The State-of-the-Art of the Humoral Memory Response to Snakebites: Insights from the Yanomami Population
Abstract
:1. Introduction
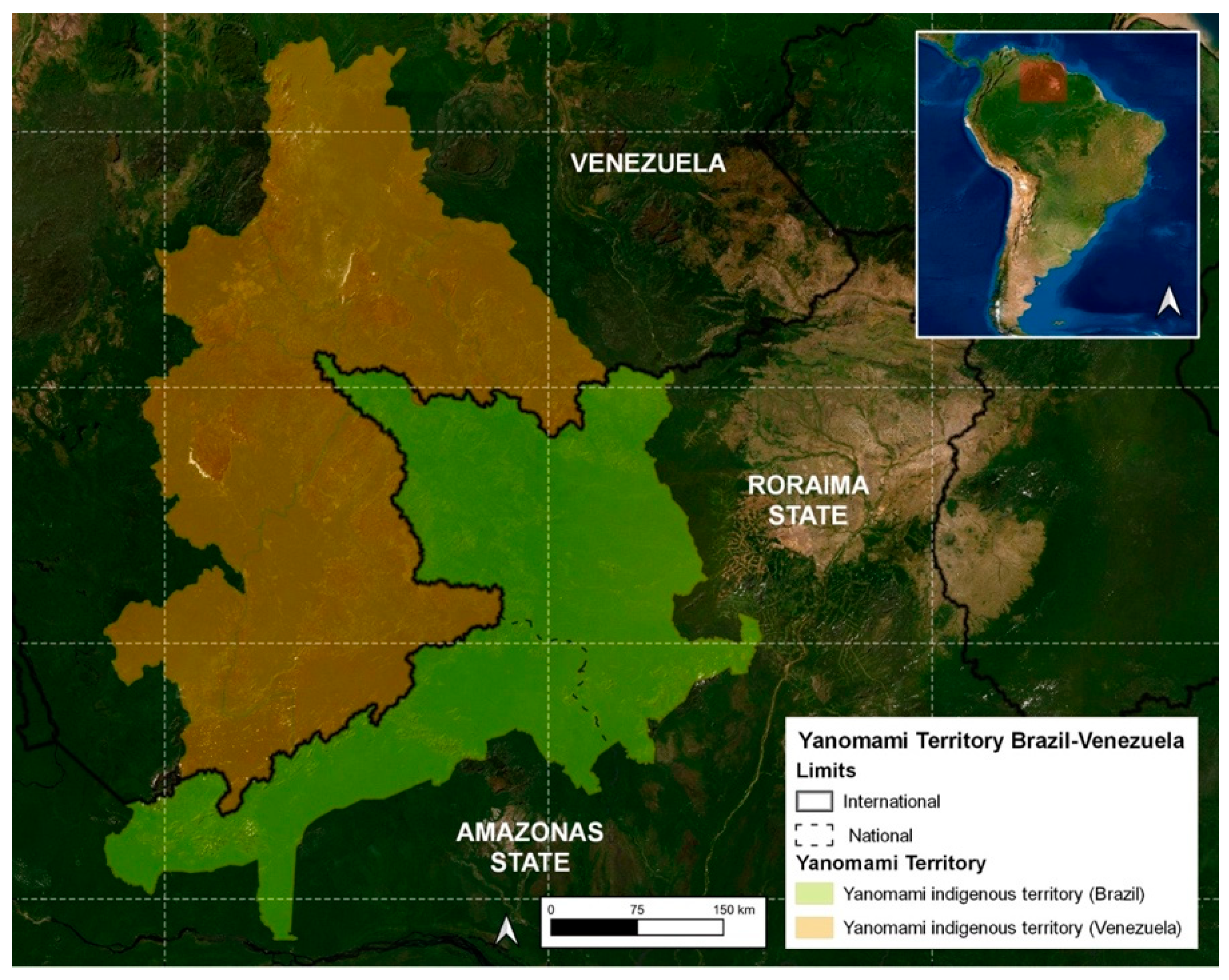
2. Snakebites in the Yanomami Indigenous Community of the Brazilian Amazon
- (i)
- In the YIL, men are mainly affected by snakebites at an average of 61.3%, which is statistically different in comparison to women (paired t-test, p = 0.034). However, compared with other studies [30,31], the percentage of Yanomami women (38.7%) who were affected by snakebites is 4.85 times higher than the national average (8%), probably due to the women-related activities in the community, as they also work in the field and harvest firewood, food, and medicinal products in the forest.
- (ii)
- Most snakebites occur in the age group of 20 to 39 years old (35.2%), followed by 15 to 19 (17.6%), 10 to 14 (16.9%), 40 to 59 (16.0%), 5 to 9 (10.4%), 60 to 79 years (2.1%), up to 4 years old (1.3% of cases), and over 80 years old (0.5% of cases), similarly to the results obtained by the pioneering Vital Brazil studies [30] and others [31,32,33].
- (iii)
- Regarding seasonality, the month with the highest snakebite incidence was May (mean of 19.8) and the one with the lowest incidence was December (mean of 9.5) (Figure 2). This is a different result compared to a previous study that marked July as the month with the highest occurrence of snakebites and October as the one with the lowest occurrence.
- (iv)
- The highest number of snakebites were reported in the municipality of Alto Alegre in the Roraima States/Brazil (50.5%), which was expected since the region includes the Serra dos Surucucus, located in the westernmost portion of this municipality, in the border region with Venezuela, known to be inhabited by snake-rich fauna [31].
3. The Immunological Response Targeting Snake Venoms
4. Evidence of Humoral Memory Response following Snakebite Envenomings
5. Vaccine Approaches for Populations Living in Areas of Snakebite Risk
6. Snakebite Immunity: A Fact Lacking Precise Assessment
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ministério da Saúde Acidente Por Animais Peçonhentos—Notificações Registradas No Sistema de Informação de Agravos de Notificação—Brasil. Available online: http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sinannet/cnv/animaisbr.def (accessed on 28 February 2023).
- Sampaio, V.S.; Gomes, A.A.; Silva, I.M.; Sachett, J.; Ferreira, L.C.L.; Oliveira, S.; Sabidò, M.; Chalkidis, H.; Barbosa Guerra, M.G.V.; Salinas, J.L.; et al. Low Health System Performance, Indigenous Status and Antivenom Underdosage Correlate with Spider Envenoming Severity in the Remote Brazilian Amazon. PLoS ONE 2016, 11, e0156386. [Google Scholar] [CrossRef]
- Calvete, J.J. Proteomic Tools against the Neglected Pathology of Snake Bite Envenoming. Expert. Rev. Proteom. 2011, 8, 739–758. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite Envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef]
- Burki, T. Resolution on Snakebite Envenoming Adopted at the WHA. Lancet 2018, 391, 2311. [Google Scholar] [CrossRef] [PubMed]
- Gold, B.S.; Dart, R.C.; Barish, R.A. Bites of Venomous Snakes. N. Engl. J. Med. 2002, 347, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Iyer, A.; Sunagar, K. Evolution Bites—Timeworn Inefficacious Snakebite Therapy in the Era of Recombinant Vaccines. Indian. Pediatr. 2021, 58, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Cook, D.A.; Renjifo, C.; Casewell, N.R.; Currier, R.B.; Wagstaff, S.C. Research Strategies to Improve Snakebite Treatment: Challenges and Progress. J. Proteom. 2011, 74, 1768–1780. [Google Scholar] [CrossRef]
- Harrison, R.; Gutiérrez, J. Priority Actions and Progress to Substantially and Sustainably Reduce the Mortality, Morbidity and Socioeconomic Burden of Tropical Snakebite. Toxins 2016, 8, 351. [Google Scholar] [CrossRef]
- Laustsen, A.H.; María Gutiérrez, J.; Knudsen, C.; Johansen, K.H.; Bermúdez-Méndez, E.; Cerni, F.A.; Jürgensen, J.A.; Ledsgaard, L.; Martos-Esteban, A.; Øhlenschlæger, M.; et al. Pros and Cons of Different Therapeutic Antibody Formats for Recombinant Antivenom Development. Toxicon 2018, 146, 151–175. [Google Scholar] [CrossRef]
- Pergolizzi, R.G.; Dragos, R.; Ropper, A.E.; Menez, A.; Crystal, R.G. Protective Immunity against α-Cobratoxin Following a Single Administration of a Genetic Vaccine Encoding a Non-Toxic Cobratoxin Variant. Hum. Gene Ther. 2005, 16, 292–298. [Google Scholar]
- Wallis, D.; Wallis, J. Rattlesnake Vaccine to Prevent Envenomation Toxicity in Dogs. In Proceedings of the Western Veterinary Conference, Las Vegas, NV, USA, 1 January 2005; Volume SIS1. [Google Scholar]
- Ayala, M.S.; Arevalo, B.; Vanegas, V. Design of a Therapeutic Vaccine against Snake Venom Disintegrins, 2015. [CrossRef]
- Freitas, T.; Diniz, C.; Frézard, F. The Use of Liposomes as Snake Venom Vehicles. Application in Protective Immunization. J. Toxicol. Toxin Rev. 1998, 17, 441–466. [Google Scholar] [CrossRef]
- de Melo, P.D.V.; Lima, S.d.A.; Araújo, P.; Santos, R.M.; Gonzalez, E.; Belo, A.A.; Machado-De-Ávila, R.A.; Costal-Oliveira, F.; Soccol, V.T.; Guerra-Duarte, C.; et al. Immunoprotection against Lethal Effects of Crotalus durissus Snake Venom Elicited by Synthetic Epitopes Trapped in Liposomes. Int. J. Biol. Macromol. 2020, 161, 299–307. [Google Scholar] [CrossRef]
- Theakston, R.D.; Reid, H.A.; Larrick, J.W.; Kaplan, J.; Yost, J.A. Snake Venom Antibodies in Ecuadorian Indians. J. Trop. Med. Hyg. 1981, 84, 199–202. [Google Scholar]
- Albert, B. URIHI: Terra, Economia e Saûde Yanomami; Universidade de Brasília: Brasília, Brazil, 1991. [Google Scholar]
- Kopenawa, D. A Queda do Céu: Palavras de um Xamã Yanomami; Companhia das Letras: São Paulo, Brazil, 2015; ISBN 978-85-359-2620-0. [Google Scholar]
- Freitas, F.P.d.P.; Luna, W.F.; Bastos, L.O.d.A.; Ávila, B.T. Experiências de Médicos Brasileiros Em Seus Primeiros Meses Na Atenção Primária à Saúde Na Terra Indígena Yanomami. Interface 2021, 25, e200212. [Google Scholar] [CrossRef]
- Cristino, J.S.; Salazar, G.M.; Machado, V.A.; Honorato, E.; Farias, A.S.; Vissoci, J.R.N.; Silva Neto, A.V.; Lacerda, M.; Wen, F.H.; Monteiro, W.M.; et al. A Painful Journey to Antivenom: The Therapeutic Itinerary of Snakebite Patients in the Brazilian Amazon (The QUALISnake Study). PLoS Negl. Trop. Dis. 2021, 15, e0009245. [Google Scholar] [CrossRef]
- Oliveira, I.S.; Ananias, C.B.; Medeiros, J.M.; Franco, M.V.S.; Ferreira, I.G.; Cerni, F.A.; Sandri, E.A.; Monteiro, W.M.; Pucca, M.B. Medical Management after Lancehead Snakebite in North Amazon: A Case Report of Long-Term Disability. Toxins 2022, 14, 494. [Google Scholar] [CrossRef]
- da Cunha, O.R.; Nascimento, F.P. Do Ofídios da Amazônia: As Cobras da Região Leste do Pará; Museu Emílio Goeldi: Belém, Brazil, 1993; Volume 9. [Google Scholar]
- da Frota, J.G.; dos Santos, A.P., Jr.; de Chalkidis, H.M.; Guedes, A.G. As Serpentes Da Regiao Do Baixo Rio Amazonas, Oeste Do Estado Do Pará, Brasil (Squamata). Biociências 2005, 13, 211–220. [Google Scholar]
- da Prudente, A.L.C.; Sarmento, J.F.M.; Avila-Pires, T.C.S.; Maschio, G.; Sturaro, M.J. How Much Do We Know about the Diversity of Squamata (Reptilia) in the Most Degraded Region of Amazonia? S. Am. J. Herpetol. 2018, 13, 117. [Google Scholar] [CrossRef]
- Prudente, A.L.; Ramos, L.; Silva, T.; Sarmento, J.; Dourado, A.; Silva, F.; Almeida, P.; Santos, C.; Sousa, M. Dataset from the Snakes (Serpentes, Reptiles) Collection of the Museu Paraense Emílio Goeldi, Pará, Brazil. Biodivers. Data J. 2019, 7, e34013. [Google Scholar] [CrossRef]
- de Fraga, R.; Stow, A.J.; Magnusson, W.E.; Lima, A.P. The Costs of Evaluating Species Densities and Composition of Snakes to Assess Development Impacts in Amazonia. PLoS ONE 2014, 9, e105453. [Google Scholar] [CrossRef]
- Martins, M.; Oliveira, M. Natural History of Snakes in Forests of the Manaus Region, Central Amazonia, Brazil. Herpetol. Nat. Hist. 1998, 6, 78–150. [Google Scholar]
- Kawaguchi, D. Lições de Um Xamã Yanomami Para a Construção de Uma Identidade Pós-Antropocêntrica. Rev. Abordagem Gestáltica Phenomenol. Stud. 2021, 27, 328–338. [Google Scholar] [CrossRef]
- Hata, L. A Potência Do Falso: A Cosmogonia Yanomami Na Obra de Claudia Andujar. Esferas 2021, 16, 16–31. [Google Scholar] [CrossRef]
- Brazil, I.V. A Defesa Contra o Ofidismo; Pocai & Weiss: São Paulo, Brazil, 1911. [Google Scholar]
- Nascimento, S.P. do Aspectos epidemiológicos dos acidentes ofídicos ocorridos no Estado de Roraima, Brasil, entre 1992 e 1998. Cad. Saúde Pública 2000, 16, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.E.R. DSEI YANOMAMI e os Acidentes Ofídicos No Norte do Brasil: Do Perfil Epidemiológico à Avaliação da Termoestabilidade dos Soros Antiofídicos como Estratégia de Saúde Pública; Doutorado em Medicina Tropical, Fundação Oswaldo Cruz; Instituto Oswaldo Cruz: Rio de Janeiro, Brazil, 2020. [Google Scholar]
- Jati, S.R.; Sousa, F.; Sant’Ana, A.C.; Mello, G.T.; Ferreira, D.E.S.; Gama, H.F.; Jati, N.S. Epidemiology of Accidents by Snakes in the Yanomami Indigenous Lands, in Roraima. In VenoRaima Abstract Book; Journal of Venomous Animals Including Tropical Diseases: Boa Vista, Brazil, 2022; Volume 28, p. 100. [Google Scholar]
- FUNAI Povos de Recente Contato. Available online: https://www.gov.br/funai/pt-br/atuacao/povos-indigenas/povos-indigenas-isolados-e-de-recente-contato-2/povos-de-recente-contato-1 (accessed on 21 May 2023).
- Leon, G.; Sanchez, L.; Hernandez, A.; Villalta, M.; Herrera, M.; Segura, A.; Estrada, R.; Maria Gutierrez, J. Immune Response towards Snake Venoms. Inflamm. Allergy-Drug Targets 2011, 10, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.Y.M.; Seymour, J.; Loukas, A.; Lopez, J.A.; Ikonomopoulou, M.P.; Miles, J.J. Immunological Responses to Envenomation. Front. Immunol. 2021, 12, 661082. [Google Scholar]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S.; Baker, D.L.; Baker, A. Cellular and Molecular Immunology, 9th ed.; Elsevier: Philadelphia, PA, USA, 2018; ISBN 978-0-323-47978-3. [Google Scholar]
- Chen, W.; Ghobrial, R.M.; Li, X.C. The Evolving Roles of Memory Immune Cells in Transplantation. Transplantation 2015, 99, 2029–2037. [Google Scholar] [CrossRef]
- Lomonte, B.; Kahan, L. Production and Partial Characterization of Monoclonal Antibodies to Bothrops Asper (Terciopelo) Myotoxin. Toxicon 1988, 26, 675–689. [Google Scholar] [CrossRef]
- Lomonte, B.; Gutiérrez, J.M.; Moreno, E.; Cerdas, L. Antibody Neutralization of a Myotoxin from the Venom of Bothrops Asper (Terciopelo). Toxicon 1987, 25, 443–449. [Google Scholar] [CrossRef]
- Garcia Denegri, M.E.; Maruñak, S.; Todaro, J.S.; Ponce-Soto, L.A.; Acosta, O.; Leiva, L. Neutralisation of the Pharmacological Activities of Bothrops Alternatus Venom by Anti-PLA2 IgGs. Toxicon 2014, 86, 89–95. [Google Scholar] [CrossRef]
- Theakston, R.D.; Lloyd-Jones, M.J.; Reid, H.A. Micro-ELISA for Detecting and Assaying Snake Venom and Venom-Antibody. Lancet 1977, 2, 639–641. [Google Scholar] [CrossRef]
- Theakston, R.D.G.; Reid, H.A.; Iddon, D.; Larrick, J.W. Protective Effect of Snake Venom Antibodies in Sera of Previous Snake Bite Victims. Ann. Trop. Med. Parasitol. 1983, 77, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Barraviera, B.; Sartori, A.; Pereira Da Silva, M.F.; Kaneno, R.; Peraçoli, M.T.S. Use of an Elisa assay to evaluate venom, antivenom, igg and igm human antibody levels in serum and cerebrospinal fluid from patients bitten by Crotalus durissus terrificus in Brazil. J. Venom. Anim. Toxins 1996, 2, 14–27. [Google Scholar] [CrossRef]
- Domingos, M.O.; Cardoso, J.L.; da Silva, A.M.; Mota, I. The Humoral Immune Responses of Patients Bitten by the Snake Bothrops Jararaca (Jararaca). Toxicon 1990, 28, 723–726. [Google Scholar] [CrossRef]
- Pe, T.; Myint, A. Humoral Response Following Russell’s Viper (Daboia russelli siamensis) Bites in Myanmar. Toxicon 1995, 33, 373–377. [Google Scholar] [CrossRef]
- Theakston, R.D.; Pugh, R.N.; Reid, H.A. Enzyme-Linked Immunosorbent Assay of Venom-Antibodies in Human Victims of Snake Bite. J. Trop. Med. Hyg. 1981, 84, 109–112. [Google Scholar]
- Theakston, R.D.; Wyatt, G.B. Venom Antibody Levels in a Patient Bitten by a Young Puff Adder (Bitis arietans) during a World Record Attempt. Ann. Trop. Med. Parasitol. 1985, 79, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.P.; Theakston, R.D. Epidemiological Studies of Snake Bite in French Guiana. Ann. Trop. Med. Parasitol. 1987, 81, 301–304. [Google Scholar] [CrossRef]
- Wadee, A.A.; Rabson, A.R. Development of Specific IgE Antibodies after Repeated Exposure to Snake Venom. J. Allergy Clin. Immunol. 1987, 80, 695–698. [Google Scholar] [CrossRef]
- Ameno, S.; Ameno, K.; Fuke, C.; Kiryu, T.; Ijiri, I. IgG Subclass Distributions of Anti-Horse Serum Antibodies and Natural Venom-Antibodies Produced in Response to Antivenom Injection or Snake Bite in Humans. Toxicon 1990, 28, 347–350. [Google Scholar] [CrossRef]
- Sells, P.G.; Theakston, R.D.; Warrell, D.A. Development of Alpha-Neurotoxin Antibodies in Patients Envenomed by the Monocellate Thai Cobra (Naja kaouthia). Toxicon 1994, 32, 1667–1671. [Google Scholar] [CrossRef]
- Pe, T.; Myint, A.A.; Warrell, D.; Myint, T. King Cobra (Ophiophagus hannah) Bites in Myanmar: Venom Antigen Levels and Development of Venom Antibodies. Toxicon 1995, 33, 379–382. [Google Scholar] [CrossRef]
- Morais, V.; Berasain, P.; Ifrán, S.; Carreira, S.; Tortorella, M.N.; Negrín, A.; Massaldi, H. Humoral Immune Responses to Venom and Antivenom of Patients Bitten by Bothrops Snakes. Toxicon 2012, 59, 315–319. [Google Scholar] [CrossRef] [PubMed]
- de Medeiros, C.R.; Barbaro, K.C.; Lira, M.S.; França, F.O.S.; Zaher, V.L.; Kokron, C.M.; Kalil, J.; Castro, F.F.M. Predictors of Bothrops jararaca Venom Allergy in Snake Handlers and Snake Venom Handlers. Toxicon 2008, 51, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Swiontek, K.; Planchon, S.; Ollert, M.; Eyer, F.; Fischer, J.; Hilger, C. Phospholipase A2 Triggers Anaphylaxis to Snake Venom by Repeated Skin Sensitization: A Case Report. J. Investig. Allergol. Clin. Immunol. 2021, 31, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Sewall, H. Experiments on the Preventive Inoculation of Rattlesnake Venom. J. Physiol. 1887, 8, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Leonard, M.; Bresee, C.; Cruikshank, A. Effects of the Canine Rattlesnake Vaccine in Moderate to Severe Cases of Canine Crotalid Envenomation. Veter. Med. Res. Rep. 2014, 5, 153–158. [Google Scholar] [CrossRef]
- Herrera, M.; González, K.; Rodríguez, C.; Gómez, A.; Segura, Á.; Vargas, M.; Villalta, M.; Estrada, R.; León, G. Active Immunization of Cattle with a Bothropic Toxoid Does Not Abrogate Envenomation by Bothrops asper. Venom, but Increases the Likelihood of Survival. Biologicals 2017, 46, 1–5. [Google Scholar] [CrossRef]
- Dolimbek, B.Z.; Zouhair Atassi, M. Protection against α-Bungarotoxin Poisoning by Immunization with Synthetic Toxin Peptides. Mol. Immunol. 1996, 33, 681–689. [Google Scholar] [CrossRef]
- Costa, T.G.F.; Costal-Oliveira, F.; de Assis, T.C.S.; Lima, S.A.; Martins, C.A.; Finco, A.B.; Veiga, S.S.; Soccol, V.T.; Machado-de-Ávila, R.A.; Figueiredo, L.F.M.; et al. Engineered Antigen Containing Epitopes from Loxosceles spp. Spider Toxins Induces a Monoclonal Antibody (Lox-mAb3) against Astacin-like Metalloproteases. Int. J. Biol. Macromol. 2020, 162, 490–500. [Google Scholar] [CrossRef]
- Cerni, F.; Oliveira, I.; Cordeiro, F.; Bordon, K.; Ferreira, I.; Monteiro, W.; Arantes, E.; Cunha, T.; Pucca, M. The Nociceptive Response Induced by Different Classes of Tityus serrulatus Neurotoxins: The Important Role of Ts5 in Venom-Induced Nociception. PLoS Neglected Trop. Dis. 2023, 17, e0011057. [Google Scholar] [CrossRef]
- Haast, W.E.; Winer, M.L. Complete and Spontaneous Recovery from the Bite of a Blue Krait Snake (Bungarus caeruleiis). Am. J. Trop. Med. Hyg. 1955, 4, 1135–1137. [Google Scholar] [CrossRef] [PubMed]
- Watt, H.F.; Parrish, H.M.; Pollard, C.B. Repeated Poisonous Snakebites in the Same Patient: An Unusual Case Report. North Carol. Med. J. 1956, 17, 174–179. [Google Scholar]
- Parrish, H.M.; Pollard, C.B. Effects of Repeated Poisonous Snakebites in Man. Am. J. Med. Sci. 1959, 237, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Flowers, H.H. Active immunization of a human being against cobra (Naja naja) venom. Nature 1963, 200, 1017–1018. [Google Scholar] [CrossRef] [PubMed]
- Canan, E.D.; Flowers, H.H. Cobra Bite Following Immunization against Cobra Venom. JAMA 1965, 193, 625–626. [Google Scholar] [CrossRef]
- Repeated Poisonous Snakebites in Man. Am. J. Public Health Nations Health 1959, 49, 946. [CrossRef]
- Sawai, Y.; Kawamura, Y.; Fukuyama, T.; Okonogi, T.; Ebisawa, I. Studies on the Improvement of Treatment of Habu (Trimeresurus flavoviridis) Bites. 8. A Field Trial of Prophylactic Inoculation of the Habu Venom Toxoid. Jap. J. Expo Med. 1969, 39, 197–203. [Google Scholar]
- Magalhães, S.F.V.; Peixoto, H.M.; De Almeida Gonçalves Sachett, J.; Oliveira, S.S.; Alves, E.C.; Dos Santos Ibiapina, H.N.; Monteiro, W.M.; De Oliveira, M.R.F. Snakebite Envenomation in the Brazilian Amazon: A Cost-of-Illness Study. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 642–649. [Google Scholar] [CrossRef]
- Brown, N.I. Consequences of Neglect: Analysis of the Sub-Saharan African Snake Antivenom Market and the Global Context. PLoS Neglected Trop. Dis. 2012, 6, e1670. [Google Scholar] [CrossRef]
- Habib, A.G.; Lamorde, M.; Dalhat, M.M.; Habib, Z.G.; Kuznik, A. Cost-Effectiveness of Antivenoms for Snakebite Envenoming in Nigeria. PLoS Neglected Trop. Dis. 2015, 9, e3381. [Google Scholar] [CrossRef]
- de Oliveira, I.S.; Cardoso, I.A.; de Bordon, K.C.F.; Carone, S.E.I.; Boldrini-França, J.; Pucca, M.B.; Zoccal, K.F.; Faccioli, L.H.; Sampaio, S.V.; Rosa, J.C.; et al. Global Proteomic and Functional Analysis of Crotalus durissus collilineatus Individual Venom Variation and Its Impact on Envenoming. J. Proteom. 2019, 191, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Pucca, M.B.; Knudsen, C.; Oliveira, I.S.; Rimbault, C.; Cerni, F.A.; Wen, F.H.; Sachett, J.; Sartim, M.A.; Laustsen, A.H.; Monteiro, W.M. Current Knowledge on Snake Dry Bites. Toxins 2020, 12, 668. [Google Scholar] [CrossRef] [PubMed]



| Snake Species | Patients (n) | Country | Main Results | Method of Detection | Antibody Type | Ref. |
|---|---|---|---|---|---|---|
| Echis carinatus and Echis schistosa | 1 | United Kingdom | In a patient bitten by two different species (Echis carinatus and E. schistosa) in different periods (2 years old and 22 years old, respectively), high antivenom antibodies against E. carinatus were identified, while a lack of antibodies were identified against E. schistosa. | ELISA | - | [42] |
| Several snakes | 223 | Ecuador | 79% of the patients exhibited antivenom antibodies; most patients had antibodies against more than one snake species. | ELISA | - | [16] |
| Echis carinatus | 12 | Nigeria | In 10 victims exposed to Echis carinatus, it antivenom antibodies were detectable in 4 patients up to 14 days after the bite. Two children previously bitten by Echis carinatus were exposed again to the same species and presented a rapid onset of antibodies (<48 h). | ELISA | - | [47] |
| Bitis arietans | 1 | United Kingdom | After the bite, the patient was followed for 81 days, with weekly sample collection. Significant antibody levels were detected on the 9th day, rising to a peak three weeks after the bite and sustained for 11 days before starting to decrease. The last sample collected on the 81st day displayed a 50% decrease in the antibody level when compared with the antibody titer peak. | ELISA | - | [48] |
| Several snakes | 43 | French Guiana | In 43 patients tested for a specific venom antibody, 51% were positive. It was observed that the degree of positivity of the ELISA and the intensity of the immune response was influenced by the degree of symptoms and by the time of exposure. No antibody was detected at periods greater than 15 years. | ELISA | - | [49] |
| Hemachatus haemachatus | 1 | South Africa | A farm worker with several previously dermatological exposures to H. huemachatus demonstrated significant amounts of IgE antibodies against a 66 kDa fraction of the snake venom and high titers of IgG antibody against the same snake, not identified in the control. | ELISA/Western blotting | IgE/IgG | [50] |
| Bothrops jararaca | 22 | Brazil | Early appearance and short duration of antivenom IgM, observed on the 3rd day post-exposure and disappearing by the 20th day; IgG antibodies became detectable starting from the 18th day and showed a progressive increase until the 80th day; in patients with previous exposure to snake venom, IgG antibodies were detected as early as the 3rd day, with significantly higher levels. | ELISA/Western blotting | IgM/IgG | [45] |
| Gloydius blomhoffii | 20 (19 with their first exposure and 1 previously exposed) | Japan | IgG was detected starting from the first week, and the presence of IgG1 and IgG4 was observed even after 15 years of exposure; in patients with prior exposure, a prompt IgG production was observed following the second contact. | ELISA | IgG subclasses (1–4) | [51] |
| Naja kaouthia | 50 | Thailand | 38 patients exhibited antibodies against the venom; a distinct group of 8 patients demonstrated the presence of specific antibodies targeting the α-neurotoxin. | EIA | - | [52] |
| Daboia russelli siamensis | 158 | Myanmar | 95.5% of patients exhibited antivenom antibodies; IgM specific to the venom was detectable as early as the 3rd day following exposure, reaching its peak on the 7th day, and becoming undetectable after 6 weeks (42 days). Patients who developed uremia showed a comparatively lower production of IgM; IgG was detected from the first week onward, indicating a relatively rapid and sustained immune response. | EIA | IgM/IgG | [46] |
| Ophiophagus hannah (King Cobra) | 2 | Myanmar | Venom IgM antibody was produced a week earlier than venom IgG antibody, peaked at day 8 and fell to a low base within 12–16 days after the bite. However, the venom IgG antibody presented a different development in each patient: the first one, with no previous contact with the species and treated with antivenom, developed a short peak in 12th day followed by a quick fall between the 16–28th day. The second one had received small doses of venoms, including from a king cobra, for years as a method of traditional immunization and did not receive antivenom after the exposure. In this subject, the venom IgG had a sustained response, reaching its highest concentration in oday 12 and maintaining a plateau for two weeks. | EIA | IgM/IgG | [53] |
| Crotalus durissus terrificus | 16 | Brazil | IgM and IgG were evident by the 3rd day, with IgM reaching its peak titer production between the 7th and 18th day, and IgG levels peaking between 30–90 days | ELISA | IgM/IgG | [44] |
| Bothrops alternatus and Bothrops pubescens | 11 | Uruguay | Comparing the immune response against both snakes, a higher production of IgM antibodies against B. pubescens venom was noted compared to those from B. alternatus venom. Similar concentrations of IgG were observed among the two groups. | ELISA/Western blotting | IgM/IgG | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jati, S.R.; dos Anjos Martins, T.A.; Rocha, A.M.; Melo-dos-Santos, G.; de Oliveira, I.S.; Ferreira, I.G.; de Farias, A.S.; Filardi, E.T.M.; Cerni, F.A.; Sartim, M.A.; et al. The State-of-the-Art of the Humoral Memory Response to Snakebites: Insights from the Yanomami Population. Toxins 2023, 15, 638. https://doi.org/10.3390/toxins15110638
Jati SR, dos Anjos Martins TA, Rocha AM, Melo-dos-Santos G, de Oliveira IS, Ferreira IG, de Farias AS, Filardi ETM, Cerni FA, Sartim MA, et al. The State-of-the-Art of the Humoral Memory Response to Snakebites: Insights from the Yanomami Population. Toxins. 2023; 15(11):638. https://doi.org/10.3390/toxins15110638
Chicago/Turabian StyleJati, Sewbert Rodrigues, Thais Andréa dos Anjos Martins, Anderson Maciel Rocha, Guilherme Melo-dos-Santos, Isadora Sousa de Oliveira, Isabela Gobbo Ferreira, Altair Seabra de Farias, Eloise T. M. Filardi, Felipe Augusto Cerni, Marco Aurélio Sartim, and et al. 2023. "The State-of-the-Art of the Humoral Memory Response to Snakebites: Insights from the Yanomami Population" Toxins 15, no. 11: 638. https://doi.org/10.3390/toxins15110638











