C is commonly described as an antioxidant that reduces reactive oxygen species (ROS) and free radicals; however, at high concentrations, it may exhibit prooxidant properties, generating ROS. So, the precise mechanism of action of vitamin C is still debatable [32,33,34].
Vitamin C demonstrated bactericidal and virulence-suppressing effects on hypervirulent Klebsiella pneumoniae, Pseudomonas aeruginosa, Bacillus subtilis, and Escherichia coli by actively suppressing the production of biofilm exopolysaccharides, as well as downregulating biofilm-associated genes and antibiotic resistance genes when treated with subinhibitory concentrations of ascorbic acid [25,34,35,36]. Additionally, vitamin C has been reported to synergize with antibiotics and improve their efficacy against gram-positive and gram-negative bacterial superbugs [27,37].
Nicotinamide is the amide water-soluble form of vitamin B3, manifesting as a precursor for the co-enzyme nicotinamide adenine dinucleotide (NAD+); thus, it is involved in thousands of metabolic pathways [38,39]. Meat, fish, corn, and wheat are the primary natural sources of nicotinamide, while vegetables and fruits may present lesser content [40].
It is a pellagra-preventing agent that demonstrates cytoprotective characteristics in degenerative and immunity-related diseases [41]. Oral supplementation of nicotinamide improved diastolic dysfunction and enhanced myocardial bioenergetics in rodent models [42]. In a clinical trial, nicotinamide mononucleotide supplements increased muscle insulin sensitivity in obese prediabetic women [43]. Moreover, vitamin B3 supplementation modulates neuroprotective roles in Alzheimer’s, Parkinson’s, and Huntington’s diseases, as well as downregulates proinflammatory cascades involving NLRP3 and ASC [44,45]. Nicotinamide also demonstrates virulence-suppressing effects against bacterial pathogens by actively restricting the intracellular growth of Mycobacterium tuberculosis in immune cells, as well as inhibiting biofilm formation and exopolysaccharides production in streptococcus mutans in a rat caries model [46,47].
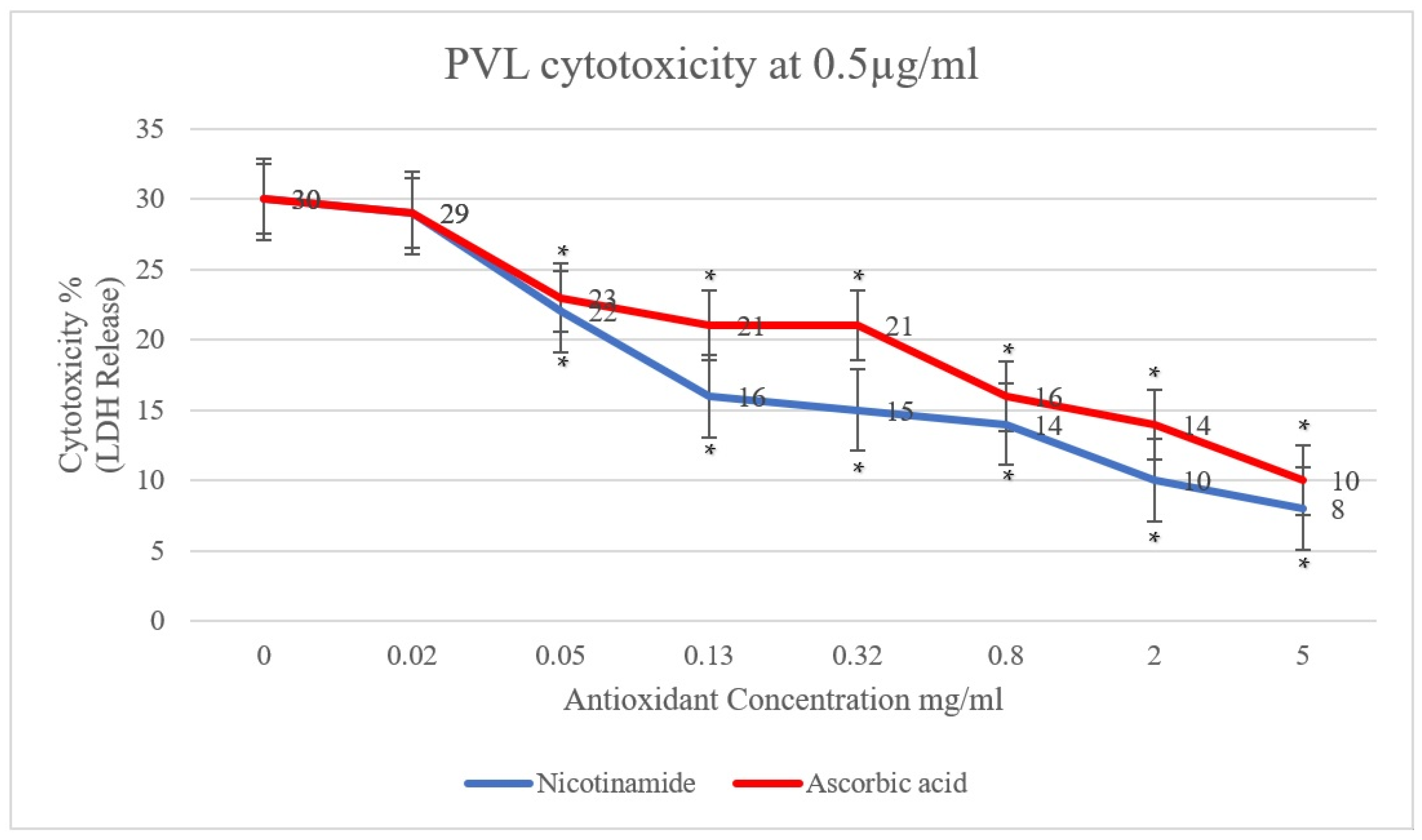
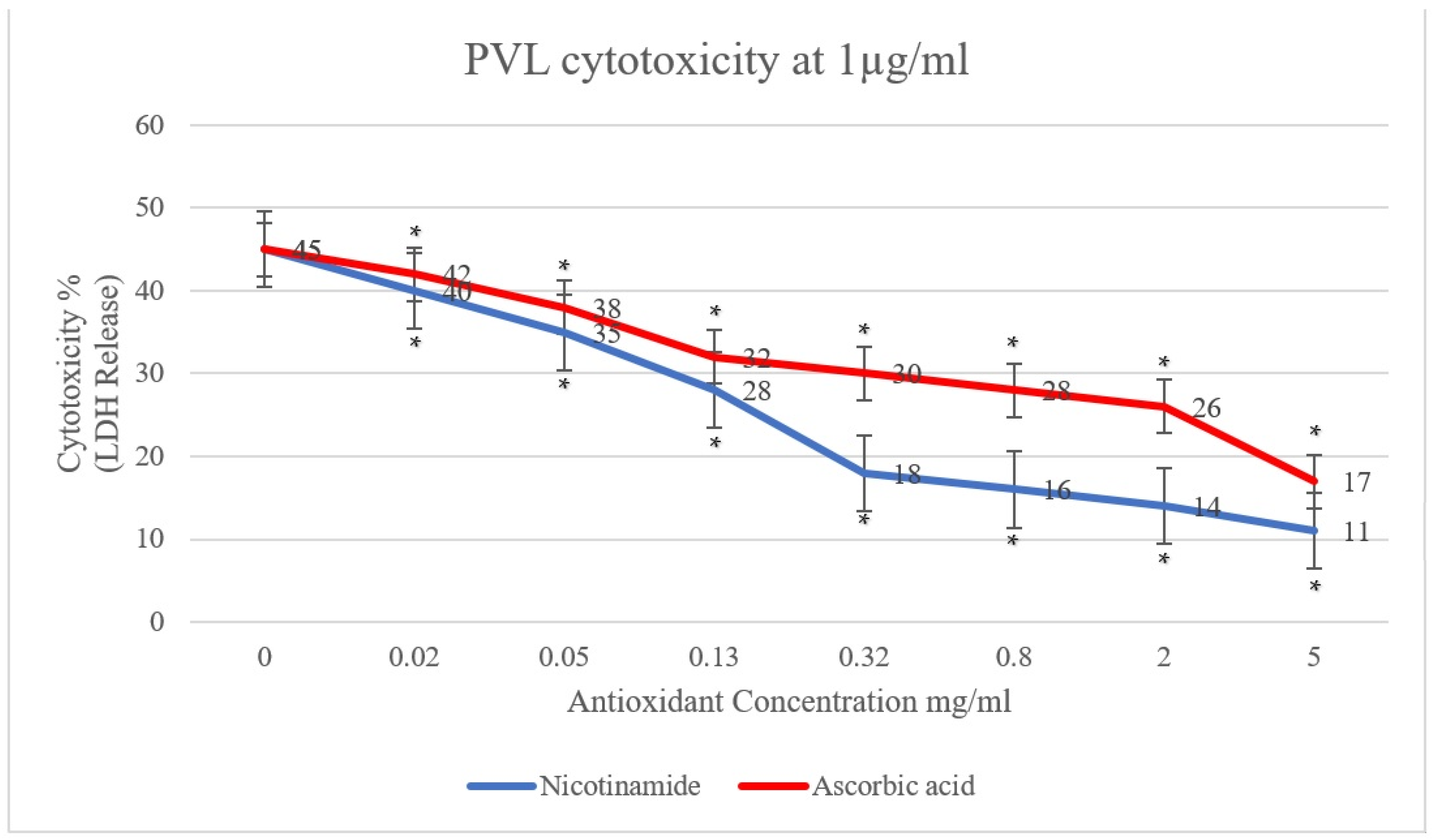
In the present study, the effect of antioxidants (ascorbic acid and nicotinamide) on PVL cytotoxicity was investigated. A clear reduction in PVL cytotoxicity was demonstrated, positively proportional to the concentration of ascorbic acid and nicotinamide individually (p < 0.001). As shown in Figure 1, PVL cytotoxicity at a toxin concentration of 0.5 µg/mL decreased from 30% to 10% and 8% when incubated with 5 mg/mL of ascorbic acid and nicotinamide, respectively (p < 0.001). Additionally, at a PVL concentration of 1 µg/mL (Figure 2), cytotoxicity decreased from 45% to 17% and 11% when incubated with 5 mg/mL of ascorbic acid and nicotinamide, respectively (p < 0.001). It is worth noting that the concentrations of ascorbic acid and nicotinamide used in this study represent a range of sub-inhibitory concentrations for PVL-producing S. aureus. Indeed, the reported minimum inhibitory concentration (MIC), S. aureus was 10–5 mg/mL and 60–30 mg/mL for ascorbic acid and nicotinamide, respectively [27].
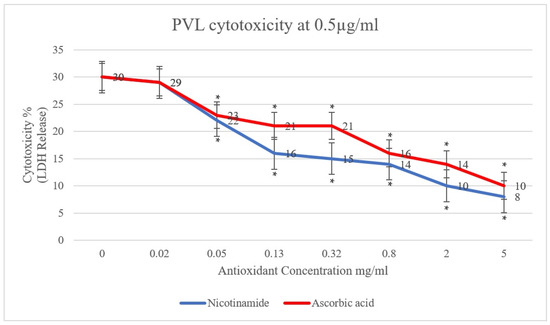
Figure 1.
PVL (0.5 µg/mL) cytotoxicity rates were determined through LDH released from white blood cells (WBCs) via the commercial Cytotoxicity Detection Kit PLUS. WBCs were incubated with serial dilutions of ascorbic acid and nicotinamide (5, 2, 0.8, 0.32, 0.13, 0.05, and 0.02 mg/mL) individually. At 0 mg/mL, the assay included WBCs and PVL only. All assays were conducted in triplicates. A t-test was conducted to determine the statistical significance of antioxidant treatment when compared with the control (0 mg/mL). Statistical significance (p < 0.001) is represented by an asterisk.
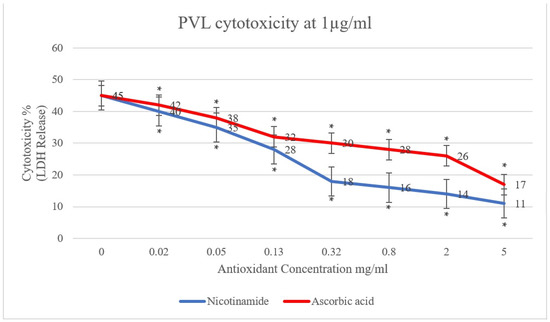
Figure 2.
PVL (1 µg/mL) cytotoxicity rates determined through LDH released from white blood cells (WBCs) via the commercial Cytotoxicity Detection Kit PLUS. WBCs were incubated with serial dilutions of ascorbic acid and nicotinamide (5, 2, 0.8, 0.32, 0.13, 0.05, and 0.02 mg/mL) individually. At 0 mg/mL, the assay included WBCs and PVL only. All assays were conducted in triplicates. A t-test was conducted to determine the statistical significance of antioxidant treatment when compared with the control (0 mg/mL). Statistical significance (p < 0.001) is represented by an asterisk.
The cytotoxic effect of PVL on granulocytes has been reported at concentrations as low as 5 ng/mL in vitro; in fact, neutrophil necrosis was induced with PVL treatment at a concentration of 0.04 µg/mL [16,19]. Additionally, PVL in clinical specimens was reported to range from 0.27 to 2 µg/mL when analyzing pus samples [48]. So, in this study, using two concentrations (0.5 and 1 µg/mL) in the mid-range of PVL concentrations in clinical specimens would provide a reasonable indication of the effectivity of the tested antioxidants. Furthermore, treatment with ascorbic acid at 5 mg/mL has shown a 3-fold consistent reduction in PVL cytotoxicity; likewise, 5 mg/mL of nicotinamide illustrated a 4-fold reduction in PVL cytotoxicity, as seen in Supplementary Tables S1 and S2. It is worth noting that a statistically significant reduction in PVL cytotoxicity with lower concentrations of the tested antioxidant has been demonstrated down to 0.05 mg/mL (Supplementary Tables S1 and S2).
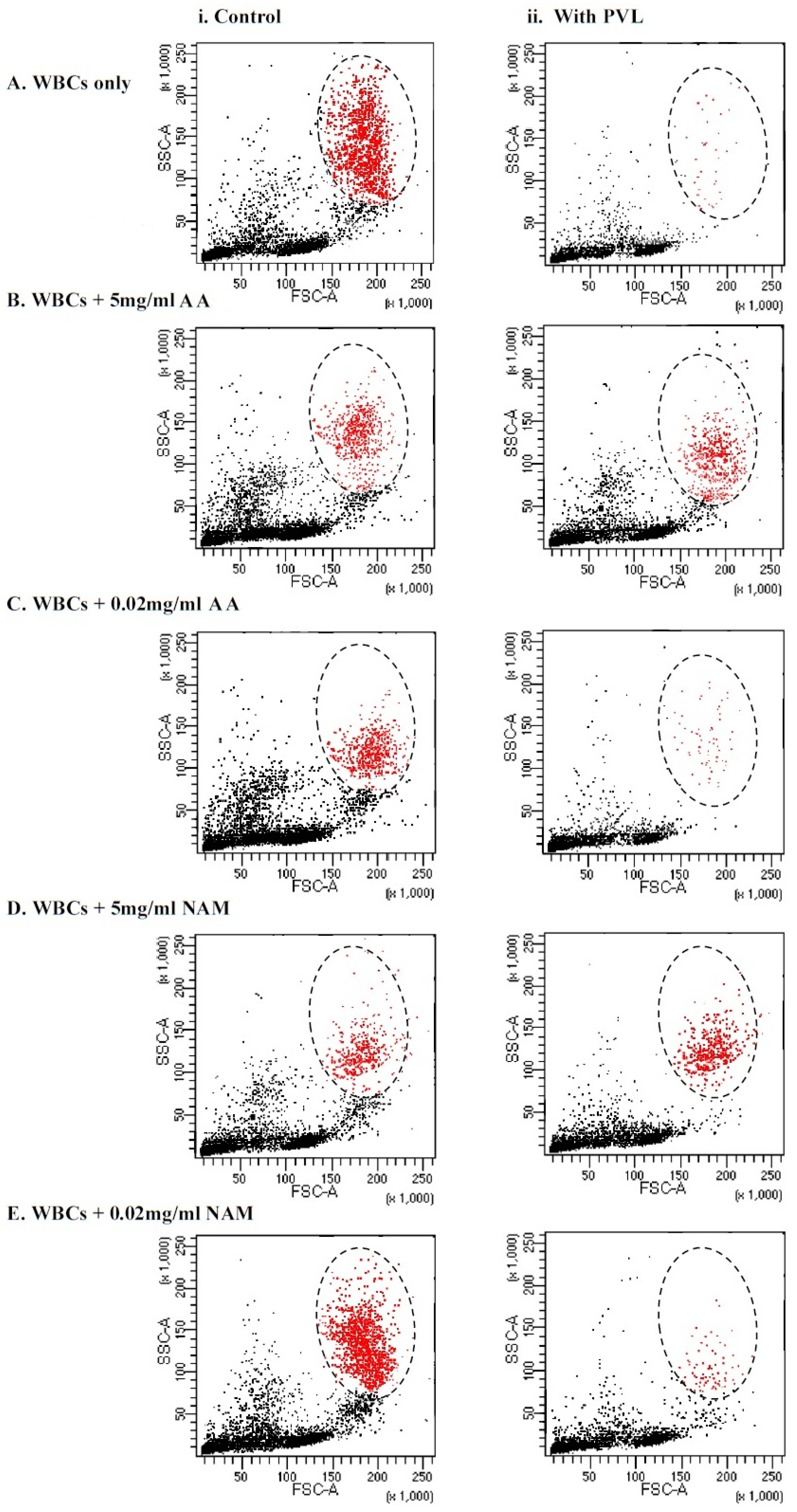
In the current study, we tested whether antioxidant incubation would improve the viability of granulocytes after PVL treatment via flow cytometry size distribution, the dot plot shown in Figure 3. As shown in Table 1, no statistically significant difference in granulocytes viability after PVL treatment was demonstrated when incubated with 5 mg/mL of ascorbic acid and nicotinamide, whereas when using a low concentration of antioxidants (0.02 mg/mL), a significant difference was demonstrated (p < 0.001). This finding may illustrate that the cytotoxic effect of the toxin was neutralized by antioxidant incubation at a concentration of 5 mg/mL, making the difference in viability between the two assays statistically insignificant. Notably, incubating WBCs with higher concentrations (25 mg/mL and 10 mg/mL) of ascorbic acid and nicotinamide induced cell death without PVL challenge (data not shown).
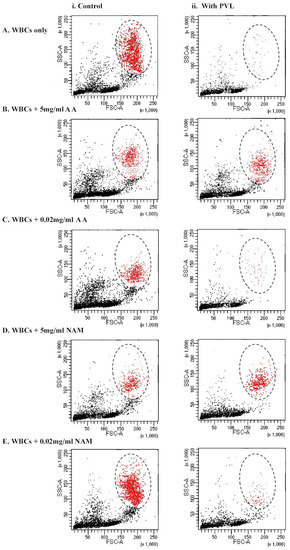
Figure 3.
Immunophenotyping of tested WBCs based on forward (FSC) and side (SSC) scatter size distribution gating. Granulocytes are colored in red and the zone of selection is illustrated by an oval. (i) The assays on the left illustrate controls that did not undergo the toxin incubation. (ii) The assays on the right illustrate test experiments where the cells were treated with 1 µg/mL of PVL for a 2 h incubation period. (A) WBCs that were not incubated with antioxidants—0 mg/mL. (B) WBCs that were incubated with 5 mg/mL of VC. (C) WBCs that were incubated with 0.02 mg/mL of VC. (D) WBCs that were incubated with 5 mg/mL of NAM. (E) WBCs that were incubated with 0.02 mg/mL of NAM. WBCs = white blood cells, PVL = Panton–Valentine Leukocidin, AA = ascorbic acid, and NAM = nicotinamide.

Table 1.
Granulocyte viability rates with or without PVL incubation were determined through forward (FSC) and side (SSC) scatter gating. Cells were incubated with 5, 0.02, and 0 mg/mL of AA or NAM. An Independent t-test was conducted to test the statistical significance of PVL treatment difference with the control (significant when p < 0.001).
Furthermore, a positive association between granulocyte viability and antioxidant incubation was determined, as granulocytes are three times more likely to be viable when incubated with 5 mg/mL of ascorbic acid and nicotinamide (OR 2.8 and 3, respectively, p < 0.01), as seen in Table 1. The cytoprotective mechanism of ascorbic acid and nicotinamide remains to be fully explained. However, many studies have illustrated this function in many cell types; for example, ascorbic acid contributed to neutrophil function recovery in furunculosis patients with defective neutrophil function [49]. In another report, ascorbic acid demonstrated amelioration of liver damage in the amoebic liver abscess (ALA) hamster model [50]. Similarly, vitamin B3 cytoprotective properties were illustrated in a stroke model study where nicotinamide ameliorated brain cell damage after focal ischemia-reperfusion [51]. Additionally, vitamin B3 increased cell viability in high glucose-treated corneal epithelial cells [52].
Unfortunately, the effect of antioxidants on the cytotoxicity of staphylococcal synergohymenotropic toxins, such as PVL, has not been investigated extensively in the literature, so we can only postulate the reason behind our findings. PVL pore-forming complex binds to C5aR1/2 on immune cells [11]. Through NLRP3 activation, the cytotoxic paradigm of PVL is suggested to be conducted [12]. Though PVL pathology and the exact cytotoxic molecular mechanism are not fully elucidated, in some instances, PVL is associated with ROS production that ultimately leads to DNA damage and cell death [53,54]. It is well-established that vitamin C and B3 have the ability to scavenge ROS and free radicals formed intracellularly [32,55]. For instance, oral and topical nicotinamide inhibited skin photocarcinogenesis that is mediated by ROS produced via UV radiations in murine models [56]. Additionally, ascorbic acid administration significantly reduced oxidative stress in the hippocampus stemming from lipid peroxidation and nitrite production in rat models after pilocarpine treatment [57]. Thus, neutralizing ROS produced from toxin treatment may have played a role in the demonstrated reduction of PVL cytotoxicity.
Furthermore, PVL cytotoxicity is associated with the release of proinflammatory cytokines, such as IL-1β, through the activation of NLRP3 inflammasome [3,12]. Many studies illustrated the inhibitory effect of vitamins B3 and C on certain components of the NLRP3 activation cascade or the mechanism overall. For instance, nicotinamide attenuated the activity of US Standard Endotoxin (EC-5), substantially reducing the production of the proinflammatory cytokines IL-1β, IL-6, and IL-8 in whole blood assays [58]. Additionally, vitamin B3 administration correlated with the downregulation of NLRP3, ASC, IL-6, and caspase-1 in diabetic mice models [45]. Similarly, ascorbic acid inhibited the inflammatory effect of lipopolysaccharide (LPS-G) antigen on human gingival mesenchymal stem cells (hGMSCs), subsequently reducing the expression of NLRP3, caspase-1, and IL-1β [59].
In conclusion, Panton–Valentine Leukocidin is a staphylococcal exotoxin that is associated with the severity of SSTIs, joint infections, and deep-seated abscesses, regardless of methicillin resistance. Liability of PVL-associated conditions transcended common risk factors of staphylococcal infections, affecting young immunocompetent individuals without underlying conditions.
Eliminating bacterial pathogens should not be the only aim in managing PVL-associated conditions. The removal of toxins, suppressing toxin production, and neutralizing the toxic effects of PVL are additional important objectives that may result in successful treatment [60]. In the current study, our findings illustrated that ascorbic acid and nicotinamide exhibit an inhibitory effect on PVL cytotoxicity and promote cell viability. Further investigations are needed to assess whether these antioxidants may be used as viable options in PVL cytotoxicity attenuation in PVL-associated diseases.
3. Materials and Methods
3.1. White Blood Cell Isolation
Peripheral blood was collected from 8 healthy donors via venipuncture in purple-capped EDTA K3 tubes (BIOTA-PCE3K8, Biota, Istanbul, Turkey). White blood cells were isolated through a gradient of Histopaque 1077 (Sigma-Aldrich, St. Louis, MO, USA), as recommended by the manufacturer’s datasheet, and pooled. Trypan blue exclusion microscopy (ab233465, Abcam, Hong Kong, China) was used to ascertain the viability of harvested WBCs (a range of 95–99%) before commencing the ‘assay procedure’. Harvested WBCs (1 × 106 cells/mL) were incubated in RPMI 1640 (R0883-6X1L, Sigma-Aldrich, MO, USA) without additives and used immediately.
3.2. Panton–Valentine Leukocidin (PVL)
Purified subunits of PVL were purchased from Integrated BioTherapeutics, Rockville, MD, USA, LukS-PV (Cat#0530-001) and LukF-PV (Cat#0536-001). A 5 µg/mL PVL stock was prepared by combining both subunits in PBS + 15% Glycerol.
3.3. Antioxidant Preparation
Stocks (20 mg/mL) of ascorbic acid (#A92902, Sigma-Aldrich, MO, USA) and nicotinamide (#N3376, Sigma-Aldrich, MO, USA) were prepared in RPMI 1640 and pH was adjusted to neutral (pH = 7.2).
3.4. Assay Procedure
WBCs (1 × 106 cells/mL) were incubated with a 2.5-fold serial dilution of ascorbic acid or nicotinamide (5, 2, 0.8, 0.32, 0.13, 0.05, and 0.02 mg/mL) in RPMI 1640 for 2 h at 37 °C and 5% CO2. Then, PVL (LuKS/F-PV) was added at a concentration of 0.5 µg/mL or 1 µg/mL to the WBCs and incubated for an additional 2 h at 37 °C and 5% CO2. Afterward, viability measurement and a cytotoxicity assay were conducted. Appropriate controls were used in each step.
Note: Determining the optimum assay procedure required a period of trials in the early stages of the study. Challenging WBCs with PVL (0.5 and 1 µg/mL) before, with, and after antioxidant treatment (starting concentrations of 25, 10, and 5 mg/mL) were attempted at 10 min, 30 min, 1 h, 2 h, and 3 h. At 10 min, no measurable changes occurred. Challenging the cells with PVL prior to antioxidant treatment (for 30 min and more) resulted in the release of LDH into the enclosed microenvironment, covering any potential ameliorating effect caused by the antioxidant (the OD was 2.5–3 at 650 nm). Likewise, applying the toxin with the antioxidant at the same time demonstrated the same observations. At 2 and 3 h incubations, the results were very similar, statistically showing no significant difference. All in all, we pursued the method that provided a clear, reliable, and repeatable association between the toxin and the antioxidant.
3.5. Cytotoxicity Assay
After the ‘assay procedure’ incubation periods, the cytotoxicity assay was performed in triplicates using the Cytotoxicity Detection Kit PLUS (LDH) (Cat#04744934001, Roche Diagnostics, Mannheim, Germany), according to the manufacturer’s manual. This assay is based on the measurement of lactate dehydrogenase (LDH) activity released from the damaged cell.
Three controls were included: the background control (media only), the low control (untreated cells), and the high control (cells treated with a lysis buffer for maximum LDH release).
To determine the experimental absorbance values, the average absorbance values of the triplicate samples and controls were calculated and subtracted from the absorbance values of the background control. The absorbance was measured via a FLUOstar® OMEGA Plate Reader (BMG LABTECH, Ortenberg, Germany) at 490 nm, and the reference wavelength was 650 nm.
The cytotoxicity rate was determined using the following equation:
3.6. Viability Measurement
After the ‘assay procedure’ incubation periods, WBCs were washed thrice and suspended in 1X PBS. A total of 10,000 events were gated and analyzed via a BD FACSCanto™ Flow Cytometer (Becton and Dickinson (BD), Franklin Lakes, NJ, USA) based on the forward scatter (FSC) and the side scatter (SSC) parameters. The viability of granulocytes was determined through size distribution (FSC X SSC). Viability was calculated by the following equation:
3.7. Statistical Analysis
Calculations and statistical analysis (descriptive statistics, unpaired t-tests, and odds ratios) were conducted using Microsoft Excel 365. All experiments were performed in triplicates and the data are expressed as Mean ± SD whenever applicable. Differences between values were considered to be significant when the p-value was <0.001. The odds ratio was used to determine the efficacy of the antioxidant treatment for the viability of PVL-challenged cells.
The odds ratio calculation was performed according to the following formula:
where
| Treatment status | PVL-challenged | Without PVL |
| Antioxidant treatment | a | b |
| Without antioxidant treatment | c | d |
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/toxins15010038/s1, Table S1: PVL (0.5 µg/mL) cytotoxicity rates determined through LDH release, Table S2: PVL (1 µg/mL) cytotoxicity rates determined through LDH release.
Author Contributions
Methodology, A.A., M.S. and E.F.; Validation, E.F.; Formal analysis, A.A.; Writing—original draft, A.A. and M.S.; Writing—review and editing, A.A., M.S., E.F. and K.B.; Supervision, M.S.; Project administration, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that there are no conflict of interest.
Ethical Statement
This study was approved by the Arabian Gulf University’s Research and Ethics Committee (REC), and also by the Research Technical Support Team (RTST) in the Ministry of Health, Bahrain. Bilingual informed consent (Arabic and English) was issued for all donors; opting out of this study was a clear option for all participants.
References
- Otto, M. Community-associated MRSA: What makes them special? Int. J. Med. Microbiol. IJMM 2013, 303, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Panton, P.N.; Valentine, F.C.O. Staphylococcal Toxin. Lancet 1932, 219, 506–508. [Google Scholar] [CrossRef]
- Spaan, A.N.; van Strijp, J.A.G.; Torres, V.J. Leukocidins: Staphylococcal bi-component pore-forming toxins find their receptors. Nat. Rev. Microbiol. 2017, 15, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Diep, B.A.; Chan, L.; Tattevin, P.; Kajikawa, O.; Martin, T.R.; Basuino, L.; Mai, T.T.; Marbach, H.; Braughton, K.R.; Whitney, A.R.; et al. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc. Natl. Acad. Sci. USA 2010, 107, 5587–5592. [Google Scholar] [CrossRef] [PubMed]
- Lipinska, U.; Hermans, K.; Meulemans, L.; Dumitrescu, O.; Badiou, C.; Duchateau, L.; Haesebrouck, F.; Etienne, J.; Lina, G. Panton-Valentine leukocidin does play a role in the early stage of Staphylococcus aureus skin infections: A rabbit model. PLoS ONE 2011, 6, e22864. [Google Scholar] [CrossRef]
- Cremieux, A.C.; Dumitrescu, O.; Lina, G.; Vallee, C.; Cote, J.F.; Muffat-Joly, M.; Lilin, T.; Etienne, J.; Vandenesch, F.; Saleh-Mghir, A. Panton-valentine leukocidin enhances the severity of community-associated methicillin-resistant Staphylococcus aureus rabbit osteomyelitis. PLoS ONE 2009, 4, e7204. [Google Scholar] [CrossRef]
- Perbet, S.; Soummer, A.; Vinsonneau, C.; Vandebrouck, A.; Rackelboom, T.; Etienne, J.; Cariou, A.; Chiche, J.D.; Mira, J.P.; Charpentier, J. Multifocal community-acquired necrotizing fasciitis caused by a Panton-Valentine leukocidin-producing methicillin-sensitive Staphylococcus aureus. Infection 2010, 38, 223–225. [Google Scholar] [CrossRef]
- Colin, D.A.; Mazurier, I.; Sire, S.; Finck-Barbancon, V. Interaction of the two components of leukocidin from Staphylococcus aureus with human polymorphonuclear leukocyte membranes: Sequential binding and subsequent activation. Infect. Immun. 1994, 62, 3184–3188. [Google Scholar] [CrossRef]
- Peraro, M.D.; van der Goot, F.G. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. Microbiol. 2016, 14, 77–92. [Google Scholar] [CrossRef]
- Jayasinghe, L.; Bayley, H. The leukocidin pore: Evidence for an octamer with four LukF subunits and four LukS subunits alternating around a central axis. Protein Sci. A Publ. Protein Soc. 2005, 14, 2550–2561. [Google Scholar] [CrossRef]
- Spaan, A.N.; Henry, T.; van Rooijen, W.J.M.; Perret, M.; Badiou, C.; Aerts, P.C.; Kemmink, J.; de Haas, C.J.C.; van Kessel, K.P.M.; Vandenesch, F.; et al. The staphylococcal toxin Panton-Valentine Leukocidin targets human C5a receptors. Cell Host. Microbe. 2013, 13, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, D.; Gieldon, L.; Mysore, V.; Nippe, N.; Taxman, D.J.; Duncan, J.A.; Broglie, P.M.; Marketon, K.; Austermann, J.; Vogl, T.; et al. Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome. J. Leukoc. Biol. 2012, 92, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Meunier, O.; Falkenrodt, A.; Monteil, H.; Colin, D.A. Application of flow cytometry in toxinology: Pathophysiology of human polymorphonuclear leukocytes damaged by a pore-forming toxin from Staphylococcus aureus. Cytometry 1995, 21, 241–247. [Google Scholar] [CrossRef]
- Chevriaux, A.; Pilot, T.; Derangere, V.; Simonin, H.; Martine, P.; Chalmin, F.; Ghiringhelli, F.; Rebe, C. Cathepsin B Is Required for NLRP3 Inflammasome Activation in Macrophages, Through NLRP3 Interaction. Front. Cell Dev. Biol. 2020, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef]
- Konig, B.; Prevost, G.; Piemont, Y.; Konig, W. Effects of Staphylococcus aureus leukocidins on inflammatory mediator release from human granulocytes. J. Infect. Dis. 1995, 171, 607–613. [Google Scholar] [CrossRef]
- Konig, B.; Prevost, G.; Konig, W. Composition of staphylococcal bi-component toxins determines pathophysiological reactions. J. Med. Microbiol. 1997, 46, 479–485. [Google Scholar] [CrossRef]
- Spaan, A.N.; Schiepers, A.; de Haas, C.J.; van Hooijdonk, D.D.; Badiou, C.; Contamin, H.; Vandenesch, F.; Lina, G.; Gerard, N.P.; Gerard, C.; et al. Differential Interaction of the Staphylococcal Toxins Panton-Valentine Leukocidin and gamma-Hemolysin CB with Human C5a Receptors. J. Immunol. 2015, 195, 1034–1043. [Google Scholar] [CrossRef]
- Loffler, B.; Hussain, M.; Grundmeier, M.; Bruck, M.; Holzinger, D.; Varga, G.; Roth, J.; Kahl, B.C.; Proctor, R.A.; Peters, G. Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 2010, 6, e1000715. [Google Scholar] [CrossRef]
- Prince, A.; Wang, H.; Kitur, K.; Parker, D. Humanized Mice Exhibit Increased Susceptibility to Staphylococcus aureus Pneumonia. J. Infect. Dis. 2017, 215, 1386–1395. [Google Scholar] [CrossRef]
- Tseng, C.W.; Biancotti, J.C.; Berg, B.L.; Gate, D.; Kolar, S.L.; Muller, S.; Rodriguez, M.D.; Rezai-Zadeh, K.; Fan, X.; Beenhouwer, D.O.; et al. Increased Susceptibility of Humanized NSG Mice to Panton-Valentine Leukocidin and Staphylococcus aureus Skin Infection. PLoS Pathog. 2015, 11, e1005292. [Google Scholar] [CrossRef] [PubMed]
- Kawata, A.; Murakami, Y.; Suzuki, S.; Fujisawa, S. Anti-inflammatory Activity of beta-Carotene, Lycopene and Tri-n-butylborane, a Scavenger of Reactive Oxygen Species. In Vivo 2018, 32, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.S.; Dombrowski, Y.; Koglin, S.; Ruzicka, T.; Schauber, J. Impact of vitamin D3 on cutaneous immunity and antimicrobial peptide expression. Derm.-Endocrinol. 2011, 3, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Tintino, S.R.; Morais-Tintino, C.D.; Campina, F.F.; Pereira, R.L.; Costa Mdo, S.; Braga, M.F.; Limaverde, P.W.; Andrade, J.C.; Siqueira-Junior, J.P.; Coutinho, H.D.; et al. Action of cholecalciferol and alpha-tocopherol on Staphylococcus aureus efflux pumps. EXCLI J. 2016, 15, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.; Ravikumar, V.; Abdel-Haleem, A.M.; Derouiche, A.; Mokkapati, V.; Sihlbom, C.; Mineta, K.; Gojobori, T.; Gao, X.; Westerlund, F.; et al. Low Concentrations of Vitamin C Reduce the Synthesis of Extracellular Polymers and Destabilize Bacterial Biofilms. Front. Microbiol. 2017, 8, 2599. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.M.; Valvano, M.A. Vitamin E Increases Antimicrobial Sensitivity by Inhibiting Bacterial Lipocalin Antibiotic Binding. mSphere 2018, 3, e00564-18. [Google Scholar] [CrossRef]
- AlSaleh, A.; Shahid, M.; Farid, E.; Kamal, N.; Bindayna, K. Synergistic antimicrobial effect of ascorbic acid and nicotinamide with rifampicin and vancomycin against SCCmec type IV Methicillin-Resistant Staphylococcus aureus (MRSA). Access Microbiol. 2022. [Google Scholar] [CrossRef]
- Mandl, J.; Szarka, A.; Banhegyi, G. Vitamin C: Update on physiology and pharmacology. Br. J. Pharmacol. 2009, 157, 1097–1110. [Google Scholar] [CrossRef]
- Kraemer, K.; Semba, R.D.; Eggersdorfer, M.; Schaumberg, D.A. Introduction: The diverse and essential biological functions of vitamins. Ann. Nutr. Metab. 2012, 61, 185–191. [Google Scholar] [CrossRef]
- Eydou, Z.; Jad, B.N.; Elsayed, Z.; Ismail, A.; Magaogao, M.; Hossain, A. Investigation on the effect of vitamin C on growth & biofilm-forming potential of Streptococcus mutans isolated from patients with dental caries. BMC Microbiol. 2020, 20, 231. [Google Scholar] [CrossRef]
- Farris, K. Cosmeceutical vitamins: Vitamin C. In Cosmeceuticals, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, pp. 37–42. [Google Scholar]
- Slade Hutton, D.; Knox Grace, A. Nutrition and the role of reducing agents in the formation of streptolysin o by a group a hemolytic streptococcus. J. Bacteriol. 1950, 60, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Vilcheze, C.; Hartman, T.; Weinrick, B.; Jacobs, W.R., Jr. Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nat. Commun. 2013, 4, 1881. [Google Scholar] [CrossRef] [PubMed]
- Shivaprasad, D.P.; Taneja, N.K.; Lakra, A.; Sachdev, D. In vitro and in situ abrogation of biofilm formation in E. coli by vitamin C through ROS generation, disruption of quorum sensing and exopolysaccharide production. Food Chem. 2021, 341, 128171. [Google Scholar] [CrossRef]
- Xu, C.; Dong, N.; Chen, K.; Yang, X.; Zeng, P.; Hou, C.; Chi Chan, E.W.; Yao, X.; Chen, S. Bactericidal, anti-biofilm, and anti-virulence activity of vitamin C against carbapenem-resistant hypervirulent Klebsiella pneumoniae. iScience 2022, 25, 103894. [Google Scholar] [CrossRef]
- Abdelraheem, W.M.; Refaie, M.M.M.; Yousef, R.K.M.; Abd El Fatah, A.S.; Mousa, Y.M.; Rashwan, R. Assessment of Antibacterial and Anti-biofilm Effects of Vitamin C Against Pseudomonas aeruginosa Clinical Isolates. Front. Microbiol. 2022, 13, 1594. [Google Scholar] [CrossRef]
- Kallio, J.; Jaakkola, M.; Mäki, M.; Kilpeläinen, P.; Virtanen, V. Vitamin C Inhibits Staphylococcus aureus Growth and Enhances the Inhibitory Effect of Quercetin on Growth of Escherichia coli In Vitro. Planta Med. 2012, 78, 1824–1830. [Google Scholar] [CrossRef] [PubMed]
- Mehmel, M.; Jovanovic, N.; Spitz, U. Nicotinamide Riboside-The Current State of Research and Therapeutic Uses. Nutrients 2020, 12, 1616. [Google Scholar] [CrossRef]
- Revollo, J.R.; Grimm, A.A.; Imai, S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J. Biol. Chem. 2004, 279, 50754–50763. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), 5–16. [Google Scholar] [CrossRef]
- Maiese, K.; Chong, Z.Z.; Hou, J.; Shang, Y.C. The vitamin nicotinamide: Translating nutrition into clinical care. Molecules 2009, 14, 3446–3485. [Google Scholar] [CrossRef]
- Abdellatif, M.; Trummer-Herbst, V.; Koser, F.; Durand, S.; Adao, R.; Vasques-Novoa, F.; Freundt, J.K.; Voglhuber, J.; Pricolo, M.R.; Kasa, M.; et al. Nicotinamide for the treatment of heart failure with preserved ejection fraction. Sci. Transl. Med. 2021, 13, abd7064. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, M.; Yoshino, J.; Kayser, B.D.; Patti, G.J.; Franczyk, M.P.; Mills, K.F.; Sindelar, M.; Pietka, T.; Patterson, B.W.; Imai, S.I.; et al. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science 2021, 372, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Fricker, R.A.; Green, E.L.; Jenkins, S.I.; Griffin, S.M. The Influence of Nicotinamide on Health and Disease in the Central Nervous System. Int. J. Tryptophan Res. IJTR 2018, 11, 1178646918776658. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Yang, S.J. Supplementation with Nicotinamide Riboside Reduces Brain Inflammation and Improves Cognitive Function in Diabetic Mice. Int. J. Mol. Sci. 2019, 20, 4196. [Google Scholar] [CrossRef] [PubMed]
- Simmons, J.D.; Peterson, G.J.; Campo, M.; Lohmiller, J.; Skerrett, S.J.; Tunaru, S.; Offermanns, S.; Sherman, D.R.; Hawn, T.R. Nicotinamide Limits Replication of Mycobacterium tuberculosis and Bacille Calmette-Guerin Within Macrophages. J. Infect. Dis. 2020, 221, 989–999. [Google Scholar] [CrossRef]
- Lin, Y.; Gong, T.; Ma, Q.; Jing, M.; Zheng, T.; Yan, J.; Chen, J.; Pan, Y.; Sun, Q.; Zhou, X.; et al. Nicotinamide could reduce growth and cariogenic virulence of Streptococcus mutans. J. Oral Microbiol. 2022, 14, 2056291. [Google Scholar] [CrossRef]
- Badiou, C.; Dumitrescu, O.; Croze, M.; Gillet, Y.; Dohin, B.; Slayman, D.H.; Allaouchiche, B.; Etienne, J.; Vandenesch, F.; Lina, G. Panton-Valentine leukocidin is expressed at toxic levels in human skin abscesses. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2008, 14, 1180–1183. [Google Scholar] [CrossRef][Green Version]
- Levy, R.; Shriker, O.; Porath, A.; Riesenberg, K.; Schlaeffer, F. Vitamin C for the Treatment of Recurrent Furunculosis in Patients with Impaired Neutrophil Functions. J. Infect. Dis. 1996, 173, 1502–1505. [Google Scholar] [CrossRef]
- Cruz-Baquero, A.; Jarillo-Luna, R.A.; Cárdenas-Jaramillo, L.M.; Drago-Serrano, M.E.; Serrano-Luna, J.d.J.; Pacheco-Yépez, J. Ascorbic Acid Ameriolates Liver Damage by Myeloperoxidase Oxidative Products in a Hamster Model of Amoebic Liver Abscess. Front. Cell. Infect. Microbiol. 2022, 12, 855822. [Google Scholar] [CrossRef]
- Yang, J.; Klaidman, L.K.; Chang, M.L.; Kem, S.; Sugawara, T.; Chan, P.; Adams, J.D. Nicotinamide therapy protects against both necrosis and apoptosis in a stroke model. Pharmacol. Biochem. Behav. 2002, 73, 901–910. [Google Scholar] [CrossRef]
- Pu, Q.; Guo, X.-X.; Hu, J.-J.; Li, A.-L.; Li, G.-G.; Li, X.-Y. Nicotinamide mononucleotide increases cell viability and restores tight junctions in high-glucose-treated human corneal epithelial cells via the SIRT1/Nrf2/HO-1 pathway. Biomed. Pharmacother. 2022, 147, 112659. [Google Scholar] [CrossRef] [PubMed]
- Genestier, A.L.; Michallet, M.C.; Prevost, G.; Bellot, G.; Chalabreysse, L.; Peyrol, S.; Thivolet, F.; Etienne, J.; Lina, G.; Vallette, F.M.; et al. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J. Clin. Investig. 2005, 115, 3117–3127. [Google Scholar] [CrossRef] [PubMed]
- Boyle-Vavra, S.; Daum, R.S. Community-acquired methicillin-resistant Staphylococcus aureus: The role of Panton-Valentine leukocidin. Lab. Investig. A J. Tech. Methods Pathol. 2007, 87, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Gehring, W. Nicotinic acid/niacinamide and the skin. J. Cosmet. Dermatol. 2004, 3, 88–93. [Google Scholar] [CrossRef]
- Gensler, H.L.; Williams, T.; Huang, A.C.; Jacobson, E.L. Oral niacin prevents photocarcinogenesis and photoimmunosuppression in mice. Nutr. Cancer 1999, 34, 36–41. [Google Scholar] [CrossRef]
- Santos, I.M.; Tome Ada, R.; Saldanha, G.B.; Ferreira, P.M.; Militao, G.C.; Freitas, R.M. Oxidative stress in the hippocampus during experimental seizures can be ameliorated with the antioxidant ascorbic acid. Oxidative Med. Cell. Longev. 2009, 2, 214–221. [Google Scholar] [CrossRef]
- Ungerstedt, J.S.; Blomback, M.; Soderstrom, T. Nicotinamide is a potent inhibitor of proinflammatory cytokines. Clin. Exp. Immunol. 2003, 131, 48–52. [Google Scholar] [CrossRef]
- Pizzicannella, J.; Fonticoli, L.; Guarnieri, S.; Marconi, G.D.; Rajan, T.S.; Trubiani, O.; Diomede, F. Antioxidant Ascorbic Acid Modulates NLRP3 Inflammasome in LPS-G Treated Oral Stem Cells through NFkappaB/Caspase-1/IL-1beta Pathway. Antioxidants 2021, 10, 797. [Google Scholar] [CrossRef]
- Gillet, Y.; Dumitrescu, O.; Tristan, A.; Dauwalder, O.; Javouhey, E.; Floret, D.; Vandenesch, F.; Etienne, J.; Lina, G. Pragmatic management of Panton-Valentine leukocidin-associated staphylococcal diseases. Int. J. Antimicrob. Agents 2011, 38, 457–464. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).