Alkaline pH, Low Iron Availability, Poor Nitrogen Sources and CWI MAPK Signaling Are Associated with Increased Fusaric Acid Production in Fusarium oxysporum
Abstract
:1. Introduction
2. Results
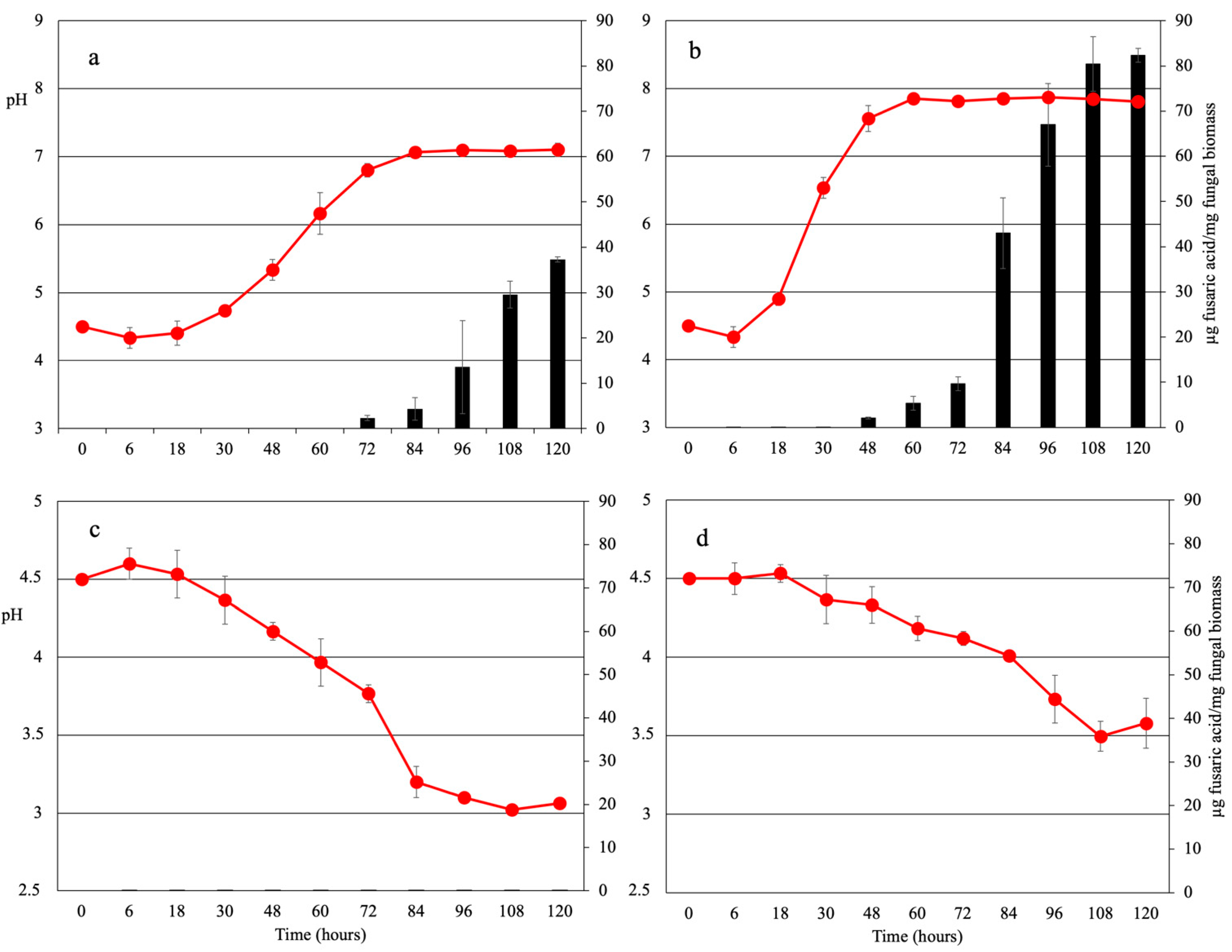
2.1. Nitrogen Source Affects Extracellular pH in F. oxysporum f. sp. lycopersici
2.2. Siderophore Production Is pH- and Iron-Sensing Dependent
2.3. Fusaric Acid Production Is Regulated by Environmental pH and Iron Availability
3. Discussion
4. Materials and Methods
4.1. Fungal Isolates and Culture Condition
4.2. Chrome Azurol S Assay
4.3. Fusaric Acid Extraction and Quantification
4.4. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gordon, T.R.; Martyn, R.D. The evolutionary biology of Fusarium oxysporum. Annu. Rev. Phytopathol. 1997, 35, 111–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuchs, J.G.; Moënne-Loccoz, Y.; Défago, G. Nonpathogenic Fusarium oxysporum strain fo47 induces resistance to Fusarium wilt in tomato. Plant Dis. 1997, 81, 492–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.; Demers, J.; del Mar Jimenez-Gasco, M.; Rep, M. Fusarium oxysporum. In Genomics of Plant-Associated Fungi and Oomycetes: Dicot Pathogens; Dean, R.A., Lichens-Park, A., Kole, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 99–119. [Google Scholar]
- Michielse, C.B.; Rep, M. Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 2009, 10, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism [mdash] from biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [Google Scholar] [CrossRef]
- Gaumann, E. Fusaric acid as a wilt toxin. Phytopathology 1957, 47, 342–357. [Google Scholar]
- Bacon, C.W.; Porter, J.K.; Norred, W.P.; Leslie, J.F. Production of fusaric acid by Fusarium species. Appl. Environ. Microbiol. 1996, 62, 4039–4043. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Ling, N.; Wang, M.; Shen, Q.; Guo, S. Fusaric acid is a crucial factor in the disturbance of leaf water imbalance in fusarium-infected banana plants. Plant. Physiol. Biochem. 2012, 60, 171–179. [Google Scholar] [CrossRef]
- Bouizgarne, B.; El-Maarouf-Bouteau, H.; Frankart, C.; Reboutier, D.; Madiona, K.; Pennarun, A.M.; Monestiez, M.; Trouverie, J.; Amiar, Z.; Briand, J.; et al. Early physiological responses of Arabidopsis thaliana cells to fusaric acid: Toxic and signalling effects. New Phytol. 2006, 169, 209–218. [Google Scholar] [CrossRef]
- Stipanovic, R.D.; Puckhaber, L.S.; Liu, J.; Bell, A.A. Phytotoxicity of fusaric acid and analogs to cotton. Toxicon 2011, 57, 176–178. [Google Scholar] [CrossRef]
- Singh, V.K.; Upadhyay, R.S. Fusaric acid induced cell death and changes in oxidative metabolism of Solanum lycopersicum. Bot. Stud. 2014, 55, 66. [Google Scholar] [CrossRef] [Green Version]
- Landa, B.B.; Cachinero-Díaz, J.M.; Lemanceau, P.; Jiménez-Díaz, R.M.; Alabouvette, C. Effect of fusaric acid and phytoanticipins on growth of rhizobacteria and Fusarium oxysporum. Can. J. Microbiol. 2002, 48, 971–985. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.A.; Bernar, E.M.; Jung, K. Production of siderophores increases resistance to fusaric acid in Pseudomonas protegens pf-5. PLoS ONE 2015, 10, e0117040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niehaus, E.M.; von Bargen, K.W.; Espino, J.J.; Pfannmuller, A.; Humpf, H.U.; Tudzynski, B. Characterization of the fusaric acid gene cluster in Fusarium fujikuroi. Appl. Microbiol. Biotechnol. 2014, 98, 1749–1762. [Google Scholar] [CrossRef] [PubMed]
- López-Díaz, C.; Rahjoo, V.; Sulyok, M.; Ghionna, V.; Martín-Vicente, A.; Capilla, J.; Di Pietro, A.; López-Berges, M.S. Fusaric acid contributes to virulence of Fusarium oxysporum on plant and mammalian hosts. Mol. Plant Pathol. 2018, 19, 440–453. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Li, M.; Sun, F.; Xi, P.; Sun, L.; Zhang, L.; Jiang, Z. Mitogen-activated protein kinases are associated with the regulation of physiological traits and virulence in Fusarium oxysporum f. sp. cubense. PLoS ONE 2015, 10, e0122634. [Google Scholar] [CrossRef] [Green Version]
- Segorbe, D.; Di Pietro, A.; Pérez-Nadales, E.; Turrà, D. Three Fusarium oxysporum mitogen-activated protein kinases (mapks) have distinct and complementary roles in stress adaptation and cross-kingdom pathogenicity. Mol. Plant Pathol. 2016, 18, 912–924. [Google Scholar] [CrossRef]
- Turrà, D.; El Ghalid, M.; Rossi, F.; Di Pietro, A. Fungal pathogen uses sex pheromone receptor for chemotropic sensing of host plant signals. Nature 2015, 527, 521–524. [Google Scholar] [CrossRef]
- Di Pietro, A.; García-Maceira, F.I.; Méglecz, E.; Roncero, M.I.G. A map kinase of the vascular wilt fungus Fusarium oxysporum is essential for root penetration and pathogenesis. Mol. Microbiol. 2001, 39, 1140–1152. [Google Scholar] [CrossRef]
- Prusky, D.; McEvoy, J.L.; Saftner, R.; Conway, W.S.; Jones, R. Relationship between host acidification and virulence of Penicillium spp. on apple and citrus fruit. Phytopathology 2004, 94, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Caracuel, Z.; Roncero, M.I.; Espeso, E.A.; Gonzalez-Verdejo, C.I.; Garcia-Maceira, F.I.; Di Pietro, A. The ph signalling transcription factor pacc controls virulence in the plant pathogen Fusarium oxysporum. Mol. Microbiol. 2003, 48, 765–779. [Google Scholar] [CrossRef]
- López-Berges, M.S.; Capilla, J.; Turrà, D.; Schafferer, L.; Matthijs, S.; Jöchl, C.; Cornelis, P.; Guarro, J.; Haas, H.; Di Pietro, A. Hapx-mediated iron homeostasis is essential for rhizosphere competence and virulence of the soilborne pathogen Fusarium oxysporum. Plant Cell 2012, 24, 3805–3822. [Google Scholar] [CrossRef] [PubMed]
- Yabuta, T.; Kambe, K.; Hayashi, T. Biochemistry of the bakanae-fungus. I. Fusarinic acid, a new product of the bakanae fungus. Agric. Chem. Soc. Jpn 1937, 10, 1059–1068. [Google Scholar]
- Brown, D.W.; Butchko, R.A.; Busman, M.; Proctor, R.H. Identification of gene clusters associated with fusaric acid, fusarin, and perithecial pigment production in Fusarium verticillioides. Fungal Genet. Biol. 2012, 49, 521–532. [Google Scholar] [CrossRef] [Green Version]
- López-Berges, M.S.; Rispail, N.; Prados-Rosales, R.C.; Di Pietro, A. A nitrogen response pathway regulates virulence functions in Fusarium oxysporum via the protein kinase tor and the bzip protein meab. Plant Cell 2010, 22, 2459–2475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masachis, S.; Segorbe, D.; Turra, D.; Leon-Ruiz, M.; Furst, U.; El Ghalid, M.; Leonard, G.; Lopez-Berges, M.S.; Richards, T.A.; Felix, G. A fungal pathogen secretes plant alkalinizing peptides to increase infection. Nat. Microbiol. 2016, 1, 16043. [Google Scholar] [CrossRef]
- Lopez-Berges, M.S.; Turra, D.; Capilla, J.; Schafferer, L.; Matthijs, S.; Jochl, C.; Cornelis, P.; Guarro, J.; Haas, H.; Di Pietro, A. Iron competition in fungus-plant interactions: The battle takes place in the rhizosphere. Plant Signal. Behav. 2013, 8, e23012. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, T.R.; Segorbe, D. How alkalinization drives fungal pathogenicity. PLoS Pathog. 2017, 13, e1006621. [Google Scholar] [CrossRef]
- Dong, X.; Wang, M.; Ling, N.; Shen, Q.; Guo, S. Effects of iron and boron combinations on the suppression of Fusarium wilt in banana. Sci. Rep. 2016, 6, 38944. [Google Scholar] [CrossRef]
- Colombo, C.; Palumbo, G.; He, J.Z.; Pinton, R.; Cesco, S. Review on iron availability in soil: Interaction of fe minerals, plants, and microbes. J. Soils Sedim. 2014, 14, 538–548. [Google Scholar] [CrossRef]
- Lemanceau, P.; Bauer, P.; Kraemer, S.; Briat, J.-F. Iron dynamics in the rhizosphere as a case study for analyzing interactions between soils, plants and microbes. Plant Soil 2009, 321, 513–535. [Google Scholar] [CrossRef]
- Eisendle, M.; Oberegger, H.; Buttinger, R.; Illmer, P.; Haas, H. Biosynthesis and uptake of siderophores is controlled by the pacc-mediated ambient-ph regulatory system in Aspergillus nidulans. Eukaryot. Cell 2004, 3, 561–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, P.-C.; Yang, C.-Y.; Lan, C.-Y. Candida albicans hap43 is a repressor induced under low-iron conditions and is essential for iron-responsive transcriptional regulation and virulence. Eukaryot. Cell 2011, 10, 207–225. [Google Scholar] [CrossRef]
- Vitale, S.; Partida-Hanon, A.; Serrano, S.; Martinez-Del-Pozo, A.; Di Pietro, A.; Turra, D.; Bruix, M. Structure-activity relationship of alpha mating pheromone from the fungal pathogen Fusarium oxysporum. J. Biol. Chem. 2017, 292, 3591–3602. [Google Scholar] [CrossRef] [Green Version]
- Vitale, S.; Di Pietro, A.; Turra, D. Autocrine pheromone signalling regulates community behaviour in the fungal pathogen Fusarium oxysporum. Nat. Microbiol. 2019, 4, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Puhalla, J.E. Compatibility reactions on solid medium and interstrain inhibition in Ustilago maydis. Genetics 1968, 60, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Miranda, S.; Cabirol, N.; George-Téllez, R.; Zamudio-Rivera, L.S.; Fernández, F.J. O-cas, a fast and universal method for siderophore detection. J. Microbiol. Meth. 2007, 70, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Amalfitano, C.; Pengue, R.; Andolfi, A.; Vurro, M.; Zonno, M.C.; Evidente, A. Hplc analysis of fusaric acid, 9,10-dehydrofusaric acid and their methyl esters, toxic metabolites from weed pathogenic Fusarium species. Phytochem. Anal. 2002, 13, 277–282. [Google Scholar] [CrossRef]
- Marzano, M.; Gallo, A.; Altomare, C. Improvement of biocontrol efficacy of Trichoderma harzianum vs. Fusarium oxysporum f. sp. lycopersici through uv-induced tolerance to fusaric acid. Biol. Control. 2013, 67, 397–408. [Google Scholar] [CrossRef]



| Fol Strain | Nitrogen Source | pH | Chelating Activity | Fusaric Acid 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wild type | Urea | 7.02 | a | 78.00 | a | 37 | a | |||
| Sodium nitrate | 7.81 | b | 68.40 | b | 82 | b | ||||
| Ammonium sulphate | 3.07 | c | 27.55 | c | n.d. | c | ||||
| Ammonium nitrate | 3.64 | d | 25.55 | c | n.d. | c | ||||
| fmk1Δ | Urea | 6.94 | a | 75.78 | a | 41 | a | |||
| Sodium nitrate | 7.85 | b | 69.46 | b | 75 | b | ||||
| Ammonium sulphate | 2.88 | c | 22.98 | c | n.d. | c | ||||
| Ammonium nitrate | 4.16 | d | 23.90 | c | n.d. | c | ||||
| hog1Δ | Urea | 6.81 | a | 75.75 | a | 36 | a | |||
| Sodium nitrate | 7.12 | b | 65.37 | b | 85 | b | ||||
| Ammonium sulphate | 2.93 | c | 25.55 | c | n.d. | c | ||||
| Ammonium nitrate | 3.60 | d | 25.16 | c | n.d. | c | ||||
| mpk1Δ | Urea | 8.95 | a | * | 89.05 | a | * | 12 | a | * |
| Sodium nitrate | 8.22 | b | * | 95.16 | a | * | 42 | b | * | |
| Ammonium sulphate | 3.01 | c | 21.09 | b | n.d. | c | ||||
| Ammonium nitrate | 4.16 | d | 23.51 | b | n.d. | c | ||||
| ste2Δ | Urea | 8.40 | a | * | 92.05 | a | * | 9 | a | * |
| Sodium nitrate | 8.33 | a | * | 91.16 | a | * | 39 | b | * | |
| Ammonium sulphate | 3.07 | b | 28.09 | b | n.d. | c | ||||
| Ammonium nitrate | 3.92 | c | 27.51 | b | n.d. | c | ||||
| bck1Δ | Urea | 8.80 | a | * | 90.59 | a | * | 11 | a | * |
| Sodium nitrate | 8.52 | a | * | 92.13 | a | * | 45 | b | * | |
| Ammonium sulphate | 2.94 | b | 21.09 | b | n.d. | c | ||||
| Ammonium nitrate | 3.91 | c | 24.76 | b | n.d. | c | ||||
| mkk2Δ | Urea | 8.71 | a | * | 93.93 | a | * | 8 | a | * |
| Sodium nitrate | 8.35 | a | * | 93.02 | a | * | 62 | b | * | |
| Ammonium sulphate | 3.12 | b | 28.43 | b | n.d. | c | ||||
| Ammonium nitrate | 4.00 | c | 23.46 | b | n.d. | c | ||||
| hapXΔ | Urea | 7.05 | a | 84.80 | a | * | 63 | a | * | |
| Sodium nitrate | 7.89 | b | 86.41 | a | * | 70 | a | |||
| Ammonium sulphate | 3.02 | c | 60.56 | b | * | 27 | b | * | ||
| Ammonium nitrate | 3.98 | d | 66.75 | b | * | 0.22 | b | * | ||
| pacCc | Urea | 7.18 | a | 77.89 | a | 37 | a | |||
| Sodium nitrate | 7.77 | b | 67.77 | b | 50 | b | * | |||
| Ammonium sulphate | 3.12 | c | 58.28 | c | * | 22 | c | * | ||
| Ammonium nitrate | 4.06 | d | 56.28 | c | * | 12 | d | * | ||
| pacCΔ | Urea | 7.08 | a | 35.55 | a | * | n.d. | a | ||
| Sodium nitrate | 7.58 | b | 32.25 | a | * | n.d. | a | |||
| Ammonium sulphate | 3.05 | c | 28.35 | b | n.d. | a | * | |||
| Ammonium nitrate | 4.18 | d | 29.25 | b | n.d. | a | * | |||
| Fol Strain | Genotype | Gene Function | Reference |
|---|---|---|---|
| FGSC 4287 | Wild type | [19] | |
| fmk1Δ | fmk1::PHLEO | MAPK | [19] |
| mpk1Δ | mpk1::HYG | MAPK | [18] |
| hog1Δ | hog1::HYG | MAPK | [17] |
| ste2Δ | ste2::HYG | GPCR | [18] |
| mkk2Δ | mkk2::HYG | MAPKK | [18] |
| bck1Δ | bck1::HYG | MAPKKK | [18] |
| hapXΔ | hapX::HYG | Transcription factor | [22] |
| pacCΔ | pacC::HYG | Transcription factor | [21] |
| pacCC | pacCC::HYG | Transcription factor | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmieri, D.; Segorbe, D.; López-Berges, M.S.; De Curtis, F.; Lima, G.; Di Pietro, A.; Turrà, D. Alkaline pH, Low Iron Availability, Poor Nitrogen Sources and CWI MAPK Signaling Are Associated with Increased Fusaric Acid Production in Fusarium oxysporum. Toxins 2023, 15, 50. https://doi.org/10.3390/toxins15010050
Palmieri D, Segorbe D, López-Berges MS, De Curtis F, Lima G, Di Pietro A, Turrà D. Alkaline pH, Low Iron Availability, Poor Nitrogen Sources and CWI MAPK Signaling Are Associated with Increased Fusaric Acid Production in Fusarium oxysporum. Toxins. 2023; 15(1):50. https://doi.org/10.3390/toxins15010050
Chicago/Turabian StylePalmieri, Davide, David Segorbe, Manuel S. López-Berges, Filippo De Curtis, Giuseppe Lima, Antonio Di Pietro, and David Turrà. 2023. "Alkaline pH, Low Iron Availability, Poor Nitrogen Sources and CWI MAPK Signaling Are Associated with Increased Fusaric Acid Production in Fusarium oxysporum" Toxins 15, no. 1: 50. https://doi.org/10.3390/toxins15010050
APA StylePalmieri, D., Segorbe, D., López-Berges, M. S., De Curtis, F., Lima, G., Di Pietro, A., & Turrà, D. (2023). Alkaline pH, Low Iron Availability, Poor Nitrogen Sources and CWI MAPK Signaling Are Associated with Increased Fusaric Acid Production in Fusarium oxysporum. Toxins, 15(1), 50. https://doi.org/10.3390/toxins15010050







