Baseline Susceptibility of the Field Populations of Ostrinia furnacalis in Indonesia to the Proteins Cry1A.105 and Cry2Ab2 of Bacillus thuringiensis
Abstract
:1. Introduction
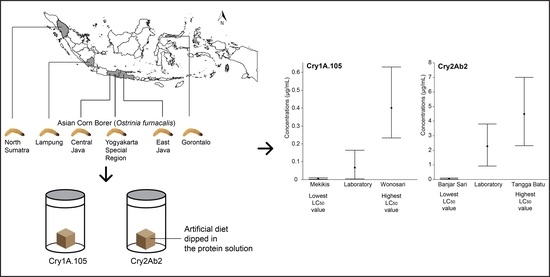
2. Results
2.1. Susceptibility of O. furnacalis to Cry1A.105
2.2. Susceptibility of O. furnacalis to Cry2Ab2
2.3. Candidate Diagnostic Concentrations
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Field-Collected Populations
5.2. Insect-Rearing Procedure
5.3. Susceptibility of O. furnacalis to Cry1A.105 and Cry2Ab2
5.4. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerpacio, R.V.; Pingali, P.L. Tropical and Subtropical Maize in Asia: Production Systems, Constraints, and Research Priorities; CIMMYT: Méx, Mexico, 2007; ISBN 978-970-648-155-9. [Google Scholar]
- Areekul, S.; Skulpanich, U.; Teeravate, P. Some studies on the control of corn borer in Thailand. Agric. Nat. Resour. 1964, 4, 110–119. [Google Scholar]
- Camarao, G.C. Population dynamics of the cornborer, Ostrinia furnacalis (Guenee), I. Life cycle, behavior, and generation cycles. Philipp. Entomol. 1976, 3, 179–200. [Google Scholar]
- Nafus, D.M.; Schreiner, I.H. Location of Ostrinia furnacalis (Lepidoptera: Pyralidae) eggs and larvae on sweet corn in relation to plant growth stage. J. Econ. Entomol. 1987, 80, 411–416. [Google Scholar] [CrossRef]
- Nafus, D.M.; Schreiner, I.H. Review of the biology and control of the Asian corn borer, Ostrinia furnacalis (Lep: Pyralidae). Trop. Pest Manag. 1991, 37, 41–56. [Google Scholar] [CrossRef]
- Da-Lopez, Y.F.; Trisyono, Y.A.; Witjaksono, W.; Subiadi, S. Distribution pattern of Ostrinia furnacalis Guenée (Lepidoptera Crambidae) egg-mass on maize-field. J. Entomol. Indones. 2014, 11, 81–92. [Google Scholar] [CrossRef]
- Trisyono, Y.A.; Aryuwandari, V.E.F.; Andika, I.P.; Sinulingga, N.G.H. Assessment on the Economic Importance of Corn Borers in Indonesia; Technical Report Submitted to Croplife Indonesia; Universitas Gadjah Mada: Yogyakarta, Indonesia, 2020; p. 41. [Google Scholar]
- Morallo-Rejesus, B.; Buctuanon, E.M.; Rejesus, R.S. Defining the economic threshold determinants for the Asian corn borer, Ostrinia furnacalis (Guenee) in the Philippines. Int. J. Pest Manag. 1990, 36, 114–121. [Google Scholar] [CrossRef]
- Subiadi, S.; Trisyono, Y.A.; Martono, E. Economic injury level (EIL) of Ostrinia furnacalis (Lepidoptera: Crambidae) larvae on three growth stages of corn. J. Entomol. Indones. 2014, 11, 19–26. [Google Scholar] [CrossRef]
- CABI. Ostrinia furnacalis (Asian Corn Borer). In: Invasive Species Compendium. Available online: https://www.cabi.org/isc/datasheet/38026 (accessed on 2 October 2021).
- Plantwise Knowledge Bank. Asian Corn Borer (Ostrinia furnacalis). Available online: https://www.plantwise.org/knowledgebank/datasheet/38026 (accessed on 2 October 2021).
- Hussein, M.Y.; Kameldeer, A.K. A field study on the oviposition of Ostrinia furnacalis Guenee (Lepidoptera: Pyralidae) on maize in Selangor, Malaysia. Int. J. Pest Manag. 1988, 34, 44–47. [Google Scholar] [CrossRef]
- Brookes, G.; Dinh, T.X. The impact of using Genetically Modified (GM) corn/maize in Vietnam: Results of the first farm-level survey. GM Crops Food 2021, 12, 71–83. [Google Scholar] [CrossRef]
- International Service for the Acquisition of Agri-Biotech Applications (ISAAA). Global Status of Commercialized Biotech/GM Crops in 2019: Biotech Crops Drive Socio-Economic Development and Sustainable Environment in the New Frontier; ISAAA Brief No. 55; ISAAA: Ithaca, NY, USA, 2019. [Google Scholar]
- ISAAA. ISAAA’s GM Approval Database. Available online: http://www.isaaa.org/gmapprovaldatabase/ (accessed on 2 October 2021).
- Biosafety Clearing-House (BCH). Country’s Decisions and Other Communications. Available online: https://bch.cbd.int/en/registries/living-modified-organisms (accessed on 17 August 2023).
- Indonesia Biosafety Clearing House. Keputusan Aman-Pangan. Available online: https://indonesiabch.menlhk.go.id/surat-keputusan/ (accessed on 17 August 2023).
- Erasmus, A.; Marais, J.; Van den Berg, J. Movement and survival of Busseola fusca (Lepidoptera: Noctuidae) larvae within maize plantings with different ratios of non-Bt and Bt seed. Pest. Manag. Sci. 2016, 72, 2287–2294. [Google Scholar] [CrossRef]
- Botha, A.S.; Erasmus, A.; du Plessis, H.; Van den Berg, J. Efficacy of Bt maize for control of Spodoptera frugiperda (Lepidoptera: Noctuidae) in South Africa. J. Econ. Entomol. 2019, 112, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, E.; Atienza, M.M.; Camacho, L.; Parimi, S. Baseline susceptibility of Philippine Ostrinia furnacalis (Lepidoptera: Crambidae) populations to insecticidal Cry1A. 105 and Cry2Ab2 proteins and validation of candidate diagnostic concentration for monitoring resistance. Biodiversitass 2021, 22, d220251. [Google Scholar] [CrossRef]
- James, C. Global Status of Commercialized Biotech/GM Crops: 2011; ISAAA Briefs no 43; ISAAA: Ithaca, NY, USA, 2011. [Google Scholar]
- International Service for the Acquisition of Agri-Biotech Applications (ISAAA). Global Status of Commercialized Biotech/GM Crops: 2016; ISAAA Brief No. 52; ISAAA: Ithaca, NY, USA, 2016. [Google Scholar]
- Department of Agriculture. List of Registered Plant-Incorporated Protectants Derived from Modern Biotechnology 2021. Department of Agriculture Quezon City, Philippines. Available online: https://fpa.da.gov.ph/NW/images/FPAfiles/DATA/Regulation/Pesticide/Files-2021/ListofRegisteredPIP12312021.pdf (accessed on 5 January 2022).
- Gould, F. Sustainability of transgenic insecticidal cultivars: Integrating pest genetics and ecology. Annu. Rev. Entomol. 1998, 43, 701–726. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E. Delaying insect resistance to transgenic crops. Proc. Natl. Acad. Sci. USA 2008, 105, 19029–19030. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Carrière, Y. Evaluating cross-resistance between Vip and Cry toxins of Bacillus thuringiensis. J. Econ. Entomol. 2020, 113, 553–561. [Google Scholar] [CrossRef]
- Roush, R.T. Two–toxin strategies for management of insecticidal transgenic crops: Can pyramiding succeed where pesticide mixtures have not? Phil. Trans. R. Soc. Lond. B 1998, 353, 1777–1786. [Google Scholar] [CrossRef]
- Zhao, J.-Z.; Cao, J.; Li, Y.; Collins, H.L.; Roush, R.T.; Earle, E.D.; Shelton, A.M. Transgenic plants expressing two Bacillus thuringiensis toxins delay insect resistance evolution. Nat. Biotechnol. 2003, 21, 1493–1497. [Google Scholar] [CrossRef]
- Carrière, Y.; Crickmore, N.; Tabashnik, B.E. Optimizing pyramided transgenic Bt crops for sustainable pest management. Nat. Biotechnol. 2015, 33, 161–168. [Google Scholar] [CrossRef]
- Blanco, C.A.; Storer, N.P.; Abel, C.A.; Jackson, R.; Leonard, R.; Lopez, J.D.; Payne, G.; Siegfried, B.D.; Spencer, T.; N-Vargas, A.P.T. Baseline susceptibility of tobacco budworm (Lepidoptera: Noctuidae) to Cry1F toxin from Bacillus thuringiensis. J. Econ. Entomol. 2008, 101, 6. [Google Scholar] [CrossRef]
- Leite, N.A.; Pereira, R.M.; Durigan, M.R.; Amado, D.; Fatoretto, J.; Medeiros, F.C.L.; Omoto, C. Susceptibility of Brazilian populations of Helicoverpa armigera and Helicoverpa zea (Lepidoptera: Noctuidae) to Vip3Aa20. J. Econ. Entomol. 2018, 111, 399–404. [Google Scholar] [CrossRef]
- Robertson, J.L.; Preisler, H.K. Pesticide Bioassays with Arthropods; CRC Press: Boca Raton, FL, USA, 1992; ISBN 0-8493-6463-9. [Google Scholar]
- Liu, X.; Liu, S.; Long, Y.; Wang, Y.; Zhao, W.; Shwe, S.M.; Wang, Z.; He, K.; Bai, S. Baseline susceptibility and resistance allele frequency in Ostrinia furnacalis in relation to Cry1Ab toxins in China. Toxins 2022, 14, 255. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Re, Y.C.; Dennehy, T.J.; Morin, S.; Sisterson, M.S.; Roush, R.T.; Shelton, A.M.; Zhao, J.-Z. Insect resistance to transgenic Bt crops: Lessons from the laboratory and field. J. Econ. Entomol. 2003, 96, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Andow, D.A.; Buschman, L.L. Success of the high-dose/refuge resistance management strategy after 15 years of Bt crop use in North America: Bt crops and resistance management. Entomol. Exp. Appl. 2011, 140, 1–16. [Google Scholar] [CrossRef]
- Alcantara, E.; Estrada, A.; Alpuerto, V.; Head, G. Monitoring Cry1Ab Susceptibility in Asian corn borer (Lepidoptera: Crambidae) on Bt corn in the Philippines. Crop Prot. 2011, 30, 554–559. [Google Scholar] [CrossRef]
- Marçon, P.C.R.G.; Young, L.J.; Steffey, K.L.; Siegfried, B.D. Baseline susceptibility of European corn borer (Lepidoptera: Crambidae) to Bacillus thuringiensis toxins. J. Econ. Entomol. 1999, 92, 279–285. [Google Scholar] [CrossRef]
- Jalali, S.K.; Yadavalli, L.; Ojha, R.; Kumar, P.; Sulaikhabeevi, S.B.; Sharma, R.; Nair, R.; Kadanur, R.C.; Kamath, S.P.; Komarlingam, M.S. Baseline sensitivity of maize borers in India to the Bacillus thuringiensis insecticidal proteins Cry1A.105 and Cry2Ab2: Bt baseline sensitivity of Indian maize Lepidopteran Pests. Pest. Manag. Sci. 2015, 71, 1082–1090. [Google Scholar] [CrossRef]
- Le, D.K.; Le, Q.K.; Tran, T.T.H.; Nguyen, D.V.; Dao, T.H.; Nguyen, T.T.; Truong, X.L.; Nguyen, Q.C.; Pham, H.P.; Phan, T.T.T.; et al. Baseline susceptibility of Asian corn borer (Ostrinia furnacalis (Guenée)) populations in Vietnam to Cry1Ab insecticidal protein. J. Asia-Pac. Entomol. 2019, 22, 493–498. [Google Scholar] [CrossRef]
- He, K.; Wang, Z.; Wen, L.; Bai, S.; Ma, X.; Yao, Z. Determination of baseline susceptibility to Cry1Ab protein for Asian corn borer (Lep., Crambidae). J. Appl. Entomol. 2005, 129, 407–412. [Google Scholar] [CrossRef]
- Li, G.; Huang, J.; Ji, T.; Tian, C.; Zhao, X.; Feng, H. Baseline susceptibility and resistance allele frequency in Ostrinia furnacalis related to Cry1 toxins in the Huanghuaihai summer corn region of China. Pest Manag. Sci. 2020, 76, 4311–4317. [Google Scholar] [CrossRef]
- Kranthi, K.R.; Kranthi, S.; Wanjari, R.R. Baseline toxicity of Cry1A toxins to Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) in India. Int. J. Pest Manag. 2001, 47, 141–145. [Google Scholar] [CrossRef]
- Bird, L.J.; Akhurst, R.J. Variation in susceptibility of Helicoverpa armigera (Hübner) and Helicoverpa punctigera (Wallengren) (Lepidoptera: Noctuidae) in Australia to two Bacillus thuringiensis Toxins. J. Invertebr. Pathol. 2007, 94, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Siegfried, B.D.; Spencer, T.; Crespo, A.L.; Storer, N.P.; Head, G.P.; Owens, E.D.; Guyer, D. Ten years of Bt resistance monitoring in the European corn borer: What we know, what we don’t know, and what we can do better. Am. Entomol. 2007, 3, 208–214. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, C.S.; Hernández-Martínez, P.; Van Rie, J.; Escriche, B.; Ferré, J. Shared midgut binding sites for Cry1A.105, Cry1Aa, Cry1Ab, Cry1Ac and Cry1Fa proteins from Bacillus thuringiensis in two important corn pests, Ostrinia nubilalis and Spodoptera frugiperda. PLoS ONE 2013, 8, e68164. [Google Scholar] [CrossRef]
- Marçon, P.C.R.G.; Siegfried, B.D.; Spencer, T.; Hutchison, W.D. Development of diagnostic concentrations for monitoring Bacillus thuringiensis resistance in European corn borer (Lepidoptera: Crambidae). J. Econ. Entomol. 2000, 93, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Monsanto Company. Petition for the Determination of Non-Regulated Status for MON 89034. Available online: https://www.aphis.usda.gov/brs/aphisdocs/06_29801p.pdf (accessed on 17 August 2023).
- Rahayu, T.; Trisyono, Y.A.; Witjaksono. Fitness of Asian corn borer, Ostrinia furnacalis (Lepidoptera: Crambidae) reared in an artificial diet. J. Asia-Pac. Entomol. 2018, 21, 823–828. [Google Scholar] [CrossRef]
- Trisyono, Y.A.; Rahayu, S.T.S.; Margino, S. Bioactivity of a Bacillus thuringiensis Cry1Ac toxin to Spodoptera litura. J. Perlindungan Tanam. Indones. 2004, 10, 53–62. [Google Scholar]
- Finney, D.J. Probit Analysis; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Oppenoorth, F.J.; Welling, W. Biochemistry and Physiology of Resistance. In Insecticide Biochemistry and Physiology; Wilkinson, C.F., Ed.; Springer: Boston, MA, USA, 1976; pp. 507–551. ISBN 978-1-4899-2214-4. [Google Scholar]
- Roush, R.T.; Miller, G.L. Considerations for design of insecticide resistance monitoring programs. J. Econ. Entomol. 1986, 79, 293–298. [Google Scholar] [CrossRef]
- Menger, J.; Beauzay, P.; Chirumamilla, A.; Dierks, C.; Gavloski, J.; Glogoza, P.; Hamilton, K.; Hodgson, E.W.; Knodel, J.J.; MacRae, I.V.; et al. Implementation of a diagnostic-concentration bioassay for detection of susceptibility to pyrethroids in soybean aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2020, 113, 932–939. [Google Scholar] [CrossRef]

| Population | N | Slope (±SE) | LC50 (95% FL) (µg/mL) | LC95 (95% FL) (µg/mL) | RR a | χ2 c | ||
|---|---|---|---|---|---|---|---|---|
| Province | District | Village | ||||||
| North Sumatra | Langkat | Pasar VI Kwala Mencirim | 440 | 2.05 ± 0.32 | 0.035 (0.012–0.066) | 0.224 (0.112–1.173) | 0.5 | 5.90 |
| Purwo Binangun | 330 | 1.44 ± 0.32 | 0.039 (0.001–108) | 0.535 (0.176–355.074) | 0.6 | 6.38 | ||
| Deli Serdang | SM Diski | 330 | 1.96 ± 0.42 | 0.010 (0.001–0.020) | 0.069 (0.031–3.746) | 0.1 | 1.31 | |
| Suka Dame | 330 | 1.27 ± 0.36 | 0.013 (N/A–N/A) * | 0.250 (N/A–N/A) * | 0.2 | 8.70 ** | ||
| Simalungun | Silenduk | 550 | 0.77 ± 0.07 | 0.058 (0.031–0.105) | 7.872 (2.647–45.801) | 0.9 | 7.74 | |
| Tangga Batu | 550 | 0.93 ± 0.10 | 0.047 (0.023–0.081) | 2.746 (1.311–8.273) | 0.7 | 5.27 | ||
| Lampung | South Lampung | Lematang | 330 | 1.57 ± 0.42 | 0.045 (0.008–0.084) | 0.495 (0.254–3.374) | 0.7 | 3.57 |
| Kelau | 550 | 1.33 ± 0.13 | 0.080 (0.044–0.132) | 1.389 (0.701–4.166) | 1.2 | 9.12 | ||
| Central Lampung | Trimurjo | 330 | 0.86 ± 0.15 | 0.185 (0.066–0.357) | 15.233 (5.345–127.917) | 2.8 | 2.49 | |
| Metro | Mulyojati | 550 | 1.46 ± 0.20 | 0.032 (0.015–0.055) | 0.429 (0.214–1.606) | 0.5 | 4.17 | |
| East Lampung | Gondang Rejo | 550 | 1.70 ± 0.21 | 0.137 (0.102–0.176) | 1.274 (0.906–2.003) | 2.0 | 1.68 | |
| Gorontalo | Pohuwato | Manawa | 550 | 1.65 ± 0.17 | 0.313 (0.238–0.403) | 3.085 (2.062–5.408) | 4.7 | 1.80 |
| Gorontalo | Tenilo | 550 | 1.37 ± 0.20 | 0.273 (0.115–0.451) | 4.316 (2.237–16.451) | 4.1 | 2.18 | |
| Central Java | Purworejo | Ketawangrejo | 550 | 1.38 ± 0.18 | 0.166 (0.071–0.292) | 2.597 (1.148–16.906) | 2.5 | 3.59 |
| Wonosari | 550 | 1.06 ± 0.11 | 0.401 (0.233–0.630) | 14.521 (7.038–45.194) | 6.0 | 4.76 | ||
| Yogyakarta | Sleman | Purwomartani | 550 | 0.96 ± 0.11 | 0.051 (0.020–0.104) | 2.604 (0.790–38.314) | 0.8 | 6.87 |
| Widodomartani | 550 | 1.02 ± 0.13 | 0.081 (0.022–0.197) | 3.336 (0.932–98.888) | 1.2 | 8.05 | ||
| Kulon Progo | Kedungsari | 550 | 0.95 ± 0.10 | 0.053 (0.028–0.090) | 2.907 (1.199–12.762) | 0.8 | 5.77 | |
| Kedundang | 550 | 0.81 ± 0.07 | 0.101 (0.059–0.173) | 0.730 (3.970–49.262) | 1.5 | 6.55 | ||
| East Java | Kediri | Papar | 550 | 1.08 ± 0.10 | 0.043 (0.029–0.063) | 1.404 (0.716–3.727) | 0.6 | 3.90 |
| Mekikis | 550 | 0.88 ± 0.11 | 0.006 (0.002–0.010) | 0.418 (0.170–2.088) | 0.1 | 3.16 | ||
| Nganjuk | Watu Dandang | 550 | 0.77 ± 0.09 | 0.014 (0.006–0.025) | 1.905 (0.659–12.081) | 0.2 | 5.24 | |
| Banjar Sari | 550 | 0.83 ± 0.11 | 0.009 (0.003–0.020) | 0.858 (0.231–20.417) | 0.1 | 6.38 | ||
| Malangsari | 550 | 0.91 ± 0.11 | 0.010 (0.004–0.021) | 0.664 (0.225–6.057) | 0.2 | 4.84 | ||
| Laboratory b | 330 | 1.47 ± 0.30 | 0.067 (0.004–0.164) | 0.890 (0.336–49.547) | 1.0 | 6.11 | ||
| Population | N | Slope (±SE) | LC50 (95% FL) (µg/mL) | LC95 (95% FL) (µg/mL) | RR a | χ2 c | ||
|---|---|---|---|---|---|---|---|---|
| Province | District | Village | ||||||
| North Sumatra | Langkat | Pasar VI Kwala Mencirim | 440 | 1.18 ± 0.16 | 1.527 (0.657–3.478) | 37.665 (11.088–1150.551) | 0.7 | 4.12 |
| Purwo Binangun | 440 | 0.91 ± 0.19 | 4.361 (1.886–10.182) | 277.584 (62.854–14,840.756) | 1.9 | 2.70 | ||
| Deli Serdang | SM Diski | 330 | 0.96 ± 0.18 | 3.416 (1.311–17.291) | 180.121 (27.658–426,921.2) | 1.5 | 3.44 | |
| Suka Dame | 440 | 2.23 ± 0.50 | 2.054 (0.490–3.540) | 11.185 (5.908–130.605) | 0.9 | 4.57 | ||
| Simalungun | Silenduk | 550 | 0.83 ± 0.10 | 1.062 (0.673–1.684) | 99.658 (36.621–502.575) | 0.5 | 2.18 | |
| Tangga Batu | 440 | 1.58 ± 0.39 | 4.490 (2.313–7.002) | 49.531 (22.220–511.071) | 2.0 | 2.24 | ||
| Lampung | South Lampung | Lematang | 550 | 1.19 ± 0.23 | 0.667 (0.268–1.181) | 16.275 (6.610–132.775) | 0.3 | 4.03 |
| Kelau | 330 | 2.17 ± 0.56 | 4.018 (1.242–6.506) | 23.015 (11.971–370.390) | 1.8 | 5.09 | ||
| Central Lampung | Trimurjo | 550 | 1.09 ± 0.19 | 2.074 (0.396–5.564) | 67.414 (17.091–12,346.001) | 0.9 | 3.89 | |
| Metro | Mulyojati | 550 | 0.99 ± 0.20 | 4.034 (0.966–12.182) | 183.278 (36.318–284,609.920) | 1.8 | 5.50 | |
| East Lampung | Gondang Rejo | 330 | 1.22 ± 0.27 | 1.662 (0.720–2.884) | 37.164 (16.372–205.583) | 0.7 | 1.71 | |
| Gorontalo | Pohuwato | Manawa | 330 | 1.17 ± 0.28 | 1.590 (0.040–4.273) | 40.540 (10.897–459,863.240) | 0.7 | 5.71 |
| Gorontalo | Tenilo | 550 | 1.01 ± 0.11 | 0.525 (0.212–1.110) | 22.666 (6.744–387.286) | 0.2 | 7.83 | |
| Central Java | Purworejo | Ketawangrejo | 550 | 1.40 ± 0.16 | 0.989 (0.481–1.824) | 14.861 (5.979–122.810) | 0.4 | 4.39 |
| Wonosari | 550 | 1.08 ± 0.11 | 0.254 (0.177–0.351) | 8.371 (4.952–16.923) | 0.1 | 2.20 | ||
| Yogyakarta | Sleman | Purwomartani | 550 | 1.09 ± 0.11 | 0.322 (0.175–0.550) | 10.463 (4.533–41.801) | 0.1 | 6.49 |
| Widodomartani | 550 | 1.26 ± 0.16 | 0.163 (0.077–0.272) | 3.320 (1.520–16.269) | 0.1 | 2.57 | ||
| Kulon Progo | Kedungsari | 550 | 1.20 ± 0.12 | 0.741 (0.401–1.340) | 17.198 (6.732–104.303) | 0.3 | 6.27 | |
| Kedundang | 550 | 1.01 ± 0.12 | 0.276 (0.134–0.477) | 11.710 (4.766–61.882) | 0.1 | 4.18 | ||
| East Java | Kediri | Papar | 330 | 0.66 ± 0.07 | 0.101 (0.058–0.176) | 30.870 (10.553–144.161) | 0.1 | 5.41 |
| Mekikis | 330 | 0.62 ± 0.07 | 0.095 (0.039–0.229) | 41.121 (8.410–667.267) | 0.1 | 11.65 | ||
| Nganjuk | Watu Dandang | 550 | 1.01 ± 0.10 | 0.585 (0.315–1.102) | 24.771 (8.645–164.730) | 0.3 | 8.48 | |
| Banjar Sari | 550 | 0.61 ± 0.06 | 0.044 (0.019–0.099) | 22.509 (4.654–375.668) | 0.1 | 12.86 | ||
| Malangsari | 550 | 0.97 ± 0.08 | 0.522 (0.309–0.907) | 25.653 (9.980–113.189) | 0.2 | 8.43 | ||
| Laboratory b | 440 | 1.64 ± 0.48 | 2.276 (0.916–3.803) | 22.984 (12.650–77.799) | 1.0 | 0.62 | ||
| Protein | Slope | No. Larvae | LC95 (95% FL) (µg/mL) | LC99 (95% FL) (µg/mL) |
|---|---|---|---|---|
| Cry1A.105 | 0.99 ± 0.03 | 11,200 | 2.720 (1.680–5.158) a | 13.240 (6.716–33.831) b |
| Cry2Ab2 | 1.04 ± 0.03 | 11,440 | 28.050 (13.600–89.795) a | 127.320 (46.616–676.792) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trisyono, Y.A.; Aryuwandari, V.E.F.; Rahayu, T.; Martinelli, S.; Head, G.P.; Parimi, S.; Camacho, L.R. Baseline Susceptibility of the Field Populations of Ostrinia furnacalis in Indonesia to the Proteins Cry1A.105 and Cry2Ab2 of Bacillus thuringiensis. Toxins 2023, 15, 602. https://doi.org/10.3390/toxins15100602
Trisyono YA, Aryuwandari VEF, Rahayu T, Martinelli S, Head GP, Parimi S, Camacho LR. Baseline Susceptibility of the Field Populations of Ostrinia furnacalis in Indonesia to the Proteins Cry1A.105 and Cry2Ab2 of Bacillus thuringiensis. Toxins. 2023; 15(10):602. https://doi.org/10.3390/toxins15100602
Chicago/Turabian StyleTrisyono, Y. Andi, Valentina E. F. Aryuwandari, Teguh Rahayu, Samuel Martinelli, Graham P. Head, Srinivas Parimi, and Luis R. Camacho. 2023. "Baseline Susceptibility of the Field Populations of Ostrinia furnacalis in Indonesia to the Proteins Cry1A.105 and Cry2Ab2 of Bacillus thuringiensis" Toxins 15, no. 10: 602. https://doi.org/10.3390/toxins15100602