The Effect of Ascorbic Acid and Nicotinamide on Panton–Valentine Leukocidin Cytotoxicity: An Ex Vivo Study
Abstract
:1. Introduction
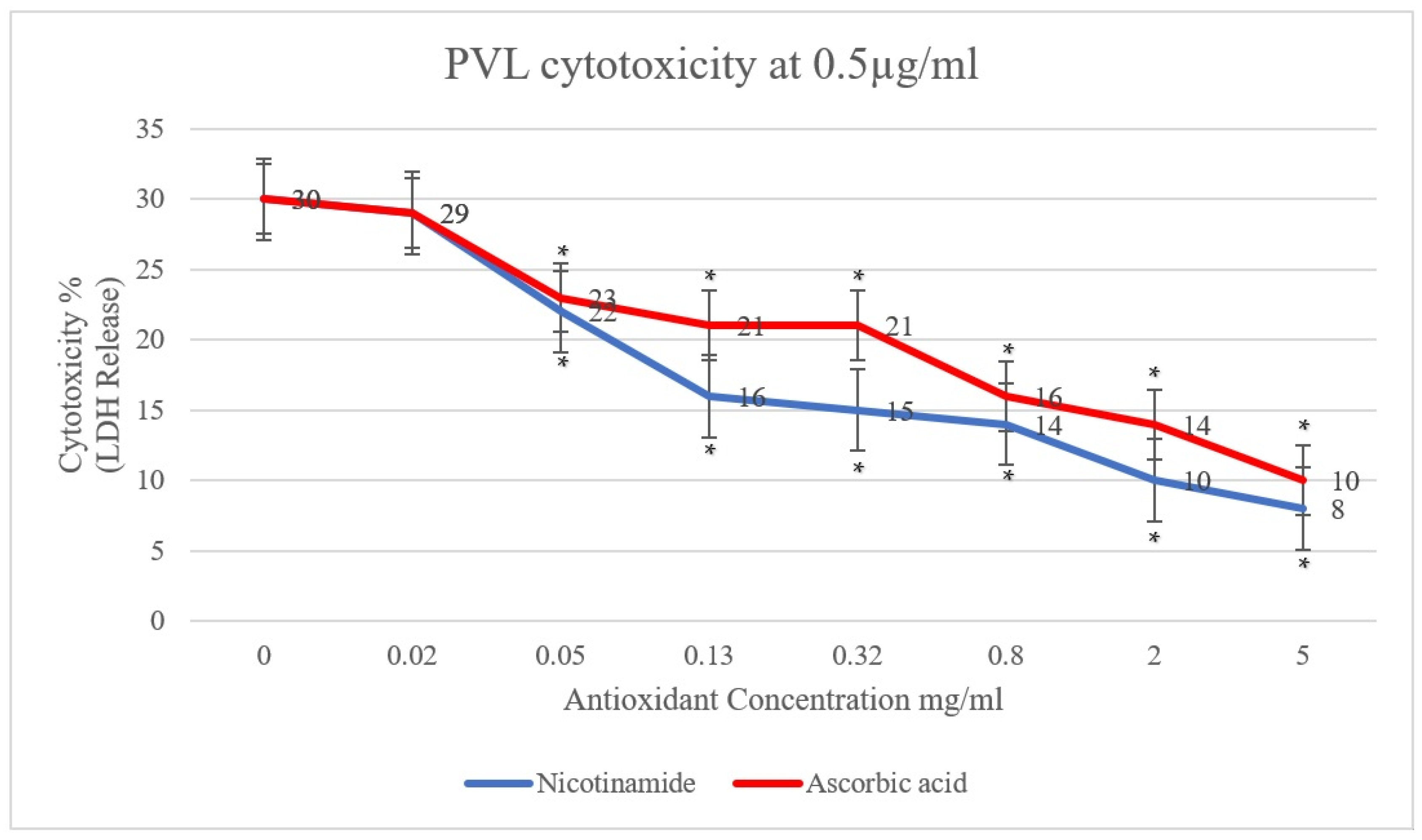
2. Results and Discussion
3. Materials and Methods
3.1. White Blood Cell Isolation
3.2. Panton–Valentine Leukocidin (PVL)
3.3. Antioxidant Preparation
3.4. Assay Procedure
3.5. Cytotoxicity Assay
3.6. Viability Measurement
3.7. Statistical Analysis
| Treatment status | PVL-challenged | Without PVL |
| Antioxidant treatment | a | b |
| Without antioxidant treatment | c | d |
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethical Statement
References
- Otto, M. Community-associated MRSA: What makes them special? Int. J. Med. Microbiol. IJMM 2013, 303, 324–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panton, P.N.; Valentine, F.C.O. Staphylococcal Toxin. Lancet 1932, 219, 506–508. [Google Scholar] [CrossRef]
- Spaan, A.N.; van Strijp, J.A.G.; Torres, V.J. Leukocidins: Staphylococcal bi-component pore-forming toxins find their receptors. Nat. Rev. Microbiol. 2017, 15, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Diep, B.A.; Chan, L.; Tattevin, P.; Kajikawa, O.; Martin, T.R.; Basuino, L.; Mai, T.T.; Marbach, H.; Braughton, K.R.; Whitney, A.R.; et al. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc. Natl. Acad. Sci. USA 2010, 107, 5587–5592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipinska, U.; Hermans, K.; Meulemans, L.; Dumitrescu, O.; Badiou, C.; Duchateau, L.; Haesebrouck, F.; Etienne, J.; Lina, G. Panton-Valentine leukocidin does play a role in the early stage of Staphylococcus aureus skin infections: A rabbit model. PLoS ONE 2011, 6, e22864. [Google Scholar] [CrossRef]
- Cremieux, A.C.; Dumitrescu, O.; Lina, G.; Vallee, C.; Cote, J.F.; Muffat-Joly, M.; Lilin, T.; Etienne, J.; Vandenesch, F.; Saleh-Mghir, A. Panton-valentine leukocidin enhances the severity of community-associated methicillin-resistant Staphylococcus aureus rabbit osteomyelitis. PLoS ONE 2009, 4, e7204. [Google Scholar] [CrossRef] [Green Version]
- Perbet, S.; Soummer, A.; Vinsonneau, C.; Vandebrouck, A.; Rackelboom, T.; Etienne, J.; Cariou, A.; Chiche, J.D.; Mira, J.P.; Charpentier, J. Multifocal community-acquired necrotizing fasciitis caused by a Panton-Valentine leukocidin-producing methicillin-sensitive Staphylococcus aureus. Infection 2010, 38, 223–225. [Google Scholar] [CrossRef]
- Colin, D.A.; Mazurier, I.; Sire, S.; Finck-Barbancon, V. Interaction of the two components of leukocidin from Staphylococcus aureus with human polymorphonuclear leukocyte membranes: Sequential binding and subsequent activation. Infect. Immun. 1994, 62, 3184–3188. [Google Scholar] [CrossRef] [Green Version]
- Peraro, M.D.; van der Goot, F.G. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. Microbiol. 2016, 14, 77–92. [Google Scholar] [CrossRef]
- Jayasinghe, L.; Bayley, H. The leukocidin pore: Evidence for an octamer with four LukF subunits and four LukS subunits alternating around a central axis. Protein Sci. A Publ. Protein Soc. 2005, 14, 2550–2561. [Google Scholar] [CrossRef]
- Spaan, A.N.; Henry, T.; van Rooijen, W.J.M.; Perret, M.; Badiou, C.; Aerts, P.C.; Kemmink, J.; de Haas, C.J.C.; van Kessel, K.P.M.; Vandenesch, F.; et al. The staphylococcal toxin Panton-Valentine Leukocidin targets human C5a receptors. Cell Host. Microbe. 2013, 13, 584–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holzinger, D.; Gieldon, L.; Mysore, V.; Nippe, N.; Taxman, D.J.; Duncan, J.A.; Broglie, P.M.; Marketon, K.; Austermann, J.; Vogl, T.; et al. Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome. J. Leukoc. Biol. 2012, 92, 1069–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meunier, O.; Falkenrodt, A.; Monteil, H.; Colin, D.A. Application of flow cytometry in toxinology: Pathophysiology of human polymorphonuclear leukocytes damaged by a pore-forming toxin from Staphylococcus aureus. Cytometry 1995, 21, 241–247. [Google Scholar] [CrossRef]
- Chevriaux, A.; Pilot, T.; Derangere, V.; Simonin, H.; Martine, P.; Chalmin, F.; Ghiringhelli, F.; Rebe, C. Cathepsin B Is Required for NLRP3 Inflammasome Activation in Macrophages, Through NLRP3 Interaction. Front. Cell Dev. Biol. 2020, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef]
- Konig, B.; Prevost, G.; Piemont, Y.; Konig, W. Effects of Staphylococcus aureus leukocidins on inflammatory mediator release from human granulocytes. J. Infect. Dis. 1995, 171, 607–613. [Google Scholar] [CrossRef]
- Konig, B.; Prevost, G.; Konig, W. Composition of staphylococcal bi-component toxins determines pathophysiological reactions. J. Med. Microbiol. 1997, 46, 479–485. [Google Scholar] [CrossRef]
- Spaan, A.N.; Schiepers, A.; de Haas, C.J.; van Hooijdonk, D.D.; Badiou, C.; Contamin, H.; Vandenesch, F.; Lina, G.; Gerard, N.P.; Gerard, C.; et al. Differential Interaction of the Staphylococcal Toxins Panton-Valentine Leukocidin and gamma-Hemolysin CB with Human C5a Receptors. J. Immunol. 2015, 195, 1034–1043. [Google Scholar] [CrossRef] [Green Version]
- Loffler, B.; Hussain, M.; Grundmeier, M.; Bruck, M.; Holzinger, D.; Varga, G.; Roth, J.; Kahl, B.C.; Proctor, R.A.; Peters, G. Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 2010, 6, e1000715. [Google Scholar] [CrossRef]
- Prince, A.; Wang, H.; Kitur, K.; Parker, D. Humanized Mice Exhibit Increased Susceptibility to Staphylococcus aureus Pneumonia. J. Infect. Dis. 2017, 215, 1386–1395. [Google Scholar] [CrossRef]
- Tseng, C.W.; Biancotti, J.C.; Berg, B.L.; Gate, D.; Kolar, S.L.; Muller, S.; Rodriguez, M.D.; Rezai-Zadeh, K.; Fan, X.; Beenhouwer, D.O.; et al. Increased Susceptibility of Humanized NSG Mice to Panton-Valentine Leukocidin and Staphylococcus aureus Skin Infection. PLoS Pathog. 2015, 11, e1005292. [Google Scholar] [CrossRef] [PubMed]
- Kawata, A.; Murakami, Y.; Suzuki, S.; Fujisawa, S. Anti-inflammatory Activity of beta-Carotene, Lycopene and Tri-n-butylborane, a Scavenger of Reactive Oxygen Species. In Vivo 2018, 32, 255–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antal, A.S.; Dombrowski, Y.; Koglin, S.; Ruzicka, T.; Schauber, J. Impact of vitamin D3 on cutaneous immunity and antimicrobial peptide expression. Derm.-Endocrinol. 2011, 3, 18–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tintino, S.R.; Morais-Tintino, C.D.; Campina, F.F.; Pereira, R.L.; Costa Mdo, S.; Braga, M.F.; Limaverde, P.W.; Andrade, J.C.; Siqueira-Junior, J.P.; Coutinho, H.D.; et al. Action of cholecalciferol and alpha-tocopherol on Staphylococcus aureus efflux pumps. EXCLI J. 2016, 15, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.; Ravikumar, V.; Abdel-Haleem, A.M.; Derouiche, A.; Mokkapati, V.; Sihlbom, C.; Mineta, K.; Gojobori, T.; Gao, X.; Westerlund, F.; et al. Low Concentrations of Vitamin C Reduce the Synthesis of Extracellular Polymers and Destabilize Bacterial Biofilms. Front. Microbiol. 2017, 8, 2599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naguib, M.M.; Valvano, M.A. Vitamin E Increases Antimicrobial Sensitivity by Inhibiting Bacterial Lipocalin Antibiotic Binding. mSphere 2018, 3, e00564-18. [Google Scholar] [CrossRef] [Green Version]
- AlSaleh, A.; Shahid, M.; Farid, E.; Kamal, N.; Bindayna, K. Synergistic antimicrobial effect of ascorbic acid and nicotinamide with rifampicin and vancomycin against SCCmec type IV Methicillin-Resistant Staphylococcus aureus (MRSA). Access Microbiol. 2022. [Google Scholar] [CrossRef]
- Mandl, J.; Szarka, A.; Banhegyi, G. Vitamin C: Update on physiology and pharmacology. Br. J. Pharmacol. 2009, 157, 1097–1110. [Google Scholar] [CrossRef] [Green Version]
- Kraemer, K.; Semba, R.D.; Eggersdorfer, M.; Schaumberg, D.A. Introduction: The diverse and essential biological functions of vitamins. Ann. Nutr. Metab. 2012, 61, 185–191. [Google Scholar] [CrossRef]
- Eydou, Z.; Jad, B.N.; Elsayed, Z.; Ismail, A.; Magaogao, M.; Hossain, A. Investigation on the effect of vitamin C on growth & biofilm-forming potential of Streptococcus mutans isolated from patients with dental caries. BMC Microbiol. 2020, 20, 231. [Google Scholar] [CrossRef]
- Farris, K. Cosmeceutical vitamins: Vitamin C. In Cosmeceuticals, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, pp. 37–42. [Google Scholar]
- Slade Hutton, D.; Knox Grace, A. Nutrition and the role of reducing agents in the formation of streptolysin o by a group a hemolytic streptococcus. J. Bacteriol. 1950, 60, 301–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilcheze, C.; Hartman, T.; Weinrick, B.; Jacobs, W.R., Jr. Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nat. Commun. 2013, 4, 1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shivaprasad, D.P.; Taneja, N.K.; Lakra, A.; Sachdev, D. In vitro and in situ abrogation of biofilm formation in E. coli by vitamin C through ROS generation, disruption of quorum sensing and exopolysaccharide production. Food Chem. 2021, 341, 128171. [Google Scholar] [CrossRef]
- Xu, C.; Dong, N.; Chen, K.; Yang, X.; Zeng, P.; Hou, C.; Chi Chan, E.W.; Yao, X.; Chen, S. Bactericidal, anti-biofilm, and anti-virulence activity of vitamin C against carbapenem-resistant hypervirulent Klebsiella pneumoniae. iScience 2022, 25, 103894. [Google Scholar] [CrossRef]
- Abdelraheem, W.M.; Refaie, M.M.M.; Yousef, R.K.M.; Abd El Fatah, A.S.; Mousa, Y.M.; Rashwan, R. Assessment of Antibacterial and Anti-biofilm Effects of Vitamin C Against Pseudomonas aeruginosa Clinical Isolates. Front. Microbiol. 2022, 13, 1594. [Google Scholar] [CrossRef]
- Kallio, J.; Jaakkola, M.; Mäki, M.; Kilpeläinen, P.; Virtanen, V. Vitamin C Inhibits Staphylococcus aureus Growth and Enhances the Inhibitory Effect of Quercetin on Growth of Escherichia coli In Vitro. Planta Med. 2012, 78, 1824–1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehmel, M.; Jovanovic, N.; Spitz, U. Nicotinamide Riboside-The Current State of Research and Therapeutic Uses. Nutrients 2020, 12, 1616. [Google Scholar] [CrossRef]
- Revollo, J.R.; Grimm, A.A.; Imai, S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J. Biol. Chem. 2004, 279, 50754–50763. [Google Scholar] [CrossRef] [Green Version]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), 5–16. [Google Scholar] [CrossRef] [Green Version]
- Maiese, K.; Chong, Z.Z.; Hou, J.; Shang, Y.C. The vitamin nicotinamide: Translating nutrition into clinical care. Molecules 2009, 14, 3446–3485. [Google Scholar] [CrossRef]
- Abdellatif, M.; Trummer-Herbst, V.; Koser, F.; Durand, S.; Adao, R.; Vasques-Novoa, F.; Freundt, J.K.; Voglhuber, J.; Pricolo, M.R.; Kasa, M.; et al. Nicotinamide for the treatment of heart failure with preserved ejection fraction. Sci. Transl. Med. 2021, 13, abd7064. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, M.; Yoshino, J.; Kayser, B.D.; Patti, G.J.; Franczyk, M.P.; Mills, K.F.; Sindelar, M.; Pietka, T.; Patterson, B.W.; Imai, S.I.; et al. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science 2021, 372, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Fricker, R.A.; Green, E.L.; Jenkins, S.I.; Griffin, S.M. The Influence of Nicotinamide on Health and Disease in the Central Nervous System. Int. J. Tryptophan Res. IJTR 2018, 11, 1178646918776658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.J.; Yang, S.J. Supplementation with Nicotinamide Riboside Reduces Brain Inflammation and Improves Cognitive Function in Diabetic Mice. Int. J. Mol. Sci. 2019, 20, 4196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, J.D.; Peterson, G.J.; Campo, M.; Lohmiller, J.; Skerrett, S.J.; Tunaru, S.; Offermanns, S.; Sherman, D.R.; Hawn, T.R. Nicotinamide Limits Replication of Mycobacterium tuberculosis and Bacille Calmette-Guerin Within Macrophages. J. Infect. Dis. 2020, 221, 989–999. [Google Scholar] [CrossRef]
- Lin, Y.; Gong, T.; Ma, Q.; Jing, M.; Zheng, T.; Yan, J.; Chen, J.; Pan, Y.; Sun, Q.; Zhou, X.; et al. Nicotinamide could reduce growth and cariogenic virulence of Streptococcus mutans. J. Oral Microbiol. 2022, 14, 2056291. [Google Scholar] [CrossRef]
- Badiou, C.; Dumitrescu, O.; Croze, M.; Gillet, Y.; Dohin, B.; Slayman, D.H.; Allaouchiche, B.; Etienne, J.; Vandenesch, F.; Lina, G. Panton-Valentine leukocidin is expressed at toxic levels in human skin abscesses. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2008, 14, 1180–1183. [Google Scholar] [CrossRef] [Green Version]
- Levy, R.; Shriker, O.; Porath, A.; Riesenberg, K.; Schlaeffer, F. Vitamin C for the Treatment of Recurrent Furunculosis in Patients with Impaired Neutrophil Functions. J. Infect. Dis. 1996, 173, 1502–1505. [Google Scholar] [CrossRef]
- Cruz-Baquero, A.; Jarillo-Luna, R.A.; Cárdenas-Jaramillo, L.M.; Drago-Serrano, M.E.; Serrano-Luna, J.d.J.; Pacheco-Yépez, J. Ascorbic Acid Ameriolates Liver Damage by Myeloperoxidase Oxidative Products in a Hamster Model of Amoebic Liver Abscess. Front. Cell. Infect. Microbiol. 2022, 12, 855822. [Google Scholar] [CrossRef]
- Yang, J.; Klaidman, L.K.; Chang, M.L.; Kem, S.; Sugawara, T.; Chan, P.; Adams, J.D. Nicotinamide therapy protects against both necrosis and apoptosis in a stroke model. Pharmacol. Biochem. Behav. 2002, 73, 901–910. [Google Scholar] [CrossRef]
- Pu, Q.; Guo, X.-X.; Hu, J.-J.; Li, A.-L.; Li, G.-G.; Li, X.-Y. Nicotinamide mononucleotide increases cell viability and restores tight junctions in high-glucose-treated human corneal epithelial cells via the SIRT1/Nrf2/HO-1 pathway. Biomed. Pharmacother. 2022, 147, 112659. [Google Scholar] [CrossRef] [PubMed]
- Genestier, A.L.; Michallet, M.C.; Prevost, G.; Bellot, G.; Chalabreysse, L.; Peyrol, S.; Thivolet, F.; Etienne, J.; Lina, G.; Vallette, F.M.; et al. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J. Clin. Investig. 2005, 115, 3117–3127. [Google Scholar] [CrossRef] [PubMed]
- Boyle-Vavra, S.; Daum, R.S. Community-acquired methicillin-resistant Staphylococcus aureus: The role of Panton-Valentine leukocidin. Lab. Investig. A J. Tech. Methods Pathol. 2007, 87, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehring, W. Nicotinic acid/niacinamide and the skin. J. Cosmet. Dermatol. 2004, 3, 88–93. [Google Scholar] [CrossRef]
- Gensler, H.L.; Williams, T.; Huang, A.C.; Jacobson, E.L. Oral niacin prevents photocarcinogenesis and photoimmunosuppression in mice. Nutr. Cancer 1999, 34, 36–41. [Google Scholar] [CrossRef]
- Santos, I.M.; Tome Ada, R.; Saldanha, G.B.; Ferreira, P.M.; Militao, G.C.; Freitas, R.M. Oxidative stress in the hippocampus during experimental seizures can be ameliorated with the antioxidant ascorbic acid. Oxidative Med. Cell. Longev. 2009, 2, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Ungerstedt, J.S.; Blomback, M.; Soderstrom, T. Nicotinamide is a potent inhibitor of proinflammatory cytokines. Clin. Exp. Immunol. 2003, 131, 48–52. [Google Scholar] [CrossRef]
- Pizzicannella, J.; Fonticoli, L.; Guarnieri, S.; Marconi, G.D.; Rajan, T.S.; Trubiani, O.; Diomede, F. Antioxidant Ascorbic Acid Modulates NLRP3 Inflammasome in LPS-G Treated Oral Stem Cells through NFkappaB/Caspase-1/IL-1beta Pathway. Antioxidants 2021, 10, 797. [Google Scholar] [CrossRef]
- Gillet, Y.; Dumitrescu, O.; Tristan, A.; Dauwalder, O.; Javouhey, E.; Floret, D.; Vandenesch, F.; Etienne, J.; Lina, G. Pragmatic management of Panton-Valentine leukocidin-associated staphylococcal diseases. Int. J. Antimicrob. Agents 2011, 38, 457–464. [Google Scholar] [CrossRef]


| Assay | Viable Granulocytes % | Viability Difference | Odds Ratio | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Δ* | Viability % ** | t-Test (p-Value) | OR | 95% CI | p-Value | |
| Control (Cells only) | 53 | 1.15 | 36 | 32 | <0.001 | - | - | - |
| Cells + PVL (1 µg/mL) | 17 | 1.15 | ||||||
| Cells + AA (5 mg/mL) | 49 | 1.15 | 5 | 90 | 0.002 | 2.8 | 1.4–5.5 | 0.003 |
| Cells + AA (5 mg/mL) + PVL (1 µg/mL) | 44 | 1 | ||||||
| Cells + AA (0.02 mg/mL) | 46 | 0.6 | 27 | 41 | <0.001 | 1.3 | 0.6–2.8 | 0.5 |
| Cells + AA (0.02 mg/mL) + PVL (1 µg/mL) | 19 | 1.5 | ||||||
| Cells + NAM (5 mg/mL) | 44 | 1.5 | 2 | 95 | 0.049 | 3 | 1.5–5.9 | 0.002 |
| Cells + NAM (5 mg/mL) + PVL (1 µg/mL) | 42 | 1.5 | ||||||
| Cells + NAM (0.02 mg/mL) | 56 | 0.5 | 34 | 39 | <0.001 | 1.2 | 0.59–2.6 | 0.6 |
| Cells + NAM (0.02 mg/mL) + PVL (1 µg/mL) | 22 | 1.15 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlSaleh, A.; Shahid, M.; Farid, E.; Bindayna, K. The Effect of Ascorbic Acid and Nicotinamide on Panton–Valentine Leukocidin Cytotoxicity: An Ex Vivo Study. Toxins 2023, 15, 38. https://doi.org/10.3390/toxins15010038
AlSaleh A, Shahid M, Farid E, Bindayna K. The Effect of Ascorbic Acid and Nicotinamide on Panton–Valentine Leukocidin Cytotoxicity: An Ex Vivo Study. Toxins. 2023; 15(1):38. https://doi.org/10.3390/toxins15010038
Chicago/Turabian StyleAlSaleh, Abdullah, Mohammed Shahid, Eman Farid, and Khalid Bindayna. 2023. "The Effect of Ascorbic Acid and Nicotinamide on Panton–Valentine Leukocidin Cytotoxicity: An Ex Vivo Study" Toxins 15, no. 1: 38. https://doi.org/10.3390/toxins15010038
APA StyleAlSaleh, A., Shahid, M., Farid, E., & Bindayna, K. (2023). The Effect of Ascorbic Acid and Nicotinamide on Panton–Valentine Leukocidin Cytotoxicity: An Ex Vivo Study. Toxins, 15(1), 38. https://doi.org/10.3390/toxins15010038





