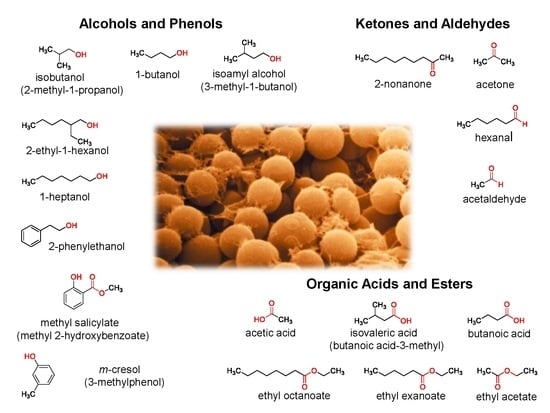
Perfume Guns: Potential of Yeast Volatile Organic Compounds in the Biological Control of Mycotoxin-Producing Fungi
Abstract
Share and Cite
Oufensou, S.; Ul Hassan, Z.; Balmas, V.; Jaoua, S.; Migheli, Q. Perfume Guns: Potential of Yeast Volatile Organic Compounds in the Biological Control of Mycotoxin-Producing Fungi. Toxins 2023, 15, 45. https://doi.org/10.3390/toxins15010045
Oufensou S, Ul Hassan Z, Balmas V, Jaoua S, Migheli Q. Perfume Guns: Potential of Yeast Volatile Organic Compounds in the Biological Control of Mycotoxin-Producing Fungi. Toxins. 2023; 15(1):45. https://doi.org/10.3390/toxins15010045
Chicago/Turabian StyleOufensou, Safa, Zahoor Ul Hassan, Virgilio Balmas, Samir Jaoua, and Quirico Migheli. 2023. "Perfume Guns: Potential of Yeast Volatile Organic Compounds in the Biological Control of Mycotoxin-Producing Fungi" Toxins 15, no. 1: 45. https://doi.org/10.3390/toxins15010045
APA StyleOufensou, S., Ul Hassan, Z., Balmas, V., Jaoua, S., & Migheli, Q. (2023). Perfume Guns: Potential of Yeast Volatile Organic Compounds in the Biological Control of Mycotoxin-Producing Fungi. Toxins, 15(1), 45. https://doi.org/10.3390/toxins15010045