Clinical Immunogenicity of DaxibotulinumtoxinA for Injection in Glabellar Lines: Pooled Data from the SAKURA Phase 3 Trials
Abstract
:1. Introduction
2. Results
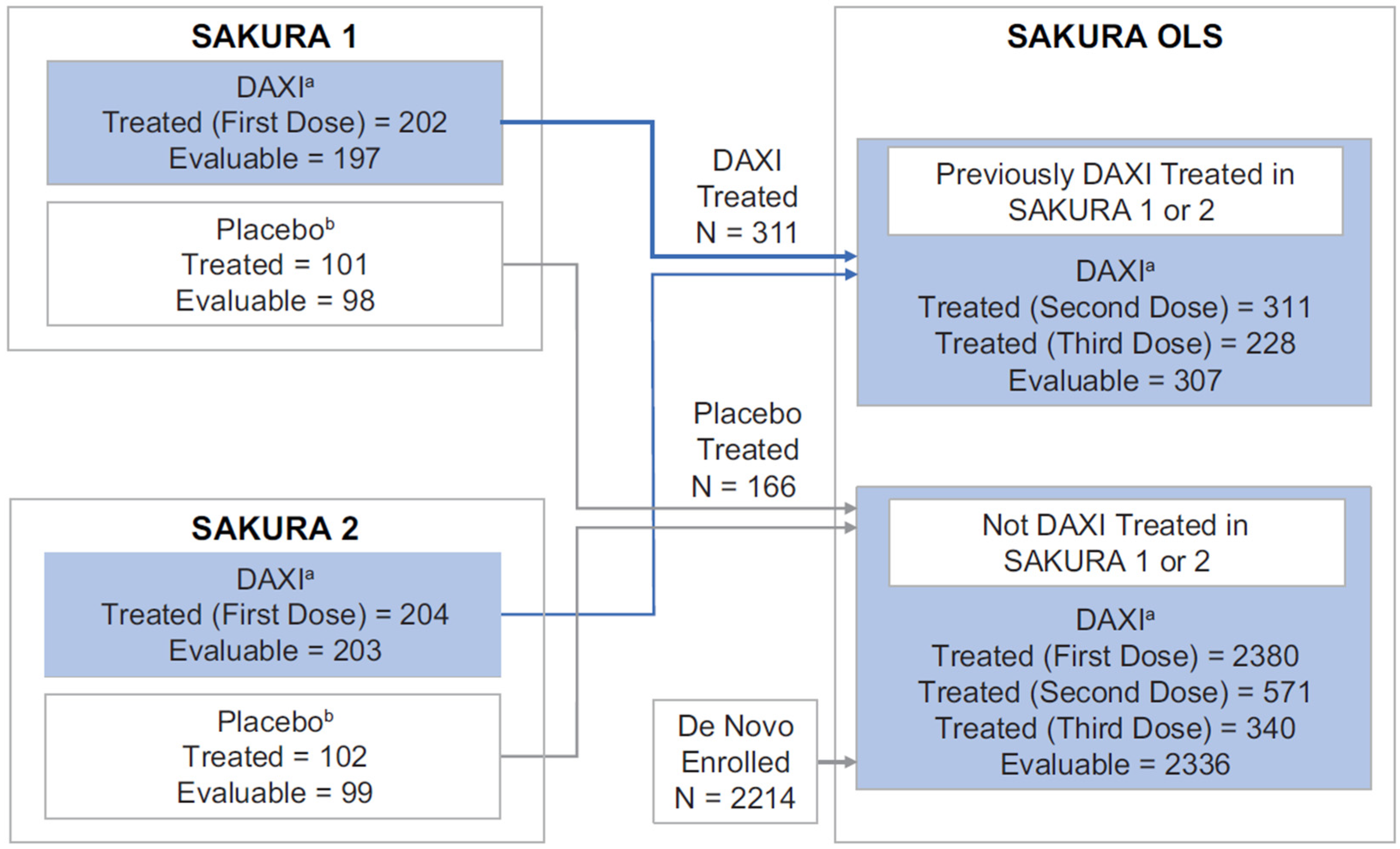
2.1. Subject Disposition and Baseline Characteristics
2.2. Binding Antibodies to DaxibotulinumtoxinA
2.3. Neutralizing Antibodies to DaxibotulinumtoxinA
2.4. Binding Antibodies to RTP004
2.5. Clinical Response and Immune-Related AEs in Subjects with Treatment-Induced Binding Antibodies to DaxibotulinumtoxinA or RTP004
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Data Source
5.2. Immunogenicity Tests
5.3. Treatment-Relationship of DaxibotulinumtoxinA and RTP004 Immunogenicity
5.4. Effect of Immunogenicity on Efficacy and Safety
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vandivort, T.C.; Horton, D.B.; Johnson, S.B. Regulatory and strategic considerations for addressing immunogenicity and related responses in biopharmaceutical development programs. J. Clin. Transl. Sci. 2020, 4, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Dolimbek, B.Z.; Aoki, K.R.; Steward, L.E.; Jankovic, J.; Atassi, M.Z. Mapping of the regions on the heavy chain of botulinum neurotoxin A (BoNT/A) recognized by antibodies of cervical dystonia patients with immunoresistance to BoNT/A. Mol. Immunol. 2007, 44, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Atassi, M.Z.; Dolimbek, B.Z.; Jankovic, J.; Steward, L.E.; Aoki, K.R. Regions of botulinum neurotoxin A light chain recognized by human anti-toxin antibodies from cervical dystonia patients immunoresistant to toxin treatment. The antigenic structure of the active toxin recognized by human antibodies. Immunobiology 2011, 216, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Krebs, K.M.; Lebeda, F.J. Comparison of the structural features of botulinum neurotoxin and NTNH, a non-toxic accessory protein of the progenitor complex. Botulinum J. 2008, 1, 116–134. [Google Scholar] [CrossRef]
- Göschel, H.; Wohlfarth, K.; Frevert, J.; Dengler, R.; Bigalke, H. Botulinum A toxin therapy: Neutralizing and nonneutralizing antibodies—Therapeutic consequences. Exp. Neurol. 1997, 147, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Glogau, R.G.; Waugh, J.M. Preclinical Transcutaneous Flux Experiments using a Macromolecule Transport System (MTS) Peptide for Delivery of Botulinum Toxin Type A. In Proceedings of the Annual Meeting of the American Academy of Dermatology, San Antonio, TX, USA, 1–5 February 2008. [Google Scholar]
- Smyth, T.; Oliyai, C.; Joshi, A. Stabilizing Effect of RTP004 on Non-Specific Surface Adsorption in Drug Product Manufacturing of Daxibotulinumtoxina (DAXI). In Proceedings of the TOXINS 2019, Copenhagen, Denmark, 16–19 January 2019. [Google Scholar]
- Weisemann, J.; Rummel, A.; Oliyai, C.; Too, P.; Joshi, A. Novel Peptide Excipient RTP004 Enhances the Binding of Botulinum Neurotoxin A Cell Binding Domain Hc to Rat Brain Synaptosomes. In Proceedings of the TOXINS 2019, Copenhagen, Denmark, 16–19 January 2019. [Google Scholar]
- Yin, L.; Masuyer, G.; Zhang, S.; Zhang, J.; Miyashita, S.I.; Burgin, D.; Lovelock, L.; Coker, S.F.; Fu, T.M.; Stenmark, P.; et al. Characterization of a membrane binding loop leads to engineering botulinum neurotoxin B with improved therapeutic efficacy. PLoS Biol. 2020, 18, e3000618. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Comella, C.; Hauser, R.A.; Patel, A.T.; Gross, T.M.; Rubio, R.G.; Vitarella, D. ASPEN-1. A Phase 3 Trial Evaluating the Efficacy, Duration of Effect, and Safety of DaxibotulinumtoxinA for Injection in the Treatment of Cervical Dystonia. In Proceedings of the TOXINS 2021, Virtual, 16–17 January 2021. [Google Scholar]
- Allergan. BOTOX (onabotulinumtoxinA) for Injection, for Intramuscular, Intradetrusor, or Intradermal Use. Prescribing Information. 2020. Available online: https://media.allergan.com/actavis/actavis/media/allergan-pdf-documents/product-prescribing/20190620-BOTOX-100-and-200-Units-v3-0USPI1145-v2-0MG1145.pdf (accessed on 12 March 2020).
- Comella, C.L.; Jankovic, J.; Shannon, K.M.; Tsui, J.; Swenson, M.; Leurgans, S.; Fan, W.; Dystonia Study Group. Comparison of botulinum toxin serotypes A and B for the treatment of cervical dystonia. Neurology 2005, 65, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Field, M.; Splevins, A.; Picaut, P.; Van der Schans, M.; Langenberg, J.; Noort, D.; Foster, K. AbobotulinumtoxinA (Dysport®), OnabotulinumtoxinA (Botox®), and IncobotulinumtoxinA (Xeomin®) neurotoxin content and potential implications for duration of response in patients. Toxins 2018, 10, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carruthers, J.; Solish, N.; Humphrey, S.; Rosen, N.; Muhn, C.; Bertucci, V.; Swift, A.; Metelitsa, A.; Rubio, R.G.; Waugh, J.; et al. Injectable daxibotulinumtoxinA for the treatment of glabellar lines: A phase 2, randomized, dose-ranging, double-blind, multicenter comparison with onabotulinumtoxinA and placebo. Dermatol. Surg. 2017, 43, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Bertucci, V.; Solish, N.; Kaufman-Janette, J.; Yoelin, S.; Shamban, A.; Schlessinger, J.; Snyder, D.; Gallagher, C.; Liu, Y.; Shears, G.; et al. DaxibotulinumtoxinA for Injection has a prolonged duration of response in the treatment of glabellar lines: Pooled data from two multicenter, randomized, double-blind, placebo-controlled, phase 3 studies (SAKURA 1 and SAKURA 2). J. Am. Acad. Dermatol. 2020, 82, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Fabi, S.G.; Cohen, J.L.; Green, L.J.; Dhawan, S.; Kontis, T.C.; Baumann, L.; Gross, T.M.; Gallagher, C.J.; Brown, J.; Rubio, R.G. DaxibotulinumtoxinA for Injection for the treatment of glabellar lines: Efficacy results from SAKURA 3, a large, open-label, phase 3 safety study. Dermatol. Surg. 2021, 47, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Allergan. BOTOX Cosmetic (onabotulinumtoxinA) for Injection, for Intramuscular Use. Prescribing Information. 2019. Available online: https://media.allergan.com/actavis/actavis/media/allergan-pdf-documents/product-prescribing/20190626-BOTOX-Cosmetic-Insert-72715US10-Med-Guide-v2-0MG1145.pdf (accessed on 12 March 2020).
- Merz. XEOMIN (incobotulinumtoxinA) for Injection, for Intramuscular or Intraglandular Use. Prescribing Information. 2019. Available online: https://www.xeominaesthetic.com/wp-content/uploads/2019/05/XEOMIN-Full-Prescribing-Information-including-MedGuide.pdf (accessed on 12 March 2020).
- Albrecht, P.; Jansen, A.; Lee, J.I.; Moll, M.; Ringelstein, M.; Rosenthal, D.; Bigalke, H.; Aktas, O.; Hartung, H.P.; Hefter, H. High prevalence of neutralizing antibodies after long-term botulinum neurotoxin therapy. Neurology 2019, 92, e48–e54. [Google Scholar] [CrossRef] [PubMed]
- Walter, U.; Mühlenhoff, C.; Benecke, R.; Dressler, D.; Mix, E.; Alt, J.; Wittstock, M.; Dudesek, A.; Storch, A.; Kamm, C. Frequency and risk factors of antibody-induced secondary failure of botulinum neurotoxin therapy. Neurology 2020, 94, e2109–e2120. [Google Scholar] [CrossRef] [PubMed]
- Naumann, M.; Boo, L.M.; Ackerman, A.H.; Gallagher, C.J. Immunogenicity of botulinum toxins. J. Neural. Transm. 2013, 120, 275–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellows, S.; Jankovic, J. Immunogenicity associated with botulinum toxin treatment. Toxins 2019, 11, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, E.; Alhitmi, H.K.; Mosahebi, A. Immunogenicity to botulinum toxin type A: A systematic review with meta-analysis across therapeutic indications. Aesthet. Surg. J. 2021, 42, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, J.D.; Fagien, S.; Joseph, J.H.; Humphrey, S.D.; Biesman, B.S.; Gallagher, C.J.; Liu, Y.; Rubio, R.G.; Sakura, T. DaxibotulinumtoxinA for Injection for the treatment of glabellar lines: Results from each of two multicenter, randomized, double-blind, placebo-controlled, phase 3 studies (SAKURA 1 and SAKURA 2). Plast. Reconstr. Surg. 2020, 145, 45–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, J.B.; Mariwalla, K.; Coleman, K.; Ablon, G.; Weinkle, S.H.; Gallagher, C.J.; Vitarella, D.; Rubio, R.G. A large, open-label, phase 3 safety study of DaxibotulinumtoxinA for Injection in glabellar lines: A focus on safety from the SAKURA 3 study. Dermatol. Surg. 2021, 47, 42–46. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Immunogenicity Testing of Therapeutic Protein Products—Developing and Validating Assays for Anti-Drug Antibody Detection. Guidance for Industry. 2019. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/immunogenicity-testing-therapeutic-protein-products-developing-and-validating-assays-anti-drug (accessed on 24 January 2020).
- Hatheway, C.; Dang, C. Immunogenicity of the Neurotoxins of Clostridium Botulinum. In Therapy with Botulinum Toxin. Neurological Disease and Therapy; Jankovic, J., Halett, M., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1994; pp. 93–107. [Google Scholar]




| Exposed to DaxibotulinumtoxinA (n = 2786) | Exposed to RTP004 (n = 2823) | |
|---|---|---|
| Female, % | 88.5 | 88.5 |
| Age, years, mean (SD) | 49.4 (11.3) | 49.4 (11.3) |
| Race, % | ||
| White | 89.2 | 89.2 |
| Black/African American | 5.0 | 4.9 |
| Asian | 2.7 | 2.7 |
| Other | 3.1 | 3.2 |
| Time since last BoNTA, months, median (range) | 18.1 (5.3–319.9) | 18.0 (5.3–319.9) |
| IGA-FWS at maximum frown, % | ||
| Moderate | 62.1 | 62.1 |
| Severe | 37.9 | 37.9 |
| PFWS at maximum frown, n (%) | ||
| Moderate | 56.7 | 56.7 |
| Treatment Cycle # (Evaluable Subjects, DaxibotulinumtoxinA /RTP004) a | Response Type | Exposed to DaxibotulinumtoxinA (n = 2737) | Exposed to RTP004 (n = 2772) |
|---|---|---|---|
| Cycle 1 (n = 2737/2770) | Negative | 2713 (99.1%) | 2680 (96.8%) |
| Treatment-induced | 12 (0.4%) | 24 (0.9%) | |
| Treatment-unaffected | 11 (0.4%) | 66 (2.4%) | |
| Treatment-boosted | 1 (0.04%) | 0 | |
| Cycle 2 (n = 873/906) | Negative | 864 (99.0%) | 865 (95.5%) |
| Treatment-induced | 4 (0.5%) | 4 (0.4%) | |
| Treatment-unaffected | 5 (0.6%) | 37 (4.1%) | |
| Treatment-boosted | 0 | 0 | |
| Cycle 3 (n = 566/696) | Negative | 557 (98.4%) | 659 (94.7%) |
| Treatment-induced | 5 (0.9%) | 10 (1.4%) | |
| Treatment-unaffected | 4 (0.7%) | 27 (3.9%) | |
| Treatment-boosted | 0 | 0 | |
| Overall b (n = 2737/2772) | Negative | 2705 (98.8%) | 2671 (96.4%) |
| Treatment-induced | 20 (0.7%) | 35 (1.3%) | |
| Treatment-unaffected | 11 (0.4%) | 66 (2.4%) | |
| Treatment-boosted | 1 (<0.1%) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallagher, C.J.; Bowsher, R.R.; Clancy, A.; Dover, J.S.; Humphrey, S.; Liu, Y.; Prawdzik, G. Clinical Immunogenicity of DaxibotulinumtoxinA for Injection in Glabellar Lines: Pooled Data from the SAKURA Phase 3 Trials. Toxins 2023, 15, 60. https://doi.org/10.3390/toxins15010060
Gallagher CJ, Bowsher RR, Clancy A, Dover JS, Humphrey S, Liu Y, Prawdzik G. Clinical Immunogenicity of DaxibotulinumtoxinA for Injection in Glabellar Lines: Pooled Data from the SAKURA Phase 3 Trials. Toxins. 2023; 15(1):60. https://doi.org/10.3390/toxins15010060
Chicago/Turabian StyleGallagher, Conor J., Ronald R. Bowsher, Amanda Clancy, Jeffrey S. Dover, Shannon Humphrey, Yan Liu, and Gregg Prawdzik. 2023. "Clinical Immunogenicity of DaxibotulinumtoxinA for Injection in Glabellar Lines: Pooled Data from the SAKURA Phase 3 Trials" Toxins 15, no. 1: 60. https://doi.org/10.3390/toxins15010060
APA StyleGallagher, C. J., Bowsher, R. R., Clancy, A., Dover, J. S., Humphrey, S., Liu, Y., & Prawdzik, G. (2023). Clinical Immunogenicity of DaxibotulinumtoxinA for Injection in Glabellar Lines: Pooled Data from the SAKURA Phase 3 Trials. Toxins, 15(1), 60. https://doi.org/10.3390/toxins15010060





