Abstract
Calamus tenuis is a shrub species distributed across South Asia. It grows well in diversified habitats and tends to dominate plants in the surrounding environment. The phytotoxicity of C. tenuis and the action of its phytochemicals against other plant species could explain its dominant behavior. Compounds with phytotoxic activity are in high demand as prospective sources of ecofriendly bioherbicides. Therefore, we investigated the phytotoxicity of C. tenuis. Aqueous methanol extracts of this plant species significantly limited the growth of four test plant species, two monocots (barnyard grass and timothy), and two dicots (alfalfa and cress), in a dose- and species-dependent manner. Bio-directed chromatographic isolation of the C. tenuis extracts yielded two major active substances: a novel compound, calamulactone {(S)-methyl 8-(5-oxo-2,5-dihydrofuran-2-yl) octanoate}, and 3-oxo-α-ionone. Both of the identified compounds exerted strong growth inhibitory effects on cress and timothy seedlings. The concentrations of 3-oxo-α-ionone and calamulactone required to limit the growth of the cress seedlings by 50% (I50) were 281.6–199.5 and 141.1–105.5 µM, respectively, indicating that the effect of calamulactone was stronger with lower I50 values. Similarly, the seedlings of timothy also showed a considerably higher sensitivity to calamulactone (I50: 40.5–84.4 µM) than to 3-oxo-α-ionone (I50: 107.8–144.7 µM). The findings indicated that the leaves of C. tenuis have marked growth-inhibitory potential, and could affect surrounding plants to exert dominance over the surrounding plant community. Moreover, the two identified phytotoxic substances might play a key role in the phytotoxicity of C. tenuis, and could be a template for bioherbicide development. This paper was the first to report calamulactone and its phytotoxicity.
Key Contribution:
Isolation of a new phytotoxic substance calamulactone and estimation of its phytotoxicity. Identification of 3-oxo-α-ionone and determination of its phytotoxic potential. C. tenuis could be proposed as a template for bioherbicide development.
1. Introduction
Weed plants are highly adaptable and proliferate in a variety of habitats, disrupt agricultural crop development, and compromise ecosystem functioning. They can easily spread from their original habitats to other distant habitats [1]. Weeds are considered a serious menace to world agroecosystems, as they reduce the quality and productivity of agricultural crops [2]. They compete with agricultural crops for various growth resources, such as space, water, nutrients, light, and gases, as well as host pests and many diseases [3,4]. The reduction in crop yields caused by weeds varies greatly depending on crop type, weed control practices, composition of weeds, period of infestation, and different climatic and soil factors [5]. Weed management strategies play a vital role in successful crop production. Different weed management techniques are applied, such as cultural, physical, chemical, and biological techniques. Due to the scarcity of labor, the use of chemical herbicides or weedicides to decrease weed populations is becoming more popular throughout the world [6]. To control weeds, chemical methods are largely used in agriculture due to their easy application, cost effectiveness, and availability [7,8]. However, synthetic herbicidal weed control is hazardous to both the environment and people [9,10]. Furthermore, the long-term use of synthetic herbicides with limited variation in the mode of action has resulted in the emergence of herbicide resistance. Presently, more than 250 species of weeds have developed resistance around the world [11]. Based on these problems, many research studies have been conducted to investigate reducing the dependency on synthetic herbicides, and subsequently, biological weed control appears to be the most suitable alternative for herbicidal weed control [12,13]. This strategy involves using substances found in crop extracts and decomposed microbes [14,15].
C. tenuis Roxb. (local name bet in Bangladesh) is a shrub-type palm belonging to the Arecaceae family. It is an evergreen perennial plant with a slender stem and forms clusters [16]. This plant is found in India, Bangladesh, Thailand, Myanmar, Indonesia, and Cambodia [17], and is a predominant species in the northeastern part of Bangladesh. This plant species grows in foothills to plain slopes, riverbanks, and shady, damp, and wet areas [18]. They can also grow in weed-infested areas without any intercultural operations. The products of this forest plant are non-woody and have high economic value [19], very light, flexible, and durable features, and considered an important raw material for the cottage and handicraft industries. The young stems or the upper part of stems are used as both a vegetable and in traditional medicine [20] to treat fevers, dyspepsia, biliousness, piles, bacterial infections, wounds [21], diabetes [22], inflammation [23], stomach disorders, and intestinal infections [24]. Moreover, researchers have documented different biological activities of C. tenuis, including antioxidant, phytochemical, cytotoxic, and antibacterial activities, and may serve as a new potential source of medicines for humans [17,25,26]. Furthermore, Thakur et al. [27] found that methanol extracts of C. tenuis contain different secondary metabolites, including flavonoids, carbohydrates, saponins, glycosides, and steroids. Although different biological activities have been recorded for this species, no documents were found in the literature about the phytotoxic potential of this plant. Thus, the purpose of this research was to assess the phytotoxic effect of C. tenuis and to identify its phytotoxic compounds that may explain the dominant behavior of this plant species, and to provide information that could be helpful for developing new bioherbicides.
2. Results
2.1. Phytotoxicity of the C. tenuis Extract
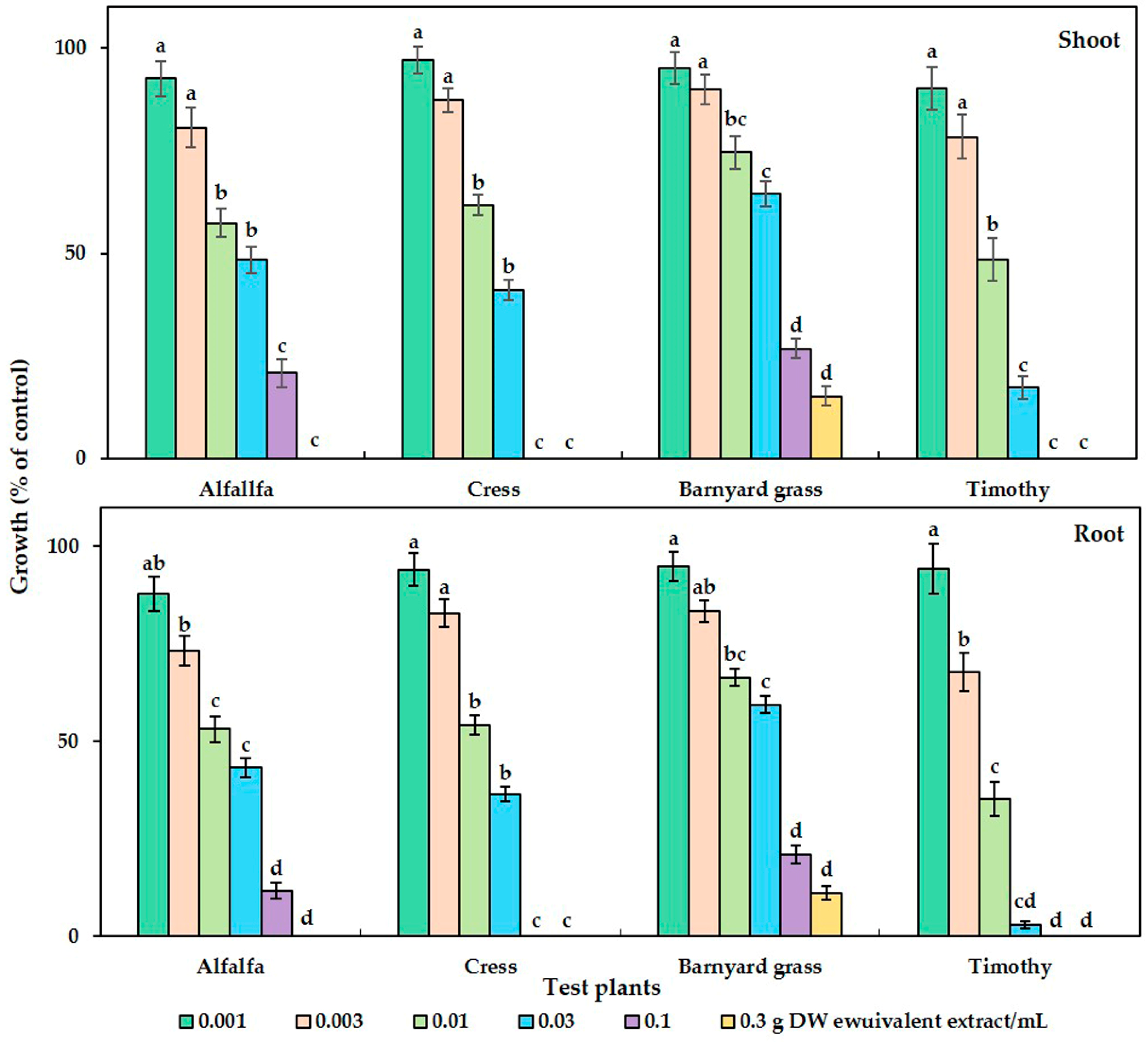
The phytotoxicity of the C. tenuis aqueous methanol extract had a significant effect on all the tested plant species (Figure 1). Dose-dependent growth inhibitory effects in parallel with the increase in extract concentration were observed, regardless of the test plant species. Although no statistically significant growth inhibitory effect was observed with the initial dose of the C. tenuis extract (0.001 g DW/mL), its inhibitory activity was increased with higher concentrations. The seedling growth of timothy was inhibited by more than 50% at 0.01 g DW of C. tenuis extract/mL, and the shoot and root was inhibited by 57.33%, 61.67 %, and 74.61%, and 53.21%, 54.18 %, and 66.42% of the control in the cases of alfalfa, cress, and barnyard grass, respectively. Notably, the seedling growth rates of cress and timothy were fully suppressed by 0.1 g DW/mL of the C. tenuis extract, although alfalfa and barnyard grass were limited to less than 30% of the control treatment. All test plant seedlings except for barnyard grass were totally inhibited at 0.3 g DW/mL of the C. tenuis extract.
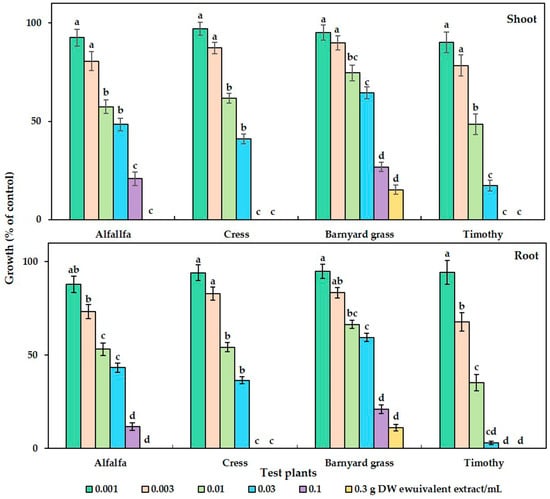
Figure 1.
The growth of alfalfa (Medicago sativa), barnyard grass (Echinochloa crus-galli), cress (Lepidium sativum), and timothy (Phleum pretense) at different C. tenuis extract concentrations. The mean values, along with their standard errors, were obtained from two separate trials, each consisting of 3 replications. Vertical bars show the standard error. Different letters denote a 5% significant difference between the treatment and control.
The concentration of C. tenuis leaf extract required to suppress 50% of the seedling growth (I50 values) of all the test plants was within the range of 5.78–47.57 mg DW equivalent C. tenuis extract/mL (Table 1). Timothy was the most sensitive species to the extract, whereas barnyard grass was the least sensitive, as indicated by their respective I50 values.

Table 1.
C. tenuis extract doses that inhibit test plant seedling growth by 50% (I50).
2.2. Isolation and Characterization of the Active Substances
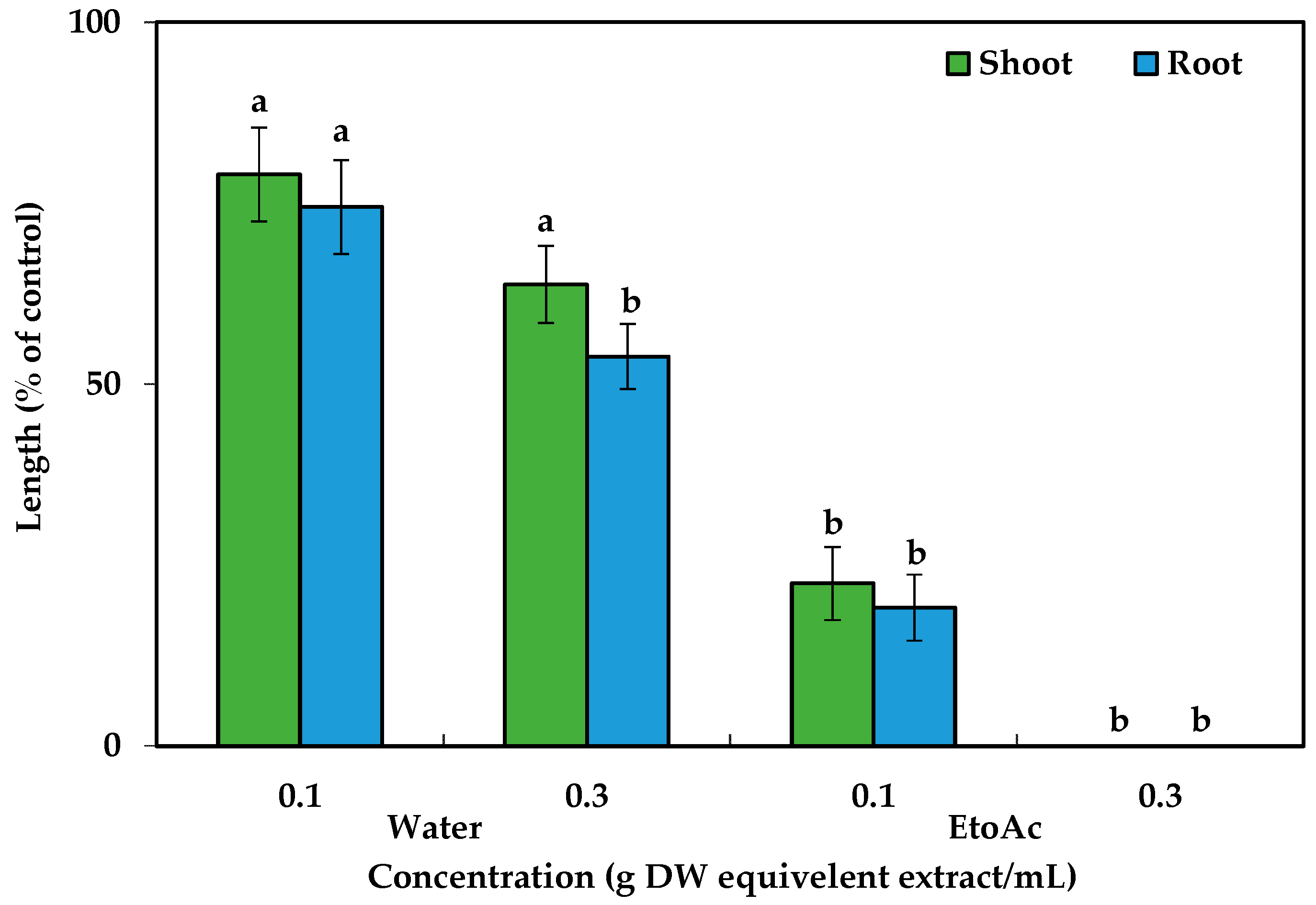
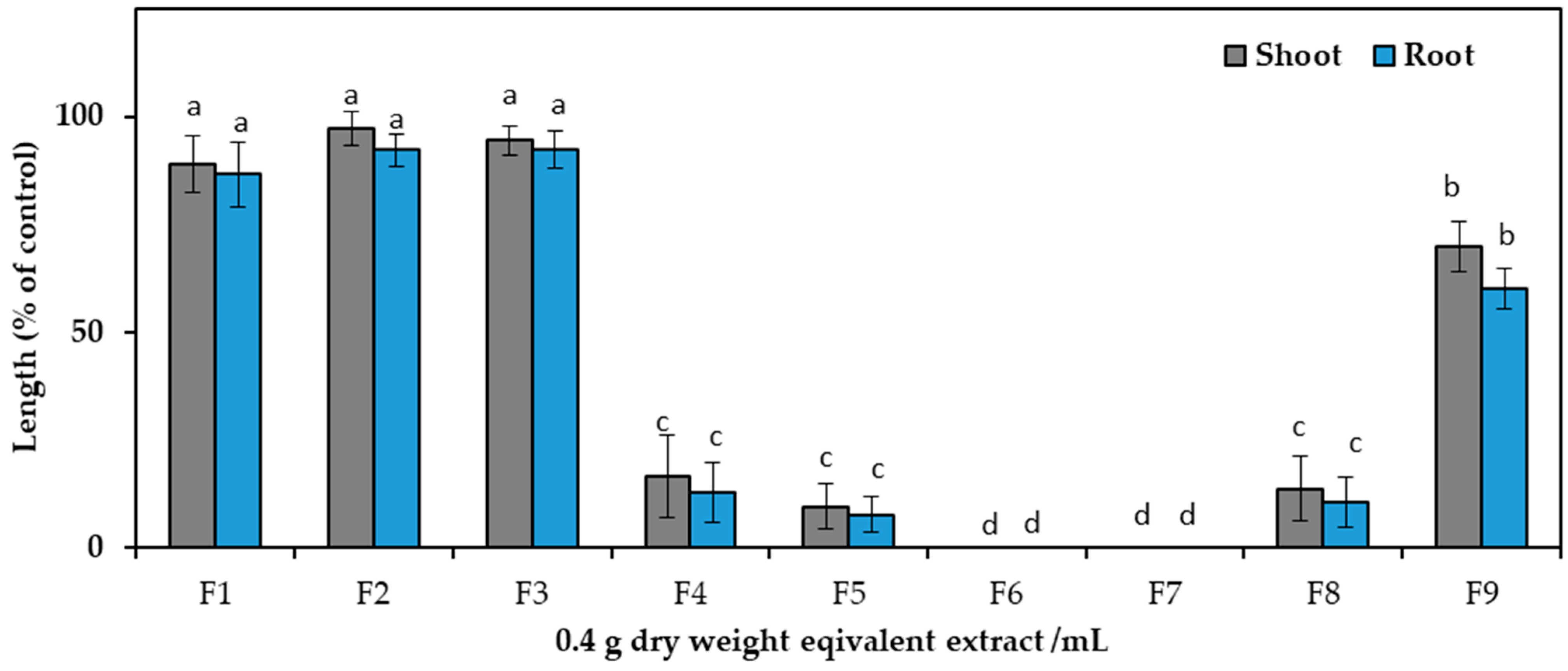
After dividing the aqueous methanol crude extracts, ethyl acetate and aqueous fractions were analyzed against cress seedlings at 0.1 g and 0.3 g DW equivalent C. tenuis extract/mL. Compared to the aqueous fraction, ethyl acetate exhibited greater phytotoxic activity (Figure 2) and was consequently chosen for the next isolation process and chromatographed on a silica gel column, yielding nine fractions through eluting with increasing amounts of ethyl acetate (10% per step, v/v) in n-hexane: F1, F2, F3, F4, F5, F6, and F7 contained 20%, 30%, 40%, 50%, 60%, 70%, and 80% ethyl acetate in n-hexane, respectively, followed by F8 (ethyl acetate), and F9 (methanol) (Figure 3). The column was among these fractions; the most phytotoxic activity was found from fractions six and seven obtained from 70% and 80% ethyl acetate/n-hexane.
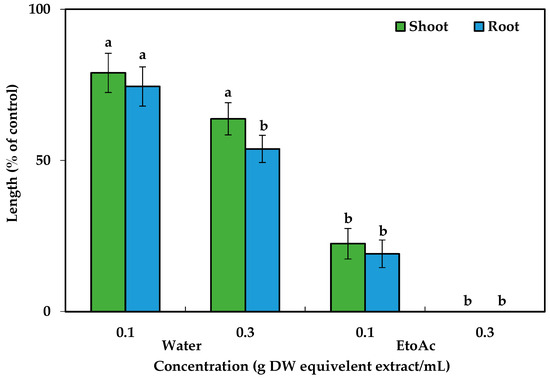
Figure 2.
Cress responses to C. tenuis aqueous and ethyl acetate extracts. The mean values, along with their standard errors, were obtained from two separate trials. Vertical bars show the mean standard error. Different letters denote a 5% significant difference between the treatment and control.
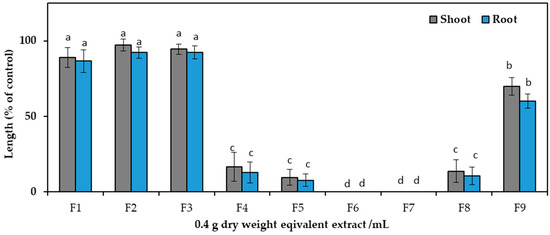
Figure 3.
Effect of silica gel column fractions on the seedling growth of cress at the concentration 0.4 g dry weight equivalent extract/mL of C. tenuis. The values are mean ± SE obtained from two independent experiments. Vertical bars show the standard error. Different alphabet letters indicate significant differences between the treatment and control at a 5% probability level.
These fractions were then purified with Sephadex LH-20, a reverse-phase C18 cartridge, and HPLC, yielding two pure compounds, substances 1 and 2. Finally, two purified substances were characterized through spectroscopic analysis. The NMR spectral data of substance 1 has been summarized in Table 2.

Table 2.
NMR spectral data for substance 1 in CDCl3.
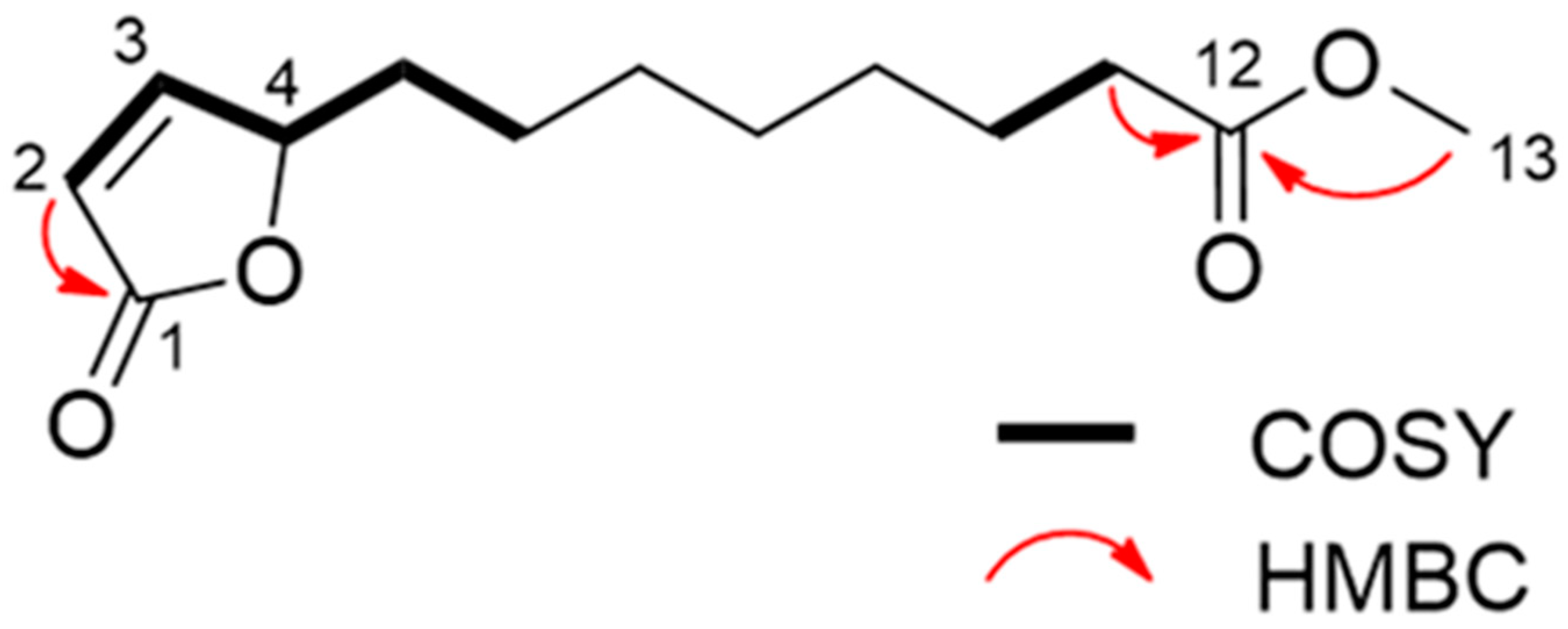
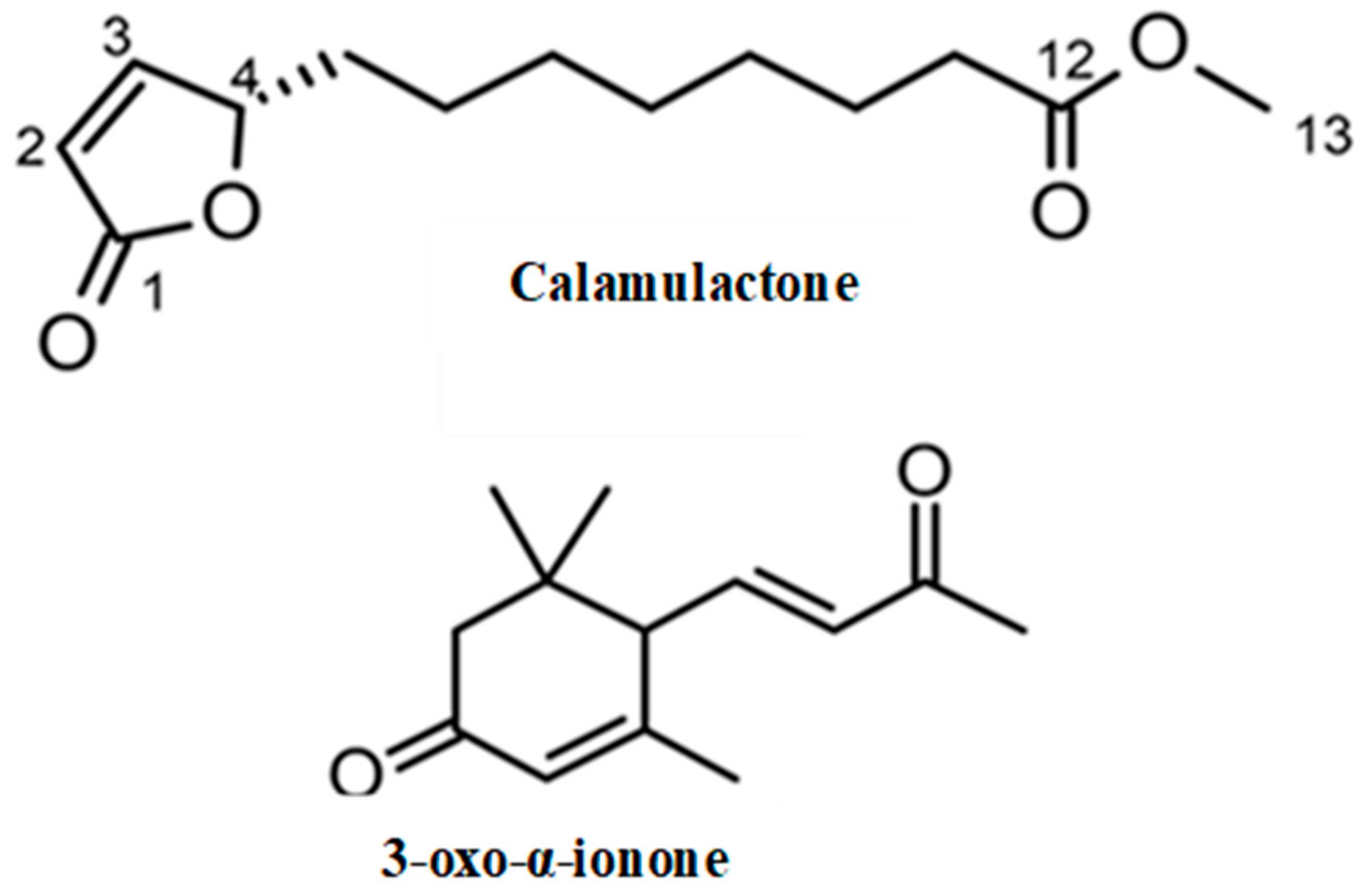
Through spectroscopic analysis, the chemical formula of substance 1, a colorless oil, was determined to be C13H20O4 via HRESIMS. The NMR data for substance 1 has been summarized in Table 2. The 1H NMR spectrum of substance 1 indicated one methoxy group (singlet at δH 3.67). The 13C NMR spectrum suggested two carbonyl carbons (δC 174.4 and 173.3) and two olefinic carbons (δC 156.3 and 121.8). The remaining carbon signals were assigned to one methoxy group, seven methylene groups, and one methine based on the results of an HSQC experiment. The gross structure was determined based on 1D and 2D NMR experiments (Figure 4). A detailed analysis of the COSY and HSQC spectra of substance 1 allowed for the identification of two partial structures, C-2 to C-6 and C-10 to C-11. The HMBC correlations between H-2/C-1 and H-11/C-12 suggested the connectivity of C-1-C-2 and C-11-C-12. The linkage of C-1 to C-4 via an oxygen atom (O2) was confirmed through the chemical shifts consistent with an α, β-unsaturated γ-lactone ring [28]. In addition, the HMBC correlation between H-13/C-12 indicated that the methoxycarbonyl part was joined to C-12. The connectivity of C-6-C-10 was confirmed through its molecular formula and degree of unsaturation. Hence, the gross structure of substance 1 was determined to be as shown in Figure 5. The absolute configuration of C-4 was determined on the basis of the ECD spectrum according to a previous report [29]. As the ECD spectra of substance 1 showed a positive π-π* transition at 204 nm, the configuration of C-4 was determined to be 4S. Thus, we assigned substance 1 as a novel compound, (S)-methyl 8-(5-oxo-2,5-dihydrofuran-2-yl) octanoate (calamulactone). On the other hand, the chemical structure of substance 2 was determined as 3-oxo-α-ionone through its 1H NMR spectrum as measured in CDCl3 in comparison with reported data [30]. The molecular structures of both substances are shown in Figure 5.
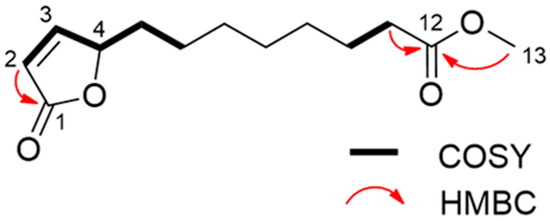
Figure 4.
Gross structure of calamulactone ((S)-methyl 8-(5-oxo-2,5-dihydrofuran-2-yl) octanoate) via 1D and 2D NMR spectroscopy.
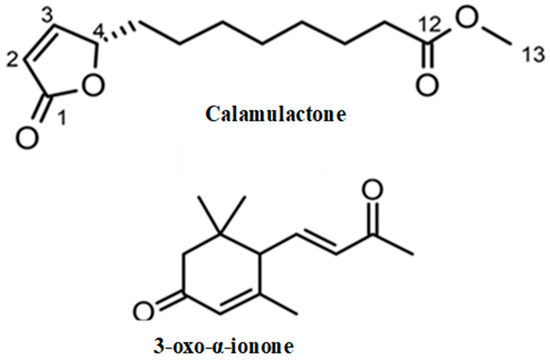
Figure 5.
Molecular structures of calamulactone ((S)-methyl 8-(5-oxo-2,5-dihydrofuran-2-yl) octanoate) and 3-oxo-α-ionone from the C. tenuis leaf extracts.
2.3. Phytotoxicity of the Identified Substances
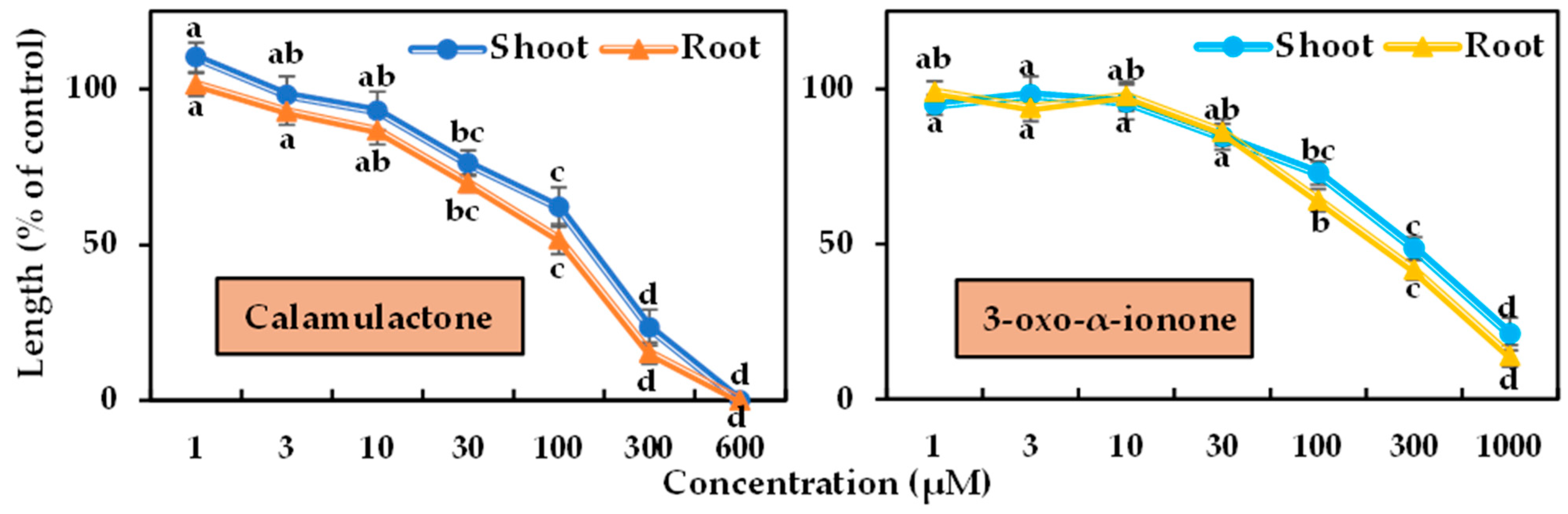
The phytotoxicity of the two isolated compounds was tested in a bioassay using cress and timothy at varying doses. Differences in phytotoxicity were observed between the two tested plants’ seedling growth, and the magnitude of phytotoxicity increased with increasing doses (Figure 6 and Figure 7). Calamulactone and 3-oxo-α-ionone started to significantly limit the seedling growth of the cress at the concentration of 30 μM. At 100 μM, calamulactone inhibited cress shoot growth by 62.14% and root growth by 51.80% from the control, while 3-oxo-α-ionone reduced the growth of cress shoots and roots to 72.89% and 64.10%, respectively. At higher concentrations, calamulactone became more toxic and completely inhibited cress growth at 600 μM. However, at 1000 μM, 3-oxo-α-ionone inhibited cress growth to just 21.02 %and 13.96% of the control in the shoot and root, respectively.
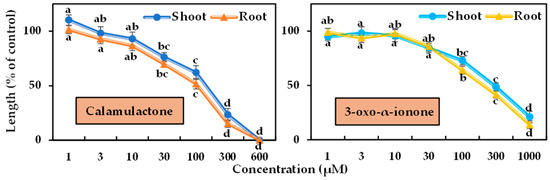
Figure 6.
Phytotoxic effects of calamulactone and 3-oxo-α-ionone on cress. Values are the means ± SE from three replications. Different letters denote statistically significant variations between the treatment and control groups.
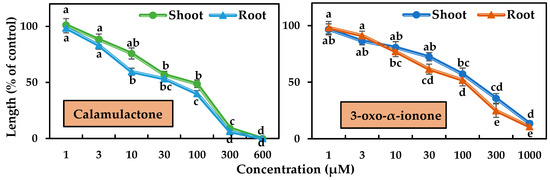
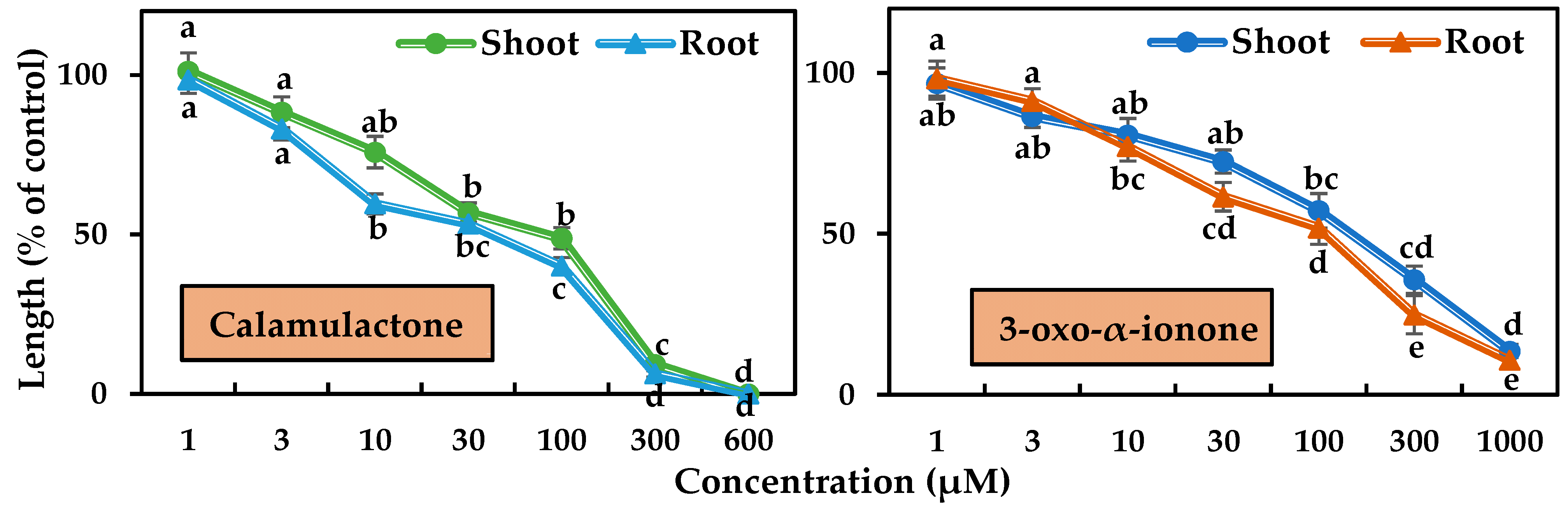
Figure 7.
Phytotoxic effects of calamulactone and 3-oxo-α-ionone on timothy. Values are the means ± SE from three replications. Different letters denote statistically significant variations between the treatment and control groups.
In the case of timothy, both compounds started to suppress the seedling growth at 10 μM. Calamulactone, at 100 μM, inhibited shoot growth by 48.79% and root growth by 40.10%, respectively, while 3-oxo-α-ionone had a comparatively less phytotoxic effect and suppressed shoot growth by 57.20% and root growth by 51.90%, respectively. At the higher concentration of 600 μM, calamulactone completely stopped timothy seedling growth, while at 1000 μM, 3-oxo-α-ionone restricted the shoot by 13.58% and the root by 10.74% of timothy seedlings of control, respectively.
Calamulactone and 3-oxo-α-ionone suppressed cress seedling growth by 50% (I50) at concentrations of 105.6–141.1 μM and 199.2–281.6 μM, respectively, where the inhibitory effect of calamulactone was clearly more pronounced than 3-oxo-α-ionone (Table 3). Similarly, in the case of timothy, 3-oxo-α-ionone inhibited growth by 50% at concentrations within the range of 107.8–144.7 μM, and calamulactone induced the same inhibitory effect at only 40.5–83.4 μM. Moreover, the root growth of both tested plants was significantly more affected by both substances compared with the corresponding shoot growth (Table 3).

Table 3.
I50 values of calamulactone and 3-oxo-α-ionone for cress and timothy.
3. Discussion
The current investigation found that the C. tenuis leaf extracts (aqueous methanol) strongly inhibited alfalfa, barnyard grass, cress, and timothy growth in a dose-dependent manner proportional to treatment concentration. Our findings corroborate the findings of several studies reporting the concentration-dependent allelopathic effects of different plant species extracts against several monocot and dicot test plants [31,32,33]. Moreover, different test plants showed different levels of sensitivity to the C. tenuis extracts. Some researchers have postulated that metabolic systems in different plant species become disordered when the concentration of a leaf extract exceeds a threshold, which is linked to the inherent tolerance mechanisms of the plants [34]. Other researches have also documented the species-specific phytotoxic action of plant extracts [35,36].
The extracts of C. tenuis may have a growth-inhibiting effect, as they may contain growth-limiting substances that interfere with the target species’ numerous physiological processes [37]. The isolation and identification of active compounds are one of the most vital areas of research in developing eco-friendly bioherbicides [38]. Thus, the current research aimed to isolate bioactive substances from the C. tenuis leaf extracts through chromatographic fractionations, with the highest active fraction containing a higher number of phytotoxic substances, imparting a higher level of phytotoxicity. Two bioactive substances 1 and 2, were isolated from the C. tenuis extracts.
The bioassays of calamulactone and 3-oxo-α-ionone against cress and timothy revealed strong dose-dependent phytotoxicity (Figure 3 and Figure 4). Many recent studies have shown such a concentration-dependent growth-limiting effect of plants containing phytotoxic substances [39,40,41,42,43,44]. Comparing I50 values, calamulactone suppressed cress shoot growth 1.99 times more than 3-oxo-α-ionone, and root growth 1.89 times more. Calamulactone, like 3-oxo-α-ionone, inhibited timothy growth, but at a 1.71- and 2.65-fold higher rate in the shoot and root, respectively. Both compounds had a more pronounced effect on the seedling growth of timothy than cress. Species specificity is demonstrated by the fact that various plant species have varied reactions to the same phytotoxic chemicals. This is due to the unique physiological and biochemical characteristics of each plant species [45]. Phytotoxic chemicals affect the early seedling growth of test plants, mostly through seed size and coat [46]. Large seeds with a prominent seed coat are less susceptible to phytotoxic substances than smaller seeds. The seed size of cress is almost five times higher than that of timothy, and consequently, it is less sensitive to both substances. These results corroborate our earlier observations that timothy is more susceptible to the toxic effects of a variety of plant metabolites [40,47].
Root growth was significantly more susceptible to the investigated substances than shoot growth in both plant species, which has been proposed as the best indicator of phytotoxicity of any growth inhibitory substance [34,48]. This finding can be explained by the roots being the first organ to emerge from a plant, and consequently, root tissues are the first to come into contact with the allelochemicals in the moist filter paper, resulting in the roots absorbing more allelochemicals than the shoots [49,50,51], which in turn leads to a limited division of cells [52] and restricts root growth.
In terms of phytotoxicity, calamulactone showed higher inhibitory activity than 3-oxo-α-ionone. Calamulactone is a derivative of an α, β-unsaturated γ-lactone. Although this report is the first on calamulactone as a novel compound, the derivatives of α, β-unsaturated γ-lactone are widely found in nature from plants, fungi, and animal sources [53]. It has been reported that compounds having a γ-lactone moiety may play a vital role in chemical defense systems [53,54]. Some of these substances exhibit different biological activities, including insecticidal, antibacterial, phytotoxic, and antifungal activities [55]. The γ-lactone rings of these substances have different patterns of saturation and substitution, and most are attached with a large carbon skeleton. In general, α, β-unsaturated γ-lactones show better phytotoxic activities than saturated ones [54]. On the other hand, 3-oxo-α-ionone is a carotenoid-derived anorisoprenoid [56]. This substance has several medicinal features, and its phytotoxicity is also well known. The allelopathic effects of the 3-oxo-α-ionone compound have been shown by different researchers and identified from many plant species, such as Albizia richardiana [57], rattail fescue [58], Withania aristate [59], and Vallisneria spiralis [60]. Although the phytotoxicity of this substance has been well documented, this report is the first on the isolation of 3-oxo-α-ionone from C. tenuis. Thus, structural variations between these two compounds might be the reason for their differential phytotoxic behavior. Moreover, calamulactone and 3-oxo-α-ionone likely account for at least a significant part of C. tenuis phytotoxicity. The strong phytotoxicity of C. tenuis may explain its dominance over surrounding plant species, which enables it to grow vigorously, even in places with considerable weed pressure.
Bioherbicides are regarded as a vital tool for the management of weeds, as they are developed from the extracts of different plants. Bioherbicides showed a promising influence on different weeds. When bioherbicides are applied in the field, they affect the germination, growth, and development of different weed species. Usually, they do not have a long residual effect, are not harmful for the soil and water, and do not affect the non-target organisms. Thus, bioherbicides could be used as an environment friendly weed management method. In Bangladesh, C. tenuis plant species are available in the northeastern region; the results of this study provide suitable information about its phytotoxicity, which will be helpful for the farmers to apply this species for weed management.
4. Conclusions
The current study revealed the strong phytotoxicity of aqueous extracts of C. tenuis against the seedling growth of monocotyledonous and dicotyledonous standard test plants. Bioassay-directed purification yielded two substances, a novel compound, calamulactone, and a known substance, 3-oxo-α-ionone. Both substances showed significant phytotoxicity against cress and timothy. In particular, with its low I50 values, calamulactone showed notable phytotoxicity against the test plants. The phytotoxicity of the C. tenuis extracts and its phytotoxic substances may play a crucial role in the defense and survival mechanisms of this species. In addition, this study provides indications for eco-friendly weed management.
5. Materials and Methods
5.1. Plant Samples
Fresh, healthy C. tenuis Roxb. leaves were obtained around Sylhet Agricultural University (24.8917° N 91.8833° E) from May to June in the year 2017. The leaves were washed with purified water to get rid of dirt, dust, and other surface impurities. They were then left to dry in the shade for 15 days, and an electric blender machine was used to grind them into a coarse powder. The powder was put in a thick plastic bag and kept at 2 °C until it was used. Four test plant species were selected for phytotoxicity assays: two monocots (Echinochloa crus-galli (L.) P. Beauv. (barnyard grass) and Phleum pratense L. (timothy)) and two dicots (Medicago sativa L. (alfalfa) and Lepidium sativum L. (cress)).
5.2. Extraction and Bioassay
An initial extraction was carried out to check the phytotoxicity of the plant materials and to design a precise isolation process. Accordingly, 100 g of C. tenuis leaves were extracted via soaking in 500 mL of methanol (70% methanol) in a dark condition for two days and filtered through filter paper (one layer, No. 2, 125 mm; Toyo Ltd., Tokyo, Japan). After a day of dissolving the solids in an equivalent volume of methanol, they were filtered once again. A Rotavapor was used to dry the combined extracts at 40 °C. Filter paper (No. 2, 28 mm; Toyo) was placed in Petri plates with a final concentration of 0.001, 0.003, 0.01, 0.03, 0.1, and 0.3 g of DW (dry weight) equivalent extract/mL, which were prepared through dissolving the extracts in 100 mL of methanol. After the methanol was removed via evaporation, 0.6 mL of a 0.05% aqueous solution of Tween 20 (polyoxyethylene sorbitan monolaurate; Nacalai Tesque, Inc., Kyoto, Japan) was added to each Petri dish to moisten the surface. Each Petri plate received ten homogeneous and ten pre-emergent seeds of dicots and monocots, respectively. The control was aqueous Tween 20-treated Petri dishes without C. tenuis extracts. After two days in a germinator at 25 °C in the dark, all Petri dishes were measured for seedling length.
5.3. Purification of the Active Substances
A separate extraction was carried out to identify any bioactive compounds. C. tenuis powder (2 kg) from the same sample source was subjected to the above-described extraction procedure, and the solvent of the produced extracts was removed using the Rotavapor (40 °C) to obtain an aqueous methanol crude extract. Following neutralization at pH 7.0 with 1 M phosphate buffer, the crude extract was partitioned four times with the same volume of ethyl acetate. Cress was used in bioassays for both the aqueous and ethyl acetate extracts. The bioactive substances were isolated and identified from the ethyl acetate fraction, since it demonstrated the highest activity. After eliminating the undesirable water with anhydrous Na2SO4, the ethyl acetate fraction was dried, separated on a silica gel column (60 g of silica gel 60, spherical, 70–230 mesh; Nacalai Tesque), and eluted with an ethyl acetate and n-hexane mixture (from 20% to 100%, increased 10% per step) and methanol. Based on the result of the bioassay, the strongest activity was obtained from the 70% and 80% ethyl acetate in n-hexane fractions. Purification of both fractions was accomplished by passing them over a Sephadex LH-20 column (GE Healthcare Bio-Sciences AB, SE-751 84, Uppsala, Sweden) and fractionating them at increasing methanol concentrations from 20% to 100%. The most impressive result was achieved with 40% aqueous methanol, which was then fractionated with a C18 reverse-phase cartridge. Eluting the cartridge with 20–80% methanol in water and methanol resulted in five distinct fractions. The highest level of activity was recorded using a solution consisting of 40% methanol in water. This solution was purified using reverse-phase high-performance liquid chromatography (HPLC) with a column of dimensions 500 × 10 mm (inner diameter) and ODS AQ-325 packing material, manufactured by YMC Ltd. in Kyoto, Japan. The purification process was carried out at a flow rate of 1.5 mL/min. The composition of the mobile phase consisted of a 40% aqueous methanol solution. The chromatogram was obtained through recording the absorbance at a wavelength of 220 nm at a temperature of 40 °C. The two most active substances, 1 and 2, had retention periods of 80–90 min and 130–140 min, respectively. To enhance purification, a precise reverse-phase HPLC system (4.6 × 250 mm I.D., S-5 µm, Inertsil® ODS-3; GL Science Inc., Tokyo, Japan) was used. A 30% aqueous methanol mobile phase was used to purify at 0.8 mL/min and isolated substances 1 and 2 at 35–40 and 60–70 min, respectively. Finally, substances 1 and 2 were identified using different spectroscopic analyses: HRESIMS, 1H-NMR, and 13C-NMR.
5.4. Characterization of the Compounds
Spectral Data
Electronic circular dichroism (ECD) spectra were recorded on a JASCO J-820 spectropolarimeter (JASCO, Tokyo, Japan). The Bruker AVANCE III was used to collect 1H (500 MHz) and 13C (125 MHz) NMR spectroscopic data. There were observed chemical shifts in comparison to the residual solvent signal (CDCl3: δH 7.26, δC 77.16). The Waters Micromass Q-TOF spectrometer (Waters Corporation, Milford, MA, USA) was used to record the HRESIMS data.
Calamulactone: colorless oil; ECD (MeOH) λext 204 nm, ∆ε +3.65; 1H NMR (500 MHz, CDCl3) δH 7.44 (dd, J = 1.5, 5.7, H-3, 1H), 6.11 (dd, J = 2.0, 5.7, H-2, 1H), 5.03 (m, H-4, 1H), 3.67 (s, 3H, H-13), 2.30 (t, J = 7.4, 2H, H-11), 1.76 (m, 1H, H-5a), 1.66 (m, 1H, H-5b), 1.62 (m, 2H, H-10), 1.44 (m, 2H, H-6), and 1.32 (m, 6H, H-7, H-8, and H-9); 13C NMR (125 MHz, CDCl3) δC 174.4, 173.3, 156.3, 121.8, 83.5, 51.6, 34.2, 33.3, 29.22, 29.12, 29.08, 25.04, and 24.98; HRESIMS m/z 241.1441 [M + H]+ (calcd for C13H21O4, 241.1440).
3-oxo-α-ionone: 1H NMR (CDCl3): 1.05 (s, 1-CH3, 3H), 1.11 (s, 1-CH3, 3H), 1.94 (d, 5-CH3,3H), 2.12 (d, 1H, J = 16 Hz, 2-H), 2.40 (d, 1H, J = 16 Hz, 2-H), 2.32 (s, 9-CH3, 3H), 2.78 (d, 1H, J = 9 Hz, 6-H), 6.04 (s, 1H, 4-H), and 6.18 (d, 1H, J = 15.5 Hz, 8-H), 6.72 (dd, 1H, J = 9 Hz, J = 15.5 Hz, 7-H); HRESIMS m/z 207.1307 [M + H]+ (calcd for C13H18O2, 206.1307) [41].
5.5. Bioassay of Calamulactone and 3-Oxo-α-Ionone
Using the same bioassay approach described before, the bioactivity of calamulactone and 3-oxo-α-ionone was evaluated against cress and timothy at doses of 1 µM, 3 µM, 10 µM, 30 µM, 100 µM, 300 µM, 600 µM, and 1000 µM dissolved in methanol.
5.6. Statistical Analysis
The assay was performed in triplicate and then replicated twice using a CRBD. The data were presented as means and standard errors. One-way analysis of variance (ANOVA) with Tukey’s post-hoc test was used to determine statistical significance between the treatment and control groups in SPSS (20.0). Statistical significance was defined as a p-value of 0.05. Data was reported as a percentage of the control group. In the assay experiments, GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA) was used to calculate species I50 values.
Author Contributions
Conceptualization, M.M.R. and K.H.; methodology, M.M.R., K.H. and H.K.-N.; software, M.M.R. and K.H.; validation, H.K.-N., K.O. and T.T.; formal analysis, M.M.R. and K.H.; investigation, H.K.-N.; resources, H.K.-N. and T.T.; data curation, M.M.R. and H.K.-N.; writing—original draft preparation, M.M.R. and K.H.; writing—review and editing, H.K.-N.; visualization, M.M.R. and K.H.; supervision, H.K.-N. All authors have read and agreed to the published version of the manuscript.
Funding
The study was financially supported by a MEXT scholarship (Grant Number MEXT-173591) in Japan to carry out this research in Japan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data from this study are included within this manuscript.
Acknowledgments
We thank Dennis Murphy for editing the English of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alvarado-Serrano, D.F.; Van Etten, M.L.; Chang, S.M.; Baucom, R.S. The relative contribution of natural landscapes and human-mediated factors on the connectivity of a noxious invasive weed. Heredity 2019, 122, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Ireland, K.B.; Van Klinken, R.; Cook, D.C.; Logan, D.; Jamieson, L.; Tyson, J.L.; Paini, D. Plant pest impact metric system (PPIMS): Framework and guidelines for a common set of metrics to classify and prioritise plant pests. Crop Prot. 2020, 128, 105003. [Google Scholar] [CrossRef]
- Nichols, V.; Verhulst, N.; Cox, R.; Govaerts, B. Weed dynamics and conservation agriculture principles: A review. Field Crops Res. 2015, 183, 56–68. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Oloke, J.K.; Bello, O.M.; Pradeep, M.; Jolly, R.S. Isolation, structural elucidation and bioherbicidal activity of an eco-friendly bioactive 2-(hydroxymethyl) phenol, from Pseudomonas aeruginosa (C1501) and its ecotoxicological evaluation on soil. Environ. Technol. Innovat. 2019, 13, 304–317. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Moss, S. Integrated weed management (IWM): Why are farmers reluctant to adopt non-chemical alternatives to herbicides? Pest Manag. Sci. 2019, 75, 1205–1211. [Google Scholar] [CrossRef]
- Hinz, H.L.; Winston, R.L.; Schwarzländer, M. A global review of target impact and direct nontarget effects of classical weed biological control. Curr. Opin. Insect. Sci. 2020, 38, 48–54. [Google Scholar] [CrossRef]
- Davis, A.M.; Neelamraju, C. Quantifying water quality improvements through use of precision herbicide application technologies in a dry-tropical, furrow-irrigated cropping system. Water 2019, 11, 2326. [Google Scholar] [CrossRef]
- Comont, D.; Lowe, C.; Hull, R.; Crook, L.; Hicks, H.L.; Onkokesung, N.; Neve, P. Evolution of generalist resistance to herbicide mixtures reveals a trade-off in resistance management. Nat. Commun. 2020, 11, 3086. [Google Scholar] [CrossRef]
- Herbicide Resistance Action Committee. Available online: https://hracglobal.com/herbicide-resistance/overview (accessed on 27 August 2023).
- Bardin, M.; Ajouz, S.; Comby, M.; Lopez-Ferber, M.; Graillot, B.; Siegwart, M.; Nicot, P.C. Is the efficacy of biological control against plant diseases likely to be more durable than that of chemical pesticides? Front. Plant Sci. 2015, 6, 566. [Google Scholar] [CrossRef]
- Kergunteuil, A.; Bakhtiari, M.; Formenti, L.; Xiao, Z.; Defossez, E.; Rasmann, S. Biological control beneath the feet: A review of crop protection against insect root herbivores. Insects 2016, 7, 70. [Google Scholar] [CrossRef]
- Carrubba, A.; Labruzzo, A.; Comparato, A.; Muccilli, S.; Spina, A. Use of plant water extracts for weed control in durum wheat (Triticum turgidum L. subsp. durum Desf.). Agronomy 2020, 10, 364. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Barbosa, B.V.; Martins, B.D.A.; Guirlanda, P.C.; Moura, A.F.M. Use of the versatility of fungal metabolism to meet modern demands for healthy aging, functional foods, and sustainability. J. Fungi 2020, 6, 223. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.M.; Henderson, A.; Nguyễn, Q.D.N.; Ledecq, T. Systematics, Ecology, and Management of Rattans in Cambodia, Laos, and Vietnam: The Biological Bases of Sustainable Use; Agricultural Publishing House: WWF-Greater Mekong and The New York Botanical Garden: New York, NY, USA, 2014; p. 235. [Google Scholar]
- Ahmed, Z.U.; Bithi, S.S.; Khan, M.M.R.; Hossain, M.M.; Sharmin, S.; Rony, S.R. Phytochemical screening, antioxidant and cytotoxic activity of fruit extracts of Calamus tenuis Roxb. J. Coast. Life Med. 2014, 2, 645–650. [Google Scholar] [CrossRef]
- Alam, M.K. Rattans of Bangladesh; Bulletin 7, Taxonomy Series; Bangladesh Forest Research Institute: Chittagong, Bangladesh, 1990; p. 35. [Google Scholar]
- Manohara, T.N. Nutritional evaluation of shoots of two rattans of northeast India—Calamus flagellum Griff. ex Mart. and C. floribundus Griff. (Arecaceae). Econ. Bot. 2013, 67, 263–268. Available online: http://www.jstor.org/stable/43304349 (accessed on 20 August 2023). [CrossRef]
- Islam, S.A.; Miah, M.A.Q.; Habib, M.A.; Rasul, M.G. Growth performance of Calamus tenuis Roxb. (Jali bet) in the coastal homesteads of Bangladesh. J. Biosci. Agric. Res. 2015, 4, 74–79. [Google Scholar] [CrossRef]
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary, 1st ed.; Springer: New York, NY, USA, 2008; p. 835. [Google Scholar] [CrossRef]
- Tag, H.; Kalita, P.; Dwivedi, P.; Das, A.K.; Namsa, N.D. Herbal medicines used in the treatment of diabetes mellitus in Arunachal Himalaya, northeast, India. J. Ethnopharmacol. 2012, 141, 786–795. [Google Scholar] [CrossRef]
- Yu, G.F.; Mulabagal, V.; Diyabalanage, T.; Hurtada, W.A.; DeWitt, D.L.; Nair, M.G. Non-nutritive functional agents in rattan-shoots, a food consumed by native people in the Philippines. Food Chem. 2008, 110, 991–996. [Google Scholar] [CrossRef]
- Saikia, P.; Khan, M.L. Diversity of medicinal plants and their uses in home gardens of upper Assam, Northeast India. Asian J. Pharm. Biol. Res. 2011, 1, 296–309. [Google Scholar]
- Sarkar, H.; Sharin, T.; Al Mamun, M.S. Assessment of Calamus tenuis fruits extract on blood glucose level elevation and its antibacterial potency. J. Appl. Pharm. Res. 2018, 6, 1–6. [Google Scholar] [CrossRef]
- Hossain, M.S. Analgesic and neuropharmacological activity of methanolic extract of Calamus tenuis Roxb. Fruits. J. Sci. Innov. 2013, 2, 1067–1072. [Google Scholar]
- Thakur, P.K.; Sheth, M.; Bhambra, G.K.; Nagar, P.S.; Upadhyay, K.; Devkar, R. Phytochemical composition and cytotoxic potential of edible rattan (Calamus tenuis Roxb.) shoot extracts on MCF7 and A549 cells. World J. Pharm. Res. 2016, 5, 1738–1746. [Google Scholar]
- Kuo, Y.H.; Huang, S.L.; Chang, C.I. A phenolic and an aliphatic lactone from Diospyros maritima. Phytochemistry 1998, 49, 2505–2507. [Google Scholar] [CrossRef]
- Uchida, I.; Kuriyama, K. The π-π circular dichroism of δβ-unsaturated γ-lactones. Tetrahedron Lett. 1974, 15, 3761–3764. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Hayashi, Y.; Arita, M.; Hieda, T.; Mikami, Y. Microbial conversion of α-ionone, α-methylionone, and α-isomethylionone. Appl. Environ. Microbiol. 1998, 54, 2354–2360. [Google Scholar] [CrossRef]
- Chai, M.; Zhu, X.; Cui, H.; Jiang, C.; Zhang, J.; Shi, L. Lily cultivars have allelopathic potential in controlling Orobanche aegyptiaca Persoon. PLoS ONE 2015, 10, e0142811. [Google Scholar] [CrossRef]
- Al-Harbi, N.A. Allelopathic effect of leaf extract of two wild plants on seed germination, shoot and root length of two weed species; Portulaca oleracea and Chenopodium murale. Biosci. Biotechnol. Res. Asia 2018, 15, 929–935. [Google Scholar] [CrossRef]
- Wang, C.; Qi, J.; Liu, Q.; Wang, Y.; Wang, H. Allelopathic potential of aqueous extracts from Fleagrass (Adenosma buchneroides Bonati) against two crop and three weed species. Agriculture 2022, 12, 1103. [Google Scholar] [CrossRef]
- Ma, H.; Chen, Y.; Chen, J.; Zhang, Y.; Zhang, T.; He, H. Comparison of allelopathic effects of two typical invasive plants: Mikania micrantha and Ipomoea cairica in Hainan Island. Sci. Rep. 2020, 10, 11332. [Google Scholar] [CrossRef]
- Popovici, J.; Bertrand, C.; Jacquemoud, D.; Bellvert, F.; Fernandez, M.P.; Comte, G.; Piola, F. An allelochemical from Myrica gale with strong phytotoxic activity against highly invasive Fallopia×bohemica taxa. Molecules 2011, 16, 2323–2333. [Google Scholar] [CrossRef] [PubMed]
- Tuyen, P.T.; Xuan, T.D.; Tu Anh, T.T.; Mai Van, T.; Ahmad, A.; Elzaawely, A.A.; Khanh, T.D. Weed suppressing potential and isolation of potent plant growth inhibitors from Castanea crenata Sieb. et Zucc. Molecules 2018, 23, 345. [Google Scholar] [CrossRef] [PubMed]
- Ladhari, A.; Omezzine, F.; DellaGreca, M.; Zarrelli, A.; Zuppolini, S.; Haouala, R. Phytotoxic activity of Cleome arabica L. and its principal discovered active compounds. S. Afr. J. Bot. 2013, 88, 341–351. [Google Scholar] [CrossRef]
- Khan, S.M.; Khanam, S.; Deepak, M.; Shivananda, B.G. Antioxidant activity of a new diarylheptanoid from Zingibero fficinale. Pharmacogn. Mag. 2006, 2, 254–257. [Google Scholar]
- Appiah, K.S.; Mardani, H.K.; Omari, R.A.; Eziah, V.Y.; Ofosu-Anim, J.; Onwona-Agyeman, S.; Fujii, Y. Involvement of carnosic acid in the phytotoxicity of Rosmarinus officinalis leaves. Toxins 2018, 10, 498. [Google Scholar] [CrossRef]
- Rob, M.; Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic activity and identification of phytotoxic substances from Schumannianthus dichotomus. Plants 2020, 9, 102. [Google Scholar] [CrossRef]
- Wei, C.; Zhou, S.; Li, W.; Jiang, C.; Yang, W.; Han, C.; Zhang, C.; Shao, H. Chemical composition and allelopathic, phytotoxic and pesticidal activities of Atriplex cana Ledeb. (Amaranthaceae) essential oil. Chem. Biodivers. 2019, 16, e1800595. [Google Scholar] [CrossRef]
- Madadi, E.; Fallah, S.; Sadeghpour, A.; Barani-Beiranvand, H. Exploring the use of chamomile (Matricaria chamomilla L.) bioactive compounds to control flixweed (Descurainia sophia L.) in bread wheat (Triticum aestivum L.): Implication for reducing chemical herbicide pollution. Saudi J. Biol. Sci. 2022, 29, 103421. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Zhu, Y.; Guo, L.; Ji, R.; Miao, Y.; Guo, L.; Du, H.; Liu, D. Caffeic acid, an allelochemical in Artemisia argyi, inhibits weed growth via suppression of mitogen-activated protein kinase signaling pathway and the biosynthesis of gibberellin and phytoalexin. Front. Plant Sci. 2022, 12, 802198. [Google Scholar] [CrossRef]
- Hossen, K.; Asato, Y.; Teruya, T.; Kato-Noguchi, H. Identification of four allelopathic compounds including a novel compound from Elaeocarpus floribundus Blume and determination of their allelopathic activity. J. Environ. Manag. 2023, 326, 116728. [Google Scholar] [CrossRef]
- Kobayashi, K. Factors affecting phytotoxic activity of allelochemicals in soil. Weed Biol. Manag. 2004, 4, 1–7. [Google Scholar] [CrossRef]
- Hanley, M.E.; Whiting, M.D. Insecticides and arable weeds: Effects on germination and seedling growth. Ecotoxicology 2005, 14, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Rob, M.M.; Iwasaki, A.; Suenaga, K.; Ozaki, K.; Teruya, T.; Kato-Noguchi, H. Potential use of Schumannianthus dichotomus waste: The phytotoxic activity of the waste and its identified compounds. J. Environ. Sci. Health Part B 2020, 55, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Omezzine, F.; Ladhari, A.; Rinez, A.; Haouala, R. Potent herbicidal activity of Inula crithmoïdes L. Sci. Hortic. 2011, 130, 853–861. [Google Scholar] [CrossRef]
- Miranda, M.A.; Varela, R.M.; Torres, A.; Molinillo, J.M.; Gualtieri, S.C.; Macías, F.A. Phytotoxins from Tithonia diversifolia. J. Nat. Prod. 2015, 78, 1083–1092. [Google Scholar] [CrossRef]
- Da Silva, B.P.; Nepomuceno, M.P.; Varela, R.M.; Torres, A.; Molinillo, J.M.; Alves, P.L.; Macías, F.A. Phytotoxicity study on Bidens sulphurea Sch. Bip. as a preliminary approach for weed control. J. Agric. Food Chem. 2017, 65, 5161–5172. [Google Scholar] [CrossRef]
- Jmii, G.; Molinillo, J.M.; Zorrilla, J.G.; Haouala, R. Allelopathic activity of Thapsia garganica L. leaves on lettuce and weeds, and identification of the active principles. S. Afr. J. Bot. 2020, 131, 188–194. [Google Scholar] [CrossRef]
- Rietjens, I.M.; Alink, G.M. Nutrition and health-toxic substances in food. Ned. Tijdschr. Geneeskd. 2003, 147, 2365–2370. [Google Scholar]
- Rauter, A.P.; Figueiredo, J.; Ismael, M.; Canda, T.; Font, J.; Figueredo, M. Efficient synthesis of α, β-unsaturated γ-lactones linked to sugars. Tetrahedron: Asymmetry 2001, 12, 1131–1146. [Google Scholar] [CrossRef]
- Resende, G.C.; Alvarenga, E.S.; Galindo, J.C.; Macias, F.A. Synthesis and phytotoxicity of 4,5 functionalized tetrahydrofuran-2-ones. J. Braz. Chem. Soc. 2012, 23, 2266–2270. [Google Scholar] [CrossRef]
- Hur, J.; Jang, J.; Sim, J. A review of the pharmacological activities and recent synthetic advances of γ-butyrolactones. Int. J. Mol. Sci. 2021, 22, 2769. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Putkaradze, N.; Bohnacker, S.; Jonczyk, R.; Fida, T.; Hoffmann, T.; Schwab, W. Six uridine-diphosphate glycosyltransferases catalyze the glycosylation of bioactive C13-apocarotenols. Plant Physiol. 2020, 184, 1744–1761. [Google Scholar] [CrossRef] [PubMed]
- Hossen, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxicity of the novel compound 3-hydroxy-4-oxo-β-dehydroionol and compound 3-oxo-α-ionone from Albizia richardiana (Voigt.) King & Prain. Environ. Technol. Innovat. 2021, 23, 101779. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Yamamoto, M.; Tamura, K.; Teruya, T.; Suenaga, K.; Fujii, Y. Isolation and identification of potent allelopathic substances in rattail fescue. Plant Growth Regul. 2010, 60, 127–131. [Google Scholar] [CrossRef]
- Llanos, G.G.; Varela, R.M.; Jiménez, I.A.; Molinillo, J.M.; Macías, F.A.; Bazzocchi, I.L. Metabolites from Withania aristata with potential phytotoxic activity. Nat. Prod. Commun. 2010, 5, 1934578X1000500712. [Google Scholar]
- Xian, Q.; Chen, H.; Liu, H.; Zou, H.; Yin, D. Isolation and identification of antialgal compounds from the leaves of Vallisneria spiralis L. by activity-guided fractionation. Environ. Sci. Pollut. Res. Int. 2006, 13, 233–237. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).