Wound Infection of Snakebite from Venomous Protobothrops mucrosquamatus, Viridovipera stejnegeri and Naja atra in Taiwan: Validation of BITE and Cobra BITE Scoring Systems and their Bacteriological Differences in Wound Cultures
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics of the Validation Cohort of the BITE Study
2.2. Patient Characteristics of the Validation Cohort of the Cobra BITE Study
2.3. Comparison of Patient Characteristics between the Derivation and Validation Cohorts for BITE and Cobra BITE Studies
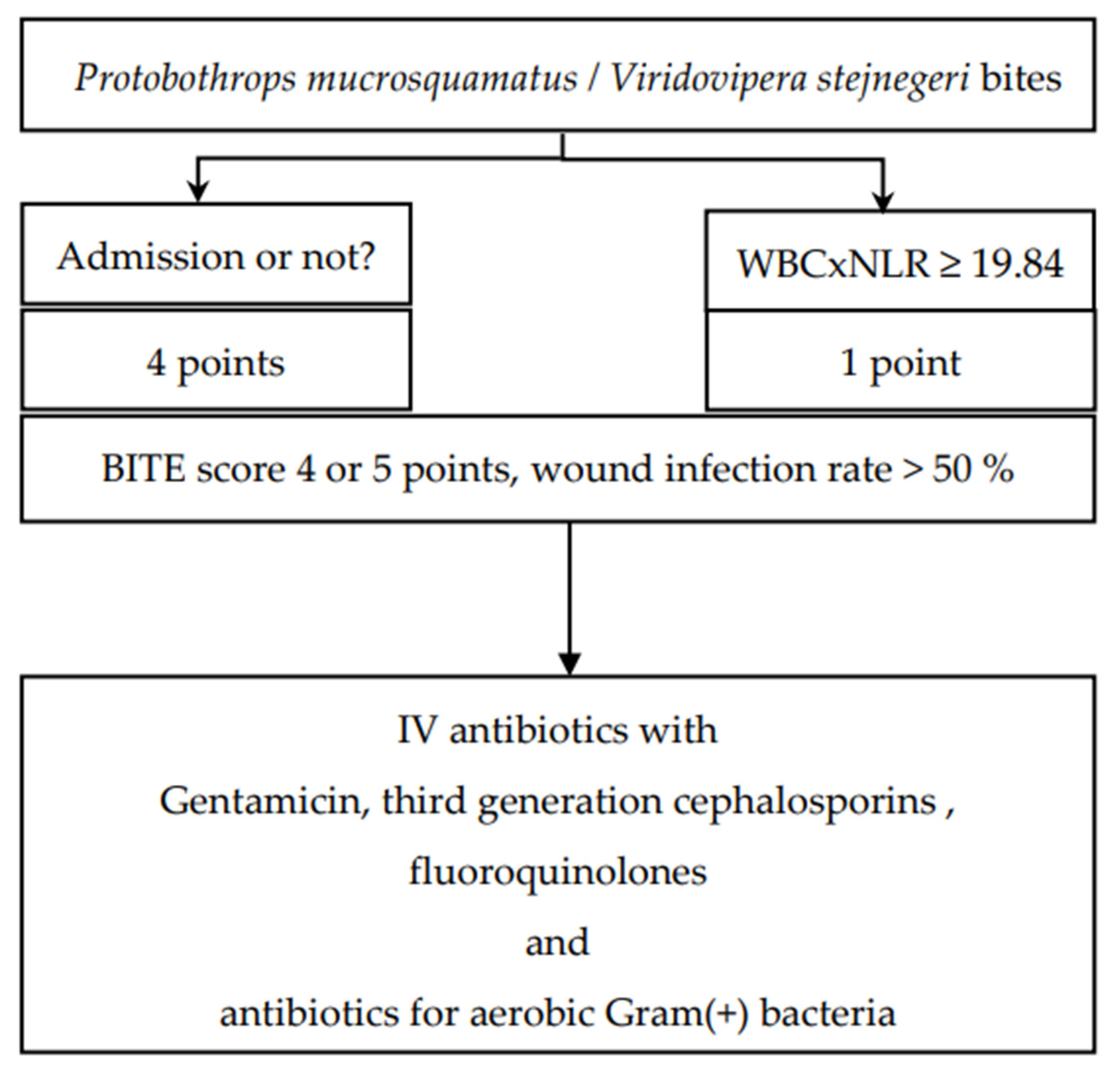
2.4. Validation of the BITE and Cobra BITE Studies
2.5. Bacteriology and Antibiotic Susceptibility Testing
2.6. Use of Empirical Antibiotics and Antibiotic Therapy for Infected Snakebite Wounds in the Validation Set
2.7. Validation of BITE and Cobra BITE Studies and the Use of Antibiotics
3. Discussion
3.1. Bacteriology of Snake Oral Cavity and Wound Culture in N. atra Bites
3.2. Bacteriology of Snake Oral Cavity and Wound Culture in P. mucrosquamatus and V. stejnegeri Bites
3.3. Antibiotic Use
3.4. Limitations
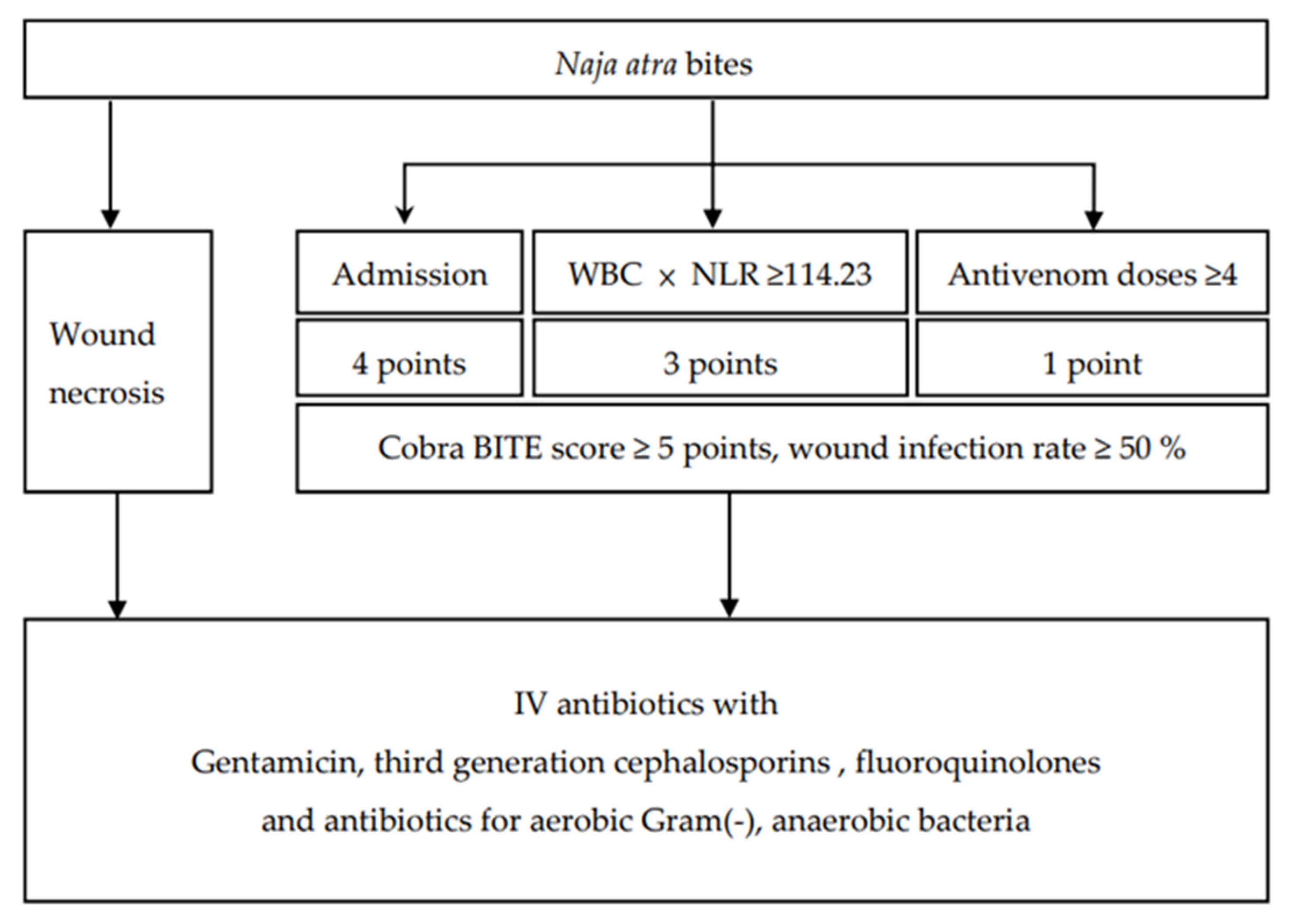
4. Conclusions
5. Materials and Methods
5.1. Patient Selection, Data Resource Setting, and Collected Variables
5.2. Current Treatment Protocol for Patients with Snakebites and Definitions of Wound Infection, Polymicrobial Infection, and Wound Necrosis
5.3. Statistical Analysis and Validation of the BITE and Cobra BITE Scores
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, C.-K.; Lin, C.-C.; Shih, F.-Y.; Chaou, C.-H.; Lin, J.C.-C.; Lai, T.-I.; Tseng, C.-Y.; Fang, C.-C. Population-based study of venomous snakebite in Taiwan. J. Acute Med. 2015, 5, 38–42. [Google Scholar] [CrossRef]
- Mao, Y.C.; Liu, P.Y.; Chiang, L.C.; Lai, C.S.; Lai, K.L.; Ho, C.H.; Wang, T.H.; Yang, C.C. Naja atra snakebite in Taiwan. Clin. Toxicol. 2018, 56, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Chou, Y.-S.; Chen, C.-Y.; Liu, K.-L.; Huang, G.-J.; Yu, J.-S.; Wu, C.-J.; Liaw, G.-W.; Hsieh, C.-H.; Chen, C.-K. Pathogenesis of local necrosis induced by Naja atra venom: Assessment of the neutralization ability of Taiwanese freeze-dried neurotoxic antivenom in animal models. PLoS Negl. Trop. Dis. 2020, 14, e0008054. [Google Scholar] [CrossRef]
- Huang, L.-W.; Wang, J.-D.; Huang, J.-A.; Hu, S.-Y.; Wang, L.-M.; Tsan, Y.-T. Wound infections secondary to snakebite in central Taiwan. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 272–276. [Google Scholar] [CrossRef]
- Seifert, S.A.; Armitage, J.O.; Sanchez, E.E. Snake Envenomation. N. Engl. J. Med. 2022, 386, 68–78. [Google Scholar] [CrossRef]
- Lin, C.-C.; Chaou, C.-H.; Tseng, C.-Y. An investigation of snakebite antivenom usage in Taiwan. J. Formos. Med Assoc. 2016, 115, 672–677. [Google Scholar] [CrossRef]
- Lin, C.C.; Chen, Y.C.; Goh, Z.N.L.; Seak, C.K.; Seak, J.C.Y.; Shi-Ying, G.; Seak, C.J.; Spot Investigators. Wound Infections of Snakebites from the Venomous Protobothrops mucrosquamatus and Viridovipera stejnegeri in Taiwan: Bacteriology, Antibiotic Susceptibility, and Predicting the Need for Antibiotics-A BITE Study. Toxins 2020, 12, 575. [Google Scholar] [CrossRef]
- Yeh, H.; Gao, S.Y.; Lin, C.C. Wound Infections from Taiwan Cobra (Naja atra) Bites: Determining Bacteriology, Antibiotic Susceptibility, and the Use of Antibiotics-A Cobra BITE Study. Toxins 2021, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Wu, K.G.; Chen, C.J.; Wang, C.M. Bacterial infection in association with snakebite: A 10-year experience in a northern Taiwan medical center. J. Microbiol. Immunol. Infect. 2011, 44, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.C.; Chuang, H.N.; Shih, C.H.; Hsieh, H.H.; Jiang, Y.H.; Chiang, L.C.; Lin, W.L.; Hsiao, T.H.; Liu, P.Y. An investigation of conventional microbial culture for the Naja atra bite wound, and the comparison between culture-based 16S Sanger sequencing and 16S metagenomics of the snake oropharyngeal bacterial microbiota. PLoS Negl. Trop. Dis. 2021, 15, e0009331. [Google Scholar] [CrossRef]
- Chuang, P.-C.; Lin, W.-H.; Chen, Y.-C.; Chien, C.-C.; Chiu, I.-M.; Tsai, T.-S. Oral Bacteria and Their Antibiotic Susceptibilities in Taiwanese Venomous Snakes. Microorganisms 2022, 10, 951. [Google Scholar] [CrossRef]
- Shek, K.C.; Tsui, K.L.; Lam, K.K.; Crow, P.; Ng, K.H.L.; Ades, G.; Yip, K.T.; Grioni, A.; Tan, K.S.; Lung, D.C.; et al. Oral bacterial flora of the Chinese cobra (Naja atra) and bamboo pit viper (Trimeresurus albolabris) in Hong Kong SAR, China. Hong Kong Med. J. 2009, 15, 183–190. [Google Scholar]
- Su, H.Y.; Wang, M.J.; Li, Y.H.; Tang, C.N.; Tsai, M.J. Can surgical need in patients with Naja atra (Taiwan or Chinese cobra) envenomation be predicted in the emergency department? Hong Kong Med. J. 2016, 22, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Padhi, L.; Sahoo, G. Oral bacterial flora of Indian cobra (Naja naja) and their antibiotic susceptibilities. Heliyon 2018, 4, e01008. [Google Scholar] [CrossRef]
- Lam, K.K.; Crow, P.; Ng, K.H.L.; Shek, K.C.; Fung, H.T.; Ades, G.; Grioni, A.; Tan, K.S.; Yip, K.T.; Lung, D.C.; et al. A cross-sectional survey of snake oral bacterial flora from Hong Kong, SAR, China. Emerg. Med. J. 2010, 28, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.C.; Liu, P.Y.; Hung, D.Z.; Lai, W.C.; Huang, S.T.; Hung, Y.M.; Yang, C.C. Bacteriology of Naja atra Snakebite Wound and Its Implications for Antibiotic Therapy. Am. J. Trop. Med. Hyg. 2016, 94, 1129–1135. [Google Scholar] [CrossRef]
- Huang, W.-H.; Kao, C.-C.; Mao, Y.-C.; Lai, C.-S.; Lai, K.-L.; Lai, C.-H.; Tseng, C.-H.; Huang, Y.-T.; Liu, P.-Y. Shewanella algae and Morganella morganii Coinfection in Cobra-Bite Wounds: A Genomic Analysis. Life 2021, 11, 329. [Google Scholar] [CrossRef] [PubMed]
- Resiere, D.; Gutiérrez, J.M.; Neviere, R.; Cabié, A.; Hossein, M.; Kallel, H. Antibiotic therapy for snakebite envenoming. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20190098. [Google Scholar] [CrossRef]
- Ngo, N.D.; Le, Q.X.; Pham, A.Q.; Nguyen, N.T.; Ha, H.T.; Dinh, M.M.Q.; Le, T.Q. Clinical Features, Bacteriology, and Antibiotic Treatment Among Patients with Presumed Naja Bites in Vietnam. Wilderness Environ. Med. 2020, 31, 151–156. [Google Scholar] [CrossRef]
- Hung, D.-Z. Taiwan’s Venomous Snakebite: Epidemiological, Evolution and Geographic Differences. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 96–101. [Google Scholar] [CrossRef]
- Chiang, L.-C.; Tsai, W.-J.; Liu, P.-Y.; Ho, C.-H.; Su, H.-Y.; Lai, C.-S.; Lai, K.-L.; Lin, W.-L.; Lee, C.-H.; Yang, Y.-Y.; et al. Envenomation by Trimeresurus stejnegeri stejnegeri: Clinical manifestations, treatment and associated factors for wound necrosis. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20200043. [Google Scholar] [CrossRef]
- Mao, Y.-C.; Liu, P.-Y.; Chiang, L.-C.; Lee, C.-H.; Lai, C.-S.; Lai, K.-L.; Lin, W.-L.; Su, H.-Y.; Ho, C.-H.; Doan, U.V.; et al. Clinical manifestations and treatments of Protobothrops mucrosquamatus bite and associated factors for wound necrosis and subsequent debridement and finger or toe amputation surgery. Clin. Toxicol. 2020, 59, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Hung, D.-Z.; Liau, M.-Y.; Lin-Shiau, S.-Y. The clinical significance of venom detection in patients of cobra snakebite. Toxicon 2003, 41, 409–415. [Google Scholar] [CrossRef]
- Blokhuis-Arkes, M.H.; Haalboom, M.; van der Palen, J.; Heinzle, A.; Sigl, E.; Guebitz, G.; Beuk, R. Rapid enzyme analysis as a diagnostic tool for wound infection: Comparison between clinical judgment, microbiological analysis, and enzyme analysis. Wound Repair Regen. 2015, 23, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Treatment of anaerobic infection. Expert Rev. Anti-Infect. Ther. 2007, 5, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.-C.; Hung, D.-Z. Management of Snake Envenomation in Taiwan. In Clinical Toxinology in Asia Pacific and Africa; Springer: Dordrecht, The Netherlands, 2015; pp. 23–52. [Google Scholar]
- Chang Gung Memorial Hospital. 2022. Available online: http://www.chang-gung.com/en/m/about.aspx?id=11&bid=1 (accessed on 2 August 2022).
- World Health Organization. Guidelines for the Management of Snakebites, 2nd ed.; WHO: Geneva, Switzerland, 2016. [Google Scholar]
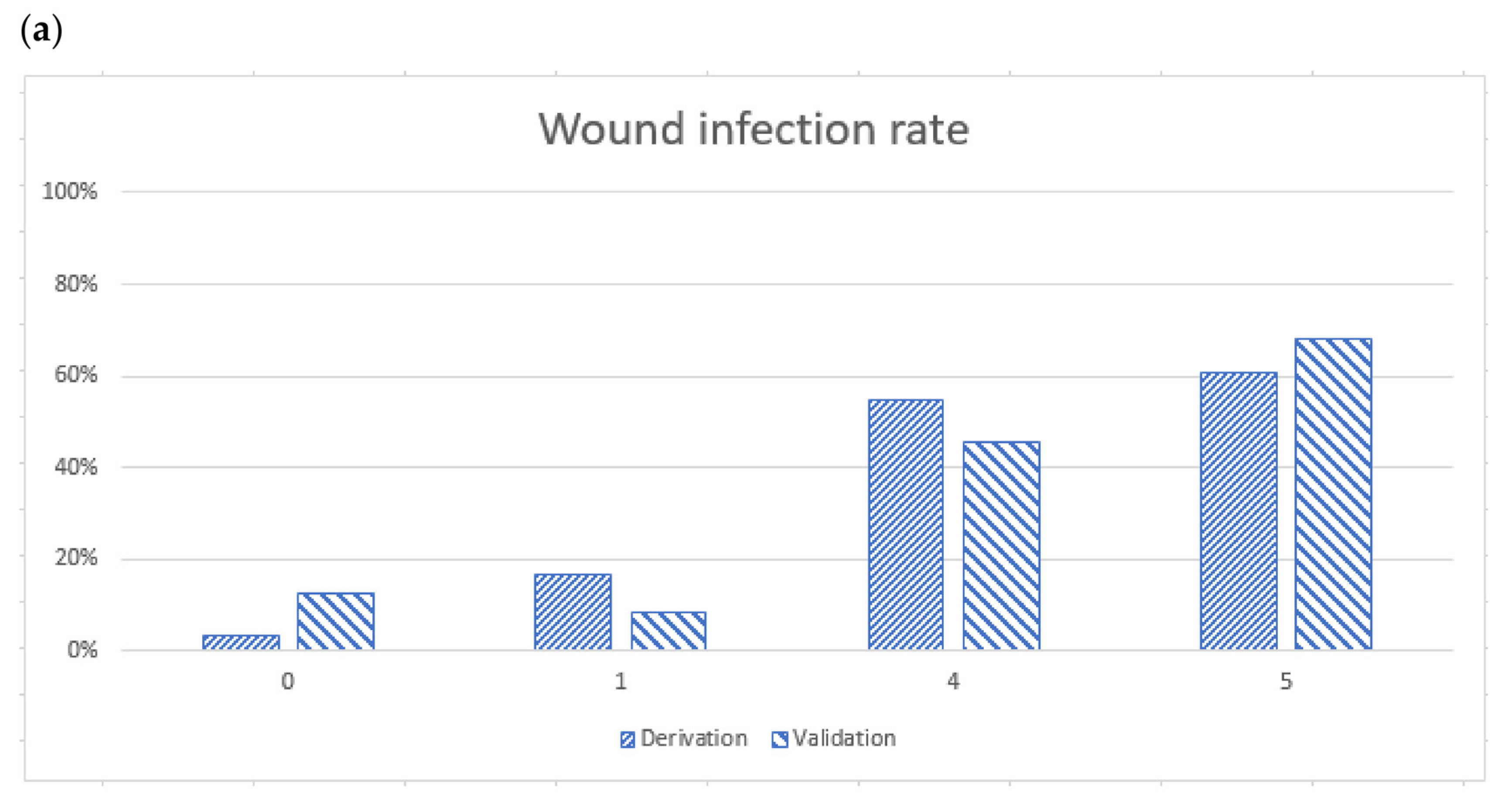
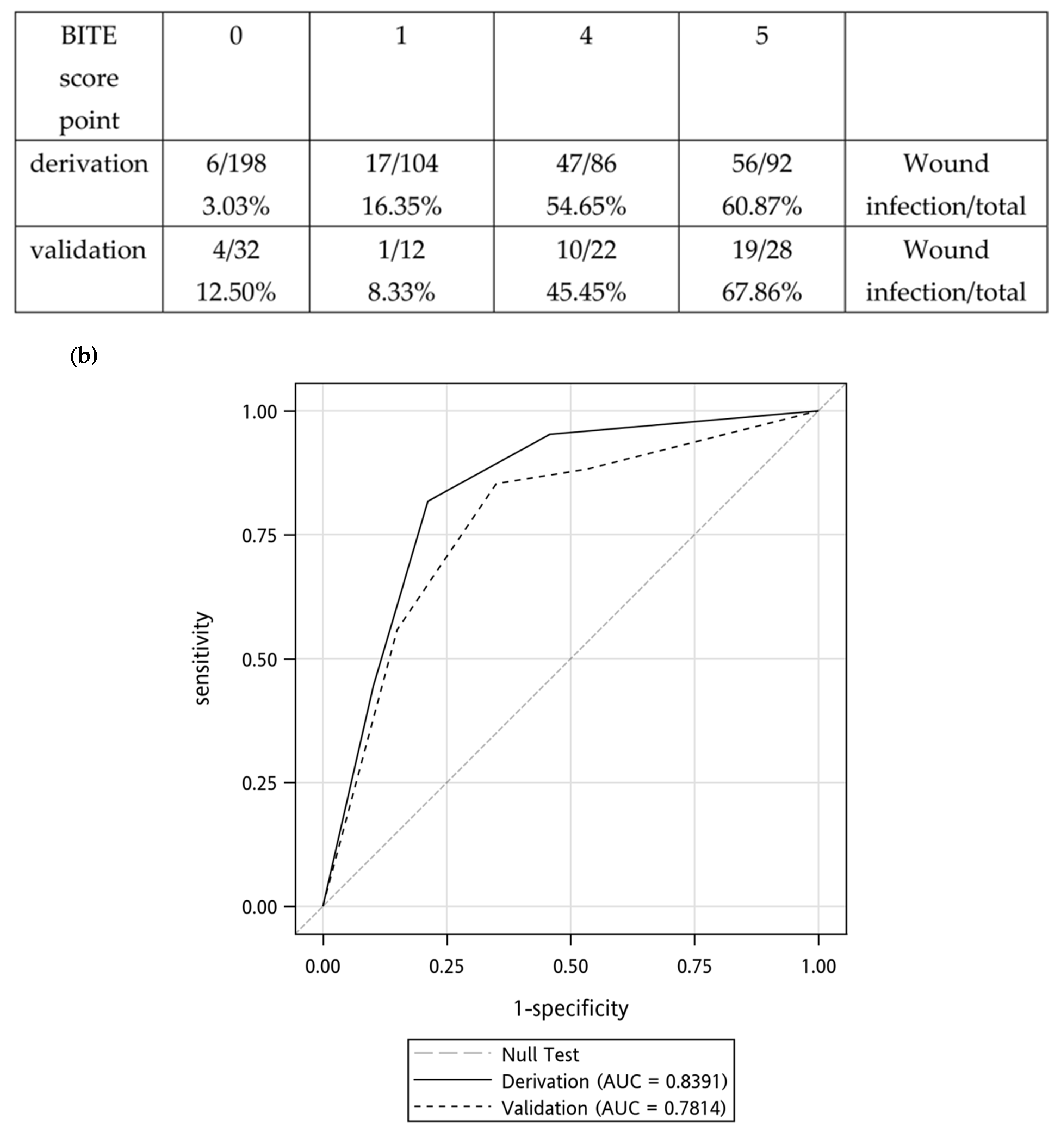
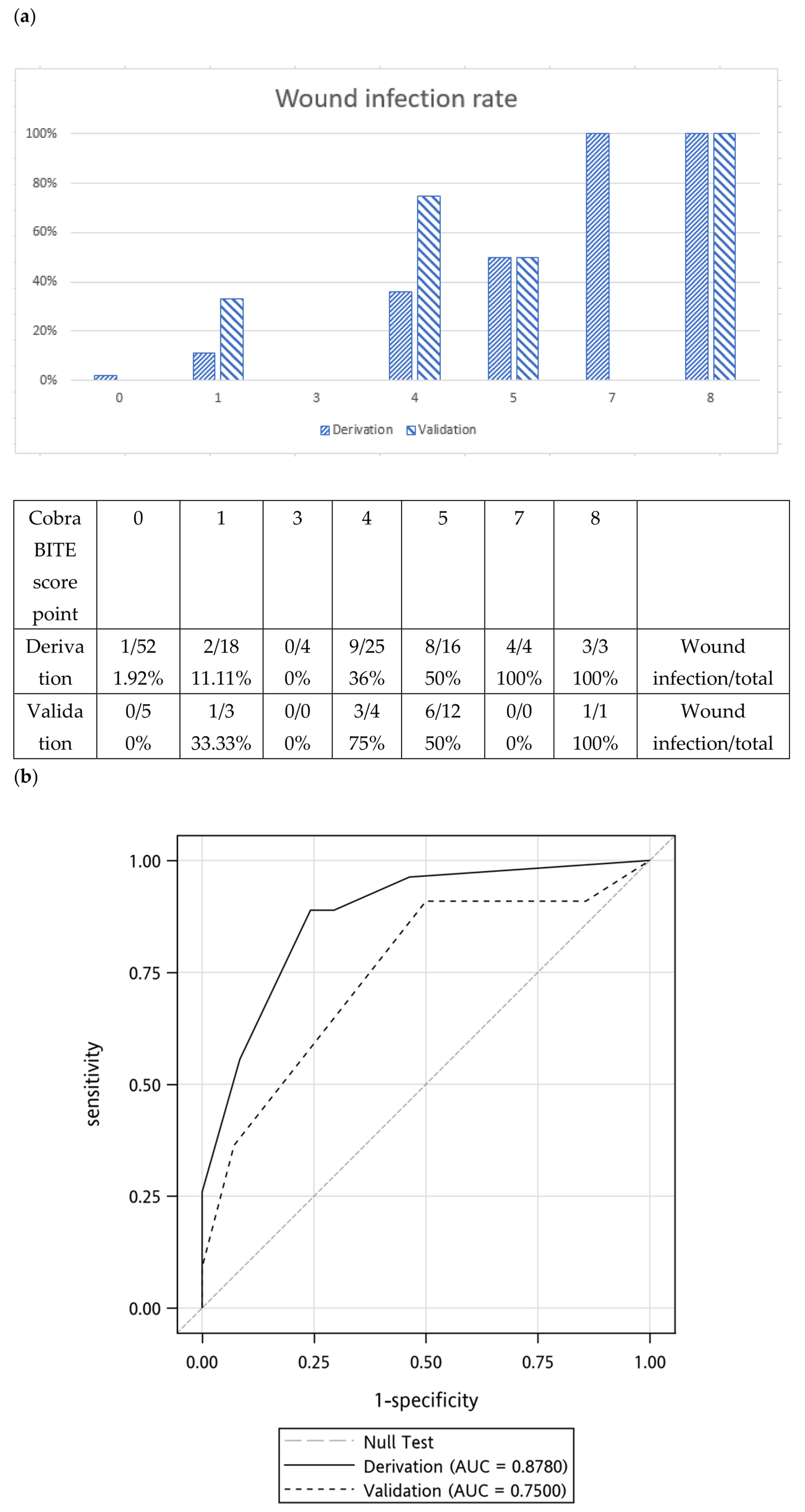
| BITE Score | |||
| Derivation (n = 726) | Validation (n = 95) | p | |
| Age, mean (SD) | 52.07(17.55) | 59.05(16.03) | <0.01 |
| Male, % | 506(69.70) | 64(67.37) | 0.64 |
| WBC (1000/μL) × NLR ※ ≥ 19.84 | 196(40.83) | 40(42.55) | 0.76 |
| Admission | 230(31.68) | 51(53.68) | <0.01 |
| Wound infection rate | 163(22.45) | 35(36.84) | <0.01 |
| Cobra BITE score | |||
| Derivation (n = 172) | Validation (n = 25) | p | |
| Age, mean (SD) | 48.94(17.43) | 56.68(19.52) | 0.04 |
| Male, % | 125(72.67) | 20(80) | 0.44 |
| WBC × NLR ※ ≥114.23 | 12(9.84) | 1(4) | 0.92 |
| Admission | 51(29.65) | 17(68) | <0.01 |
| Antivenin dose ≥4 vials | 43(25) | 16(64) | <0.01 |
| Wound infection rate | 30(17.44) | 11(44) | 0.01 |
| Microorganism | Naja atra Bites, n | Protobothrops mucrosquamatus/Viridovipera stejnegeri Bites, n |
|---|---|---|
| Aerobic Gram-positive bacteria | ||
| Bacillus | 1 | - |
| Coag_staphylococcus | 3 | 1 + 1 |
| Enterococcus_faecalis | 13 + 4 # | 3 |
| Corynebacterium jeikeium | - | 2 |
| Corynebacterium spp | - | 1 |
| Staphylococcus saprophyticus | - | 1 |
| Viridans streptococcus | - | 1 |
| Staph_aureus | 1 | 2 |
| Aerobic Gram-negative bacteria | ||
| Acineto_baumannii | 1 | - |
| Enterobacteriaceae | 1 | - |
| Acinetobacter_sp | 1 | - |
| Shewanella_algae | 2 | - |
| Morganella_morganii | 16 + 2 | 3 |
| Aeromonas hydrophila | - | 1 |
| Stenotrophomonas maltophilia | - | 1 |
| Klebsiella pneumoniae | 1 | - |
| Anaerobic bacteria | ||
| Peptostreptococcus micros | 1 | |
| Proteus vulgaris | 3 | - |
| Citrob_freundii | 1 | - |
| B_fragilis | 2 | 1 |
| B_thetaiotaomicron | 1 | - |
| Serratia_marcescens | 2 | - |
| Providencia_rettgeri | 1 | - |
| Enterobacter cloacae | 1 + 1 | |
| Enterobacter cloacae CR strain | 1 | |
| Others | ||
| Yeast_like | 1 | - |
| Cultured Bacteria | Empirical Antibiotics * | Bacteria Resistant Antibiotics |
|---|---|---|
| Taiwan Cobra infected wound | ||
| Morganella morganii | AMC | CXM, CFZ, SAM |
| Proteus vulgaris | OX, FLO | CXM, CFZ |
| Enterococcus.faecalis | OX, FLO | -- |
| Morganella morganii | AMC | CXM, CFZ |
| Proteus vulgaris | AMC | CXM, CFZ |
| Enterococcus faecalis | OX, CRO | -- |
| Proteus vulgaris | OX, CRO | CXM, CFZ |
| Enterococcus faecalis | AMC, CXM, CC | -- |
| Enterobacteriaceae | AMC, CXM, CC | CFZ |
| Kleb.pneumoniae | AMC, CXM, CC | -- |
| Kleb.pneumoniae | AMC, CXM, CC | -- |
| Enterococcus. Faecalis | AMC, CXM, CC | -- |
| Protobothrops mucrosquamatus and Viridovipera stejnegeri infected wound | ||
| Enterobacter cloacae complex | CFZ, AMC | CXM, CFZ, SAM |
| Coagulase(-) staphylococcus | CFZ, AMC | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, H.; Gao, S.-Y.; Lin, C.-C. Wound Infection of Snakebite from Venomous Protobothrops mucrosquamatus, Viridovipera stejnegeri and Naja atra in Taiwan: Validation of BITE and Cobra BITE Scoring Systems and their Bacteriological Differences in Wound Cultures. Toxins 2023, 15, 78. https://doi.org/10.3390/toxins15010078
Yeh H, Gao S-Y, Lin C-C. Wound Infection of Snakebite from Venomous Protobothrops mucrosquamatus, Viridovipera stejnegeri and Naja atra in Taiwan: Validation of BITE and Cobra BITE Scoring Systems and their Bacteriological Differences in Wound Cultures. Toxins. 2023; 15(1):78. https://doi.org/10.3390/toxins15010078
Chicago/Turabian StyleYeh, Heng, Shi-Ying Gao, and Chih-Chuan Lin. 2023. "Wound Infection of Snakebite from Venomous Protobothrops mucrosquamatus, Viridovipera stejnegeri and Naja atra in Taiwan: Validation of BITE and Cobra BITE Scoring Systems and their Bacteriological Differences in Wound Cultures" Toxins 15, no. 1: 78. https://doi.org/10.3390/toxins15010078
APA StyleYeh, H., Gao, S.-Y., & Lin, C.-C. (2023). Wound Infection of Snakebite from Venomous Protobothrops mucrosquamatus, Viridovipera stejnegeri and Naja atra in Taiwan: Validation of BITE and Cobra BITE Scoring Systems and their Bacteriological Differences in Wound Cultures. Toxins, 15(1), 78. https://doi.org/10.3390/toxins15010078





