The Important Role of Membrane Fluidity on the Lytic Mechanism of the α-Pore-Forming Toxin Sticholysin I
Abstract
:1. Introduction
2. Results
2.1. Binding to Lipid Membranes and Pore-Forming Activity of St I Is Optimal at an Equimolar Mixture of POPC:eggSM
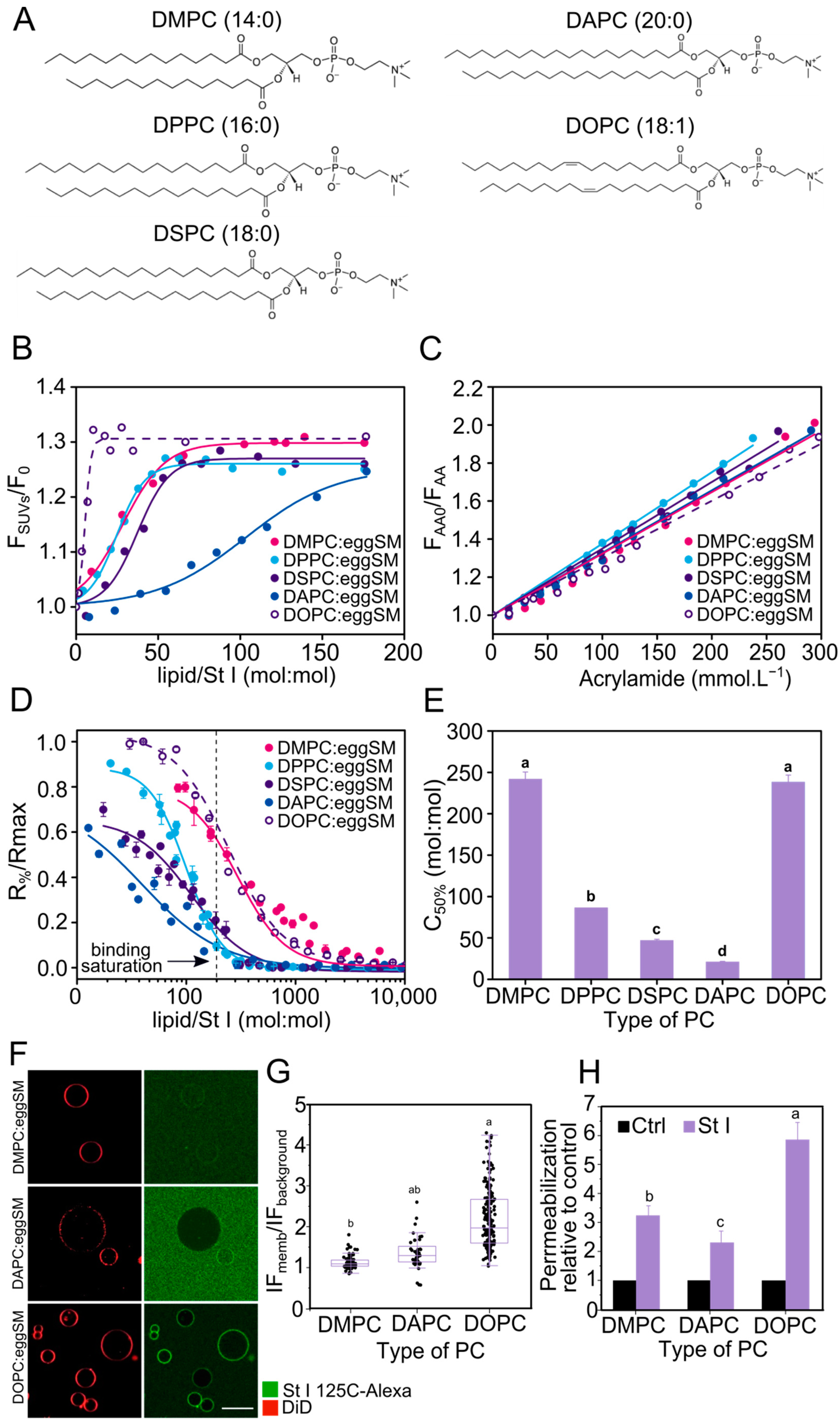
2.2. Membrane Fluidity Favors the Binding and Permeabilizing Activity of St I in Lipid Vesicles
2.3. Membrane Fluidity Regulates the Mechanism of Membrane Permeabilization by St I
2.4. The Stability of the St I Pore Increases with the Decrease in the Fluidity of the Membrane
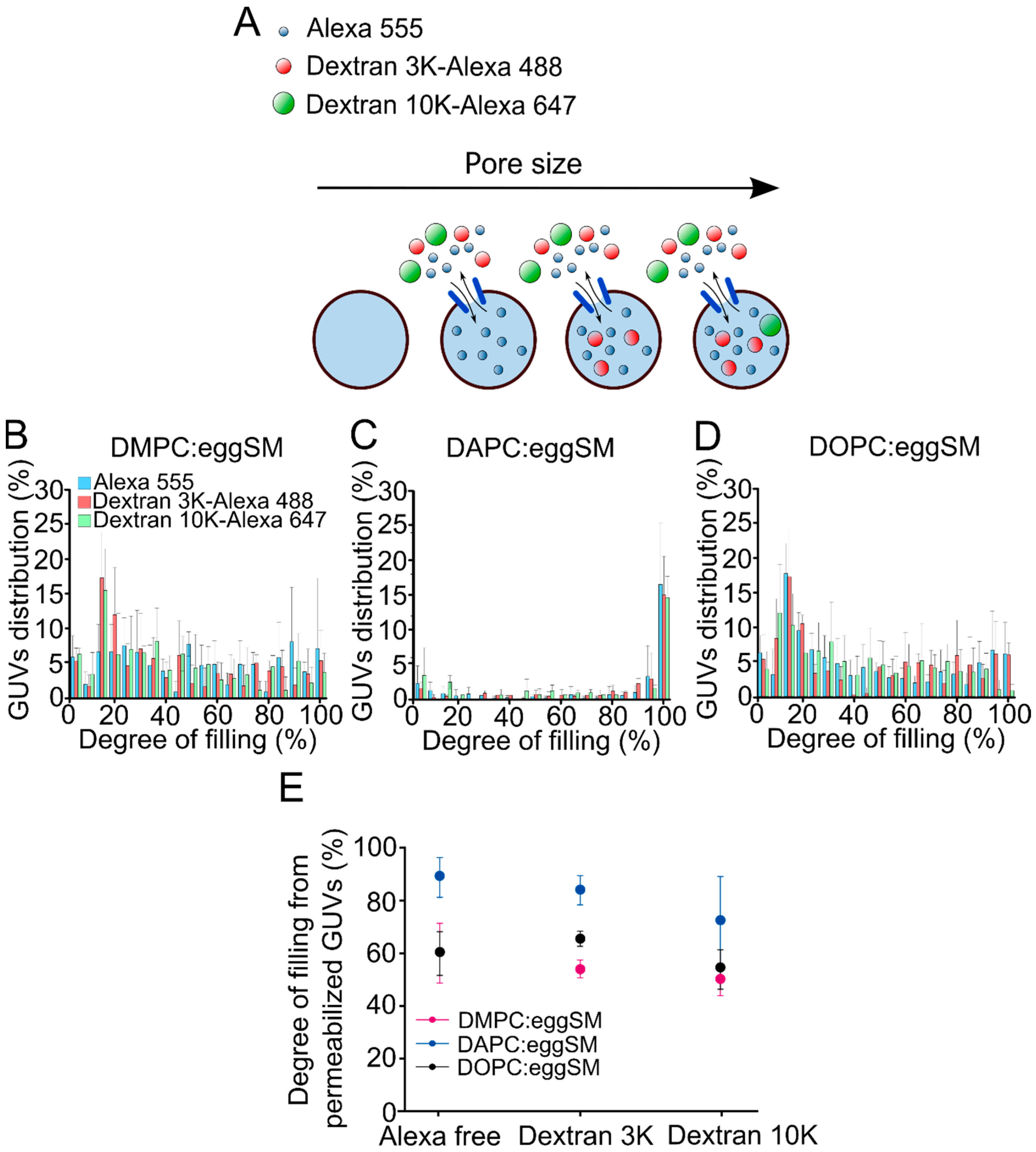
2.5. The Size of St I Pores Decreases with Membrane Fluidity
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Lipid Vesicles
4.3. Binding of St I to SUVs
4.4. Leakage Studies of CF-Containing SUVs
4.5. Binding of St I to GUVs
4.6. Permeabilization of GUVs
4.7. Membrane Fluidity Determined by Pyrene Fluorescence
4.8. Differential Scanning Calorimetric (DSC) Measurements
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lanio, M.E.; Morera, V.; Alvarez, C.; Tejuca, M.; Gómez, T.; Pazos, F.; Besada, V.; Martínez, D.; Huerta, V.; Padrón, G.; et al. Purification and characterization of two hemolysins from Stichodactyla helianthus. Toxicon 2001, 39, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Anderluh, G.; Maček, P. Cytolytic peptide and protein toxins from sea anemones (Anthozoa: Actiniaria). Toxicon 2002, 40, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Mancheño, J.M.; Martín-Benito, J.; Martínez-Ripoll, M.; Gavilanes, J.G.; A Hermoso, J. Crystal and Electron Microscopy Structures of Sticholysin II Actinoporin Reveal Insights into the Mechanism of Membrane Pore Formation. Structure 2003, 11, 1319–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.; Caaveiro, J.M.; Morante, K.; González-Mañas, J.M.; Tsumoto, K. Structural basis for self-assembly of a cytolytic pore lined by protein and lipid. Nat. Commun. 2015, 6, 6337. [Google Scholar] [CrossRef] [Green Version]
- Rojko, N.; Kristan, K.Č.; Viero, G.; Žerovnik, E.; Maček, P.; Dalla Serra, M.; Anderluh, G. Membrane damage by an α-helical pore-forming protein, Equinatoxin II, proceeds through a succession of ordered steps. J. Biol. Chem. 2013, 288, 23704–23715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonini, V.; Pérez-Barzaga, V.; Bampi, S.; Pentón, D.; Martínez, D.; Serra, M.D.; Tejuca, M. Functional Characterization of Sticholysin I and W111C Mutant Reveals the Sequence of the Actinoporin’s Pore Assembly. PLoS ONE 2014, 9, e110824. [Google Scholar] [CrossRef] [Green Version]
- Subburaj, Y.; Ros, U.; Hermann, E.; Tong, R.; García-Sáez, A.J. Toxicity of an α-Pore-forming Toxin Depends on the Assembly Mechanism on the Target Membrane as Revealed by Single Molecule Imaging. J. Biol. Chem. 2015, 290, 4856–4865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojko, N.; Dalla Serra, M.; Maček, P.; Anderluh, G. Pore formation by actinoporins, cytolysins from sea anemones. Biochim. Biophys. Acta 2016, 1858, 446–456. [Google Scholar] [CrossRef]
- Baker, M.A.B.; Rojko, N.; Cronin, B.; Anderluh, G.; Wallace, M.I. Photobleaching Reveals Heterogeneous Stoichiometry for Equinatoxin II Oligomers. ChemBioChem 2014, 15, 2139–2145. [Google Scholar] [CrossRef] [Green Version]
- Pedrera, L.; Fanani, M.L.; Ros, U.; Lanio, M.E.; Maggio, B.; Álvarez, C. Sticholysin I–membrane interaction: An interplay between the presence of sphingomyelin and membrane fluidity. Biochim. Biophys. Acta Biomembr. 2014, 1838, 1752–1759. [Google Scholar] [CrossRef]
- Palacios-Ortega, J.; García-Linares, S.; Rivera-De-Torre, E.; Gavilanes, J.G.; Martínez-Del-Pozo, A.; Slotte, J.P. Differential Effect of Bilayer Thickness on Sticholysin Activity. Langmuir 2017, 33, 11018–11027. [Google Scholar] [CrossRef]
- Pedrera, L.; Gomide, A.B.; Sánchez, R.E.; Ros, U.; Wilke, N.; Pazos, F.; Lanio, M.E.; Itri, R.; Fanani, M.L.; Alvarez, C. The Presence of Sterols Favors Sticholysin I-Membrane Association and Pore Formation Regardless of Their Ability to Form Laterally Segregated Domains. Langmuir 2015, 31, 9911–9923. [Google Scholar] [CrossRef]
- Barlič, A.; Gutiérrez-Aguirre, I.; Caaveiro, J.M.M.; Cruz, A.; Ruiz-Argüello, M.-B.; Pérez-Gil, J.; González-Mañas, J.M. Lipid Phase Coexistence Favors Membrane Insertion of Equinatoxin-II, a Pore-forming Toxin from Actinia equina. J. Biol. Chem. 2004, 279, 34209–34216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schön, P.; García-Sáez, A.J.; Malovrh, P.; Bacia, K.; Anderluh, G.; Schwille, P. Equinatoxin II Permeabilizing Activity Depends on the Presence of Sphingomyelin and Lipid Phase Coexistence. Biophys. J. 2008, 95, 691–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ros, U.; Edwards, M.A.; Epand, R.F.; Lanio, M.E.; Schreier, S.; Yip, C.M.; Alvarez, C.; Epand, R.M. The sticholysin family of pore-forming toxins induces the mixing of lipids in membrane domains. Biochim. Biophys. Acta 2013, 1828, 2757–2762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosentino, K.; Hermann, E.; von Kügelgen, N.; Unsay, J.D.; Ros, U.; García-Sáez, A.J. Force Mapping Study of Actinoporin Effect in Membranes Presenting Phase Domains. Toxins 2021, 13, 669. [Google Scholar] [CrossRef]
- Wilson-Ashworth, H.A.; Bahm, Q.; Erickson, J.; Shinkle, A.; Vu, M.P.; Woodbury, D.; Bell, J.D. Differential Detection of Phospholipid Fluidity, Order, and Spacing by Fluorescence Spectroscopy of Bis-pyrene, Prodan, Nystatin, and Merocyanine. Biophys. J. 2006, 91, 4091–4101. [Google Scholar] [CrossRef] [Green Version]
- Wheaten, S.A.; Lakshmanan, A.; Almeida, P.F. Statistical Analysis of Peptide-Induced Graded and All-or-None Fluxes in Giant Vesicles. Biophys. J. 2013, 105, 432–443. [Google Scholar] [CrossRef] [Green Version]
- Gregory, S.M.; Cavenaugh, A.; Journigan, V.B.; Pokorny, A.; Almeida, P.F. A Quantitative Model for the All-or-None Permeabilization of Phospholipid Vesicles by the Antimicrobial Peptide Cecropin A. Biophys. J. 2008, 94, 1667–1680. [Google Scholar] [CrossRef] [Green Version]
- Apellániz, B.; Nieva, J.L.; Schwille, P.; García-Sáez, A.J. All-or-None versus Graded: Single-Vesicle Analysis Reveals Lipid Composition Effects on Membrane Permeabilization. Biophys. J. 2010, 99, 3619–3628. [Google Scholar] [CrossRef]
- Belmonte, G.; Pederzolli, C.; Maček, P.; Menestrina, G. Pore formation by the sea anemone cytolysin equinatoxin II in red blood cells and model lipid membranes. J. Membr. Biol. 1993, 131, 11–22. [Google Scholar] [CrossRef] [PubMed]
- De Los Rios, V.; Mancheno, J.M.; Lanio, M.E.; Oñaderra, M.; Gavilanes, J.G. Mechanism of the leakage induced on lipid model membranes by the hemolytic protein sticholysin II from the sea anemone Stichodactyla helianthus. Eur. J. Biochem. 1998, 252, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Tejuca, M.; Díaz, I.; Figueredo, R.; Roque, L.; Pazos, F.; Martínez, D.; Iznaga-Escobar, N.; Pérez, R.; Alvarez, C.; Lanio, M. Construction of an immunotoxin with the pore forming protein StI and ior C5, a monoclonal antibody against a colon cancer cell line. Int. Immunopharmacol. 2004, 4, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Tejuca, M.; Anderluh, G.; Serra, M.D. Sea anemone cytolysins as toxic components of immunotoxins. Toxicon 2009, 54, 1206–1214. [Google Scholar] [CrossRef]
- Laborde, R.J.; Sanchez-Ferras, O.; Luzardo, M.C.; Cruz-Leal, Y.; Fernández, A.; Mesa, C.; Oliver, L.; Canet, L.; Abreu-Butin, L.; Nogueira, C.V.; et al. Novel Adjuvant Based on the Pore-Forming Protein Sticholysin II Encapsulated into Liposomes Effectively Enhances the Antigen-Specific CTL-Mediated Immune Response. J. Immunol. 2017, 198, 2772–2784. [Google Scholar] [CrossRef] [Green Version]
- Laborde, R.J.; Ishimura, M.E.; Abreu-Butin, L.; Nogueira, C.V.; Grubaugh, D.; Cruz-Leal, Y.; Luzardo, M.C.; Fernández, A.; Mesa, C.; Pazos, F.; et al. Sticholysins, pore-forming proteins from a marine anemone can induce maturation of dendritic cells through a TLR4 dependent-pathway. Mol. Immunol. 2021, 131, 144–154. [Google Scholar] [CrossRef]
- Valcarcel, C.A.; Serra, M.D.; Potrich, C.; Bernhart, I.; Tejuca, M.; Martinez, D.; Pazos, F.; Lanio, M.E.; Menestrina, G. Effects of Lipid Composition on Membrane Permeabilization by Sticholysin I and II, Two Cytolysins of the Sea Anemone Stichodactyla helianthus. Biophys. J. 2001, 80, 2761–2774. [Google Scholar] [CrossRef] [Green Version]
- Palacios-Ortega, J.; García-Linares, S.; Åstrand, M.; Al Sazzad, A.; Gavilanes, J.G.; Martínez-Del-Pozo, A.; Slotte, J.P. Regulation of Sticholysin II-Induced Pore Formation by Lipid Bilayer Composition, Phase State, and Interfacial Properties. Langmuir 2016, 32, 3476–3484. [Google Scholar] [CrossRef]
- Tejuca, M.; Dalla Serra, M.; Ferreras, M.; Lanio, M.E.; Menestrina, G. Mechanism of membrane permeabilization by sticholysin I, a cytolysin isolated from the venom of the sea anemone Stichodactyla helianthus. Biochemistry 1996, 35, 14947–14957. [Google Scholar] [CrossRef]
- Bellomio, A.; Morante, K.; Barlič, A.; Gutiérrez-Aguirre, I.; Viguera, A.R.; González-Mañas, J.M. Purification, cloning and characterization of fragaceatoxin C, a novel actinoporin from the sea anemone Actinia fragacea. Toxicon 2009, 54, 869–880. [Google Scholar] [CrossRef]
- Bakrač, B.; Gutiérrez-Aguirre, I.; Podlesek, Z.; Sonnen, A.F.-P.; Gilbert, R.J.; Maček, P.; Lakey, J.H.; Anderluh, G. Molecular Determinants of Sphingomyelin Specificity of a Eukaryotic Pore-forming Toxin. J. Biol. Chem. 2008, 283, 18665–18677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Linares, S.; Palacios-Ortega, J.; Yasuda, T.; Åstrand, M.; Gavilanes, J.G.; Martínez-Del-Pozo, A.; Slotte, J.P. Toxin-induced pore formation is hindered by intermolecular hydrogen bonding in sphingomyelin bilayers. Biochim. Biophys. Acta 2016, 1858, 1189–1195. [Google Scholar] [CrossRef] [Green Version]
- Maula, T.; Isaksson, Y.J.E.; García-Linares, S.; Niinivehmas, S.; Pentikäinen, O.T.; Kurita, M.; Yamaguchi, S.; Yamamoto, T.; Katsumura, S.; Gavilanes, J.G.; et al. 2NH and 3OH are crucial structural requirements in sphingomyelin for sticholysin II binding and pore formation in bilayer membranes. Biochim. Biophys. Acta Biomembr. 2013, 1828, 1390–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goñi, F.M.; Alonso, A.; Bagatolli, L.A.; Brown, R.E.; Marsh, D.; Prieto, M.; Thewalt, J.L. Phase diagrams of lipid mixtures relevant to the study of membrane rafts. Biochim. Biophys. Acta 2008, 1781, 665–684. [Google Scholar] [CrossRef] [Green Version]
- Lewis, B.A.; Engelman, D.M. Lipid bilayer thickness varies linearly with acyl chain length in fluid phosphatidylcholine vesicles. J. Mol. Biol. 1983, 166, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.; Bergado, G.; Blanco, R.; Griñán, T.; Rodríguez, H.; Ros, U.; Pazos, F.; Lanio, M.E.; Hernández, A.M.; Álvarez, C. Sticholysin II-mediated cytotoxicity involves the activation of regulated intracellular responses that anticipates cell death. Biochimie 2018, 148, 18–35. [Google Scholar] [CrossRef]
- Cabezas, S.; Ho, S.; Ros, U.; Lanio, M.E.; Alvarez, C.; van der Goot, F.G. Damage of eukaryotic cells by the pore-forming toxin sticholysin II: Consequences of the potassium efflux. Biochim. Biophys. Acta Biomembr. 2017, 1859, 982–992. [Google Scholar] [CrossRef]
- Rouser, G.; Fleischer, S.; Yamamoto, A. Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 1970, 5, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Bleicken, S.; Wagner, C.; García-Sáez, A.J. Mechanistic Differences in the Membrane Activity of Bax and Bcl-xL Correlate with Their Opposing Roles in Apoptosis. Biophys. J. 2013, 104, 421–431. [Google Scholar] [CrossRef] [Green Version]
- Lakowicz, J.R. Principles of Frequency-Domain Fluorescence Spectroscopy and Applications to Cell Membranes. Subcell. Biochem. 1988, 13, 89–126. [Google Scholar] [CrossRef]
- Hermann, E.; Bleicken, S.; Subburaj, Y.; García-Sáez, A.J. Automated analysis of giant unilamellar vesicles using circular Hough transformation. Bioinformatics 2014, 30, 1747–1754. [Google Scholar] [CrossRef] [PubMed]



| Composition | FSUVs/F0 max | L50% (mol:mol) | ΔλSUVs-sol (nm) | KSV (M−1) |
|---|---|---|---|---|
| Solution | - | - | - | 5.1 ± 0.1 |
| POPC | 1.15 ± 0.01 | 51.3 ± 7.7 | 2 ± 1 | 4.2 ± 0.1 |
| POPC:eggSM (85:15) | 1.23 ± 0.01 | 33.5 ± 2.7 | 2 ± 1 | - |
| POPC:eggSM (70:30) | 1.30 ± 0.01 | 9.9 ± 0.5 | 6 ± 1 | 3.6 ± 0.1 |
| POPC:eggSM (50:50) | 1.30 ± 0.01 | 8.2 ± 0.2 | 5 ± 1 | 3.5 ± 0.1 |
| POPC:eggSM (30:70) | 1.27 ± 0.01 | 8.1 ± 0.4 | 6 ± 1 | 3.7 ± 0.1 |
| Composition | FSUVs/F0 max | L50% (mol:mol) | ΔλSUVs-sol (nm) | KSV (M−1) |
|---|---|---|---|---|
| DMPC:eggSM | 1.30 ± 0.01 | 28.3 ± 2.4 | 5 ± 1 | 3.2 ± 0.1 |
| DPPC:eggSM | 1.26 ± 0.01 | 24.9 ± 1.1 | 5 ± 1 | 3.7 ± 0.1 |
| DSPC:eggSM | 1.27 ± 0.01 | 37.6 ± 1.8 | 3 ± 1 | 3.5 ± 0.1 |
| DAPC:eggSM | 1.25 ± 0.01 | 103.0 ± 8.0 | 5 ± 1 | 3.3 ± 0.1 |
| DOPC:eggSM | 1.31 ± 0.01 | 5.4 ± 0.5 | 5 ± 1 | 3.0 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrera, L.; Ros, U.; Fanani, M.L.; Lanio, M.E.; Epand, R.M.; García-Sáez, A.J.; Álvarez, C. The Important Role of Membrane Fluidity on the Lytic Mechanism of the α-Pore-Forming Toxin Sticholysin I. Toxins 2023, 15, 80. https://doi.org/10.3390/toxins15010080
Pedrera L, Ros U, Fanani ML, Lanio ME, Epand RM, García-Sáez AJ, Álvarez C. The Important Role of Membrane Fluidity on the Lytic Mechanism of the α-Pore-Forming Toxin Sticholysin I. Toxins. 2023; 15(1):80. https://doi.org/10.3390/toxins15010080
Chicago/Turabian StylePedrera, Lohans, Uris Ros, Maria Laura Fanani, María E. Lanio, Richard M. Epand, Ana J. García-Sáez, and Carlos Álvarez. 2023. "The Important Role of Membrane Fluidity on the Lytic Mechanism of the α-Pore-Forming Toxin Sticholysin I" Toxins 15, no. 1: 80. https://doi.org/10.3390/toxins15010080
APA StylePedrera, L., Ros, U., Fanani, M. L., Lanio, M. E., Epand, R. M., García-Sáez, A. J., & Álvarez, C. (2023). The Important Role of Membrane Fluidity on the Lytic Mechanism of the α-Pore-Forming Toxin Sticholysin I. Toxins, 15(1), 80. https://doi.org/10.3390/toxins15010080





