Comparative Assessment of Different Yeast Cell Wall-Based Mycotoxin Adsorbents Using a Model- and Bioassay-Based In Vitro Approach
Abstract
:1. Introduction
2. Results
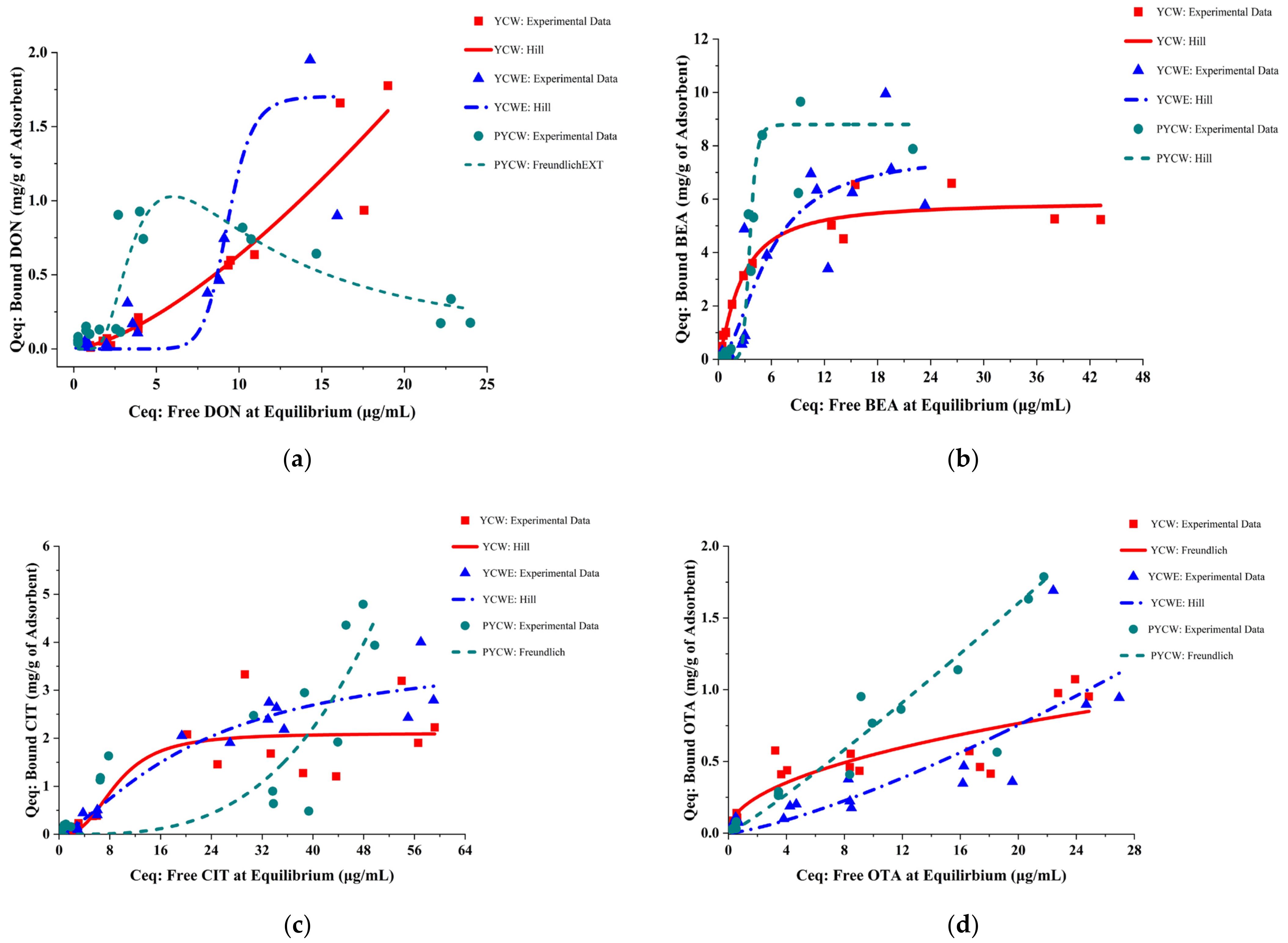
2.1. The Goodness of Fit of Adsorption Isotherm Models
2.2. Isotherm Parameters of Best-Fitting Models
2.2.1. Hill Isotherm Model Fitting and Binding Potential
2.2.2. Freundlich Isotherm Model Fitting
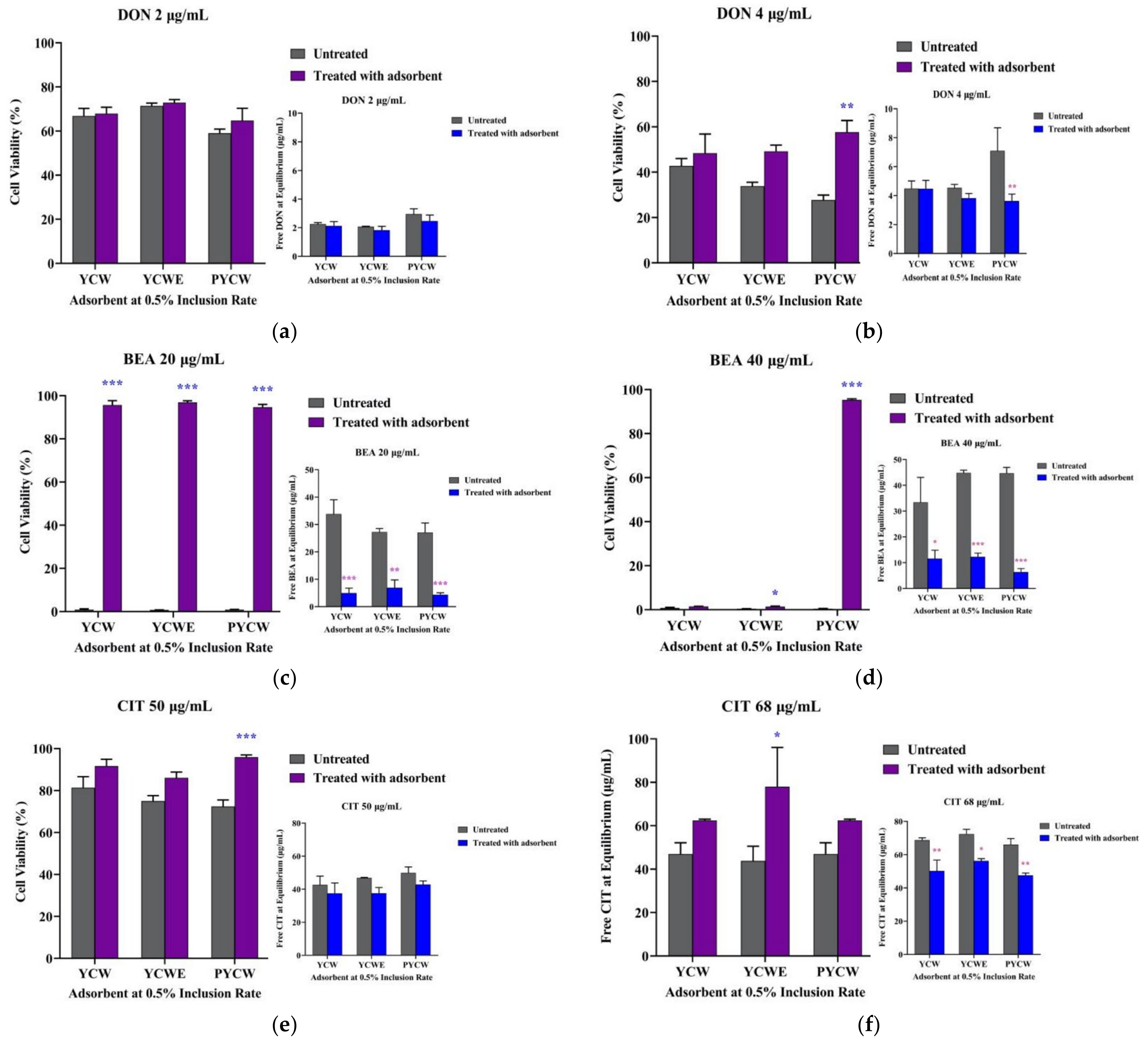
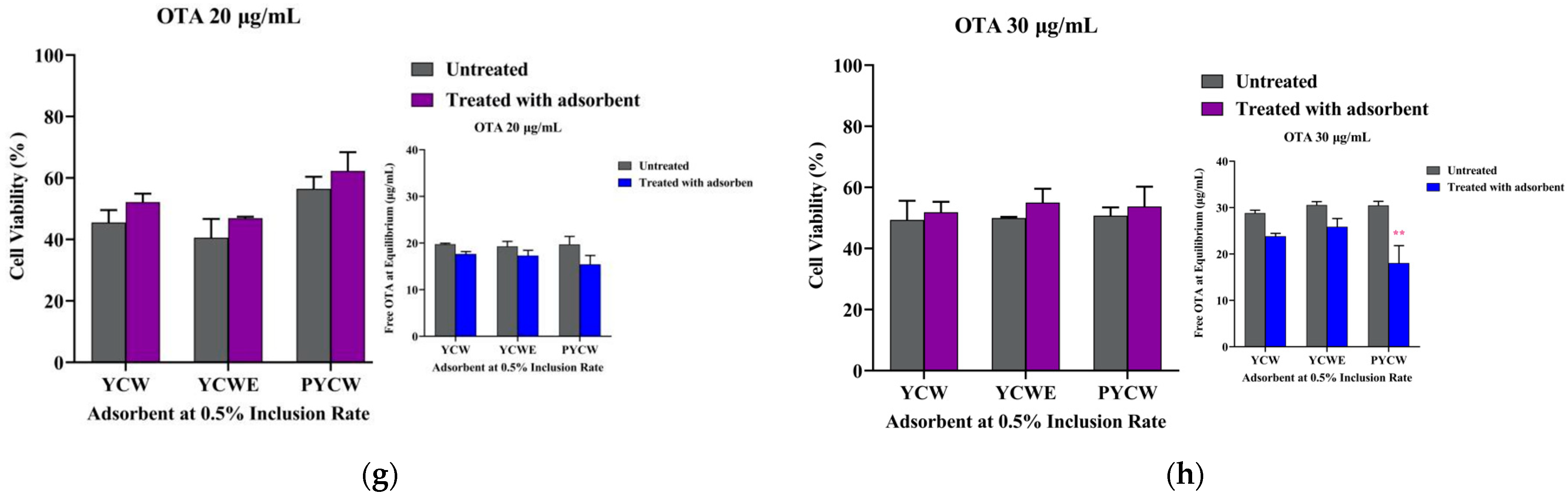
2.3. Adsorption Efficiency
2.4. Bioassay Adsorbent Efficacy Assessment
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Mycotoxin Adsorbents
5.2. Mycotoxin Adsorption Experiment in Aqueous Buffer
5.3. Analytical Methodology for Mycotoxin Quantification
5.4. Equilibrium Adsorption Isotherms
5.5. Adsorption Efficiency
5.6. Bioassay
5.6.1. Cell Culture
5.6.2. Cytotoxicity Assay
5.7. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reisinger, N.; Schürer-Waldheim, S.; Mayer, E.; Debevere, S.; Antonissen, G.; Sulyok, M.; Nagl, V. Mycotoxin Occurrence in Maize Silage—A Neglected Risk for Bovine Gut Health? Toxins 2019, 11, 577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Kiarie, E.G.; Yiannikouris, A.; Sun, L.; Karrow, N.A. Nutritional Impact of Mycotoxins in Food Animal Production and Strategies for Mitigation. J. Anim. Sci. Biotechnol. 2022, 13, 69. [Google Scholar] [CrossRef] [PubMed]
- Kępińska-Pacelik, J.; Biel, W. Mycotoxins—Prevention, Detection, Impact on Animal Health. Processes 2021, 9, 2035. [Google Scholar] [CrossRef]
- Antonissen, G.; Martel, A.; Pasmans, F.; Ducatelle, R.; Verbrugghe, E.; Vandenbroucke, V.; Li, S.; Haesebrouck, F.; Van Immerseel, F.; Croubels, S. The Impact of Fusarium Mycotoxins on Human and Animal Host Susceptibility to Infectious Diseases. Toxins 2014, 6, 430–452. [Google Scholar] [CrossRef] [Green Version]
- Brennan, K.M.; Oh, S.-Y.; Yiannikouris, A.; Graugnard, D.E.; Karrow, N.A. Differential Gene Expression Analysis of Bovine Macrophages after Exposure to the Penicillium Mycotoxins Citrinin and/or Ochratoxin A. Toxins 2017, 9, 366. [Google Scholar] [CrossRef]
- Brown, R.; Priest, E.; Naglik, J.R.; Richardson, J.P. Fungal Toxins and Host Immune Responses. Front. Microbiol. 2021, 12, 697. [Google Scholar] [CrossRef]
- EFSA; Maggiore, A.; Afonso, A.; Barrucci, F.; Sanctis, G.D. Climate Change as a Driver of Emerging Risks for Food and Feed Safety, Plant, Animal Health and Nutritional Quality. EFSA Support. Publ. 2020, 17, 1881E. [Google Scholar] [CrossRef]
- FAO. Climate Change: Unpacking the Burden on Food Safety; Food Safety and Quality Series; FAO: Rome, Italy, 2020; ISBN 978-92-5-132293-2. [Google Scholar]
- Adegbeye, M.J.; Reddy, P.R.K.; Chilaka, C.A.; Balogun, O.B.; Elghandour, M.M.M.Y.; Rivas-Caceres, R.R.; Salem, A.Z.M. Mycotoxin Toxicity and Residue in Animal Products: Prevalence, Consumer Exposure and Reduction Strategies—A Review. Toxicon 2020, 177, 96–108. [Google Scholar] [CrossRef]
- Becker-Algeri, T.A.; Castagnaro, D.; Bortoli, K.; Souza, C.; Drunkler, D.A.; Badiale-Furlong, E. Mycotoxins in Bovine Milk and Dairy Products: A Review. J. Food Sci. 2016, 81, R544–R552. [Google Scholar] [CrossRef] [Green Version]
- Piątkowska, M.; Sulyok, M.; Pietruszka, K.; Panasiuk, Ł. Pilot Study for the Presence of Fungal Metabolites in Sheep Milk from First Spring Milking. J. Vet. Res. 2018, 62, 167–172. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Opinion of the Scientific Panel on Contaminants in the Food Chain [CONTAM] Related to Ochratoxin A (OTA) as Undesirable Substance in Animal Feed. EFSA J. 2004, 2, 101. [CrossRef]
- Binder, S.B.; Schwartz-Zimmermann, H.E.; Varga, E.; Bichl, G.; Michlmayr, H.; Adam, G.; Berthiller, F. Metabolism of Zearalenone and Its Major Modified Forms in Pigs. Toxins 2017, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Bouhet, S.; Hourcade, E.; Loiseau, N.; Fikry, A.; Martinez, S.; Roselli, M.; Galtier, P.; Mengheri, E.; Oswald, I.P. The Mycotoxin Fumonisin B1 Alters the Proliferation and the Barrier Function of Porcine Intestinal Epithelial Cells. Toxicol. Sci. 2004, 77, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Weaver, A.C.; Weaver, D.M.; Adams, N.; Yiannikouris, A. Co-Occurrence of 35 Mycotoxins: A Seven-Year Survey of Corn Grain and Corn Silage in the United States. Toxins 2021, 13, 516. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.-C.; Nebbia, C.S.; et al. Risk Assessment of Ochratoxin A in Food. EFSA J. 2020, 18, e06113. [Google Scholar] [CrossRef]
- Deng, J.; Zhao, L.; Zhang, N.-Y.; Karrow, N.A.; Krumm, C.S.; Qi, D.-S.; Sun, L.-H. Aflatoxin B1 Metabolism: Regulation by Phase I and II Metabolizing Enzymes and Chemoprotective Agents. Mutat. Res./Rev. Mutat. Res. 2018, 778, 79–89. [Google Scholar] [CrossRef]
- Yiannikouris, A.; Apajalahti, J.; Kettunen, H.; Ojanperä, S.; Bell, A.N.W.; Keegan, J.D.; Moran, C.A. Efficient Aflatoxin B1 Sequestration by Yeast Cell Wall Extract and Hydrated Sodium Calcium Aluminosilicate Evaluated Using a Multimodal In-Vitro and Ex-Vivo Methodology. Toxins 2021, 13, 24. [Google Scholar] [CrossRef]
- Boudergue, C.; Burel, C.; Dragacci, S.; Favrot, M.-C.; Fremy, J.-M.; Massimi, C.; Prigent, P.; Debongnie, P.; Pussemier, L.; Boudra, H.; et al. Review of Mycotoxin-Detoxifying Agents Used as Feed Additives: Mode of Action, Efficacy and Feed/Food Safety. EFSA Support. Publ. 2009, 6, 22E. [Google Scholar] [CrossRef] [Green Version]
- Kolawole, O.; Meneely, J.; Greer, B.; Chevallier, O.; Jones, D.S.; Connolly, L.; Elliott, C. Comparative In Vitro Assessment of a Range of Commercial Feed Additives with Multiple Mycotoxin Binding Claims. Toxins 2019, 11, 659. [Google Scholar] [CrossRef] [Green Version]
- Dillon, G.P.; Yiannikouris, A.; Moran, C.A. Toxicological Evaluation of a Glycan Preparation from an Enzymatic Hydrolysis of Saccharomyces Cerevisiae. Regul. Toxicol. Pharmacol. 2021, 123, 104924. [Google Scholar] [CrossRef]
- Holanda, D.M.; Yiannikouris, A.; Kim, S.W. Investigation of the Efficacy of a Postbiotic Yeast Cell Wall-Based Blend on Newly-Weaned Pigs under a Dietary Challenge of Multiple Mycotoxins with Emphasis on Deoxynivalenol. Toxins 2020, 12, 504. [Google Scholar] [CrossRef] [PubMed]
- Kudupoje, M.B.; Malathi, V.; Yiannikouris, A. Impact of a Natural Fusarial Multi-Mycotoxin Challenge on Broiler Chickens and Mitigation Properties Provided by a Yeast Cell Wall Extract and a Postbiotic Yeast Cell Wall-Based Blend. Toxins 2022, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-Y.; Quinton, V.M.; Boermans, H.J.; Swamy, H.V.L.N.; Karrow, N.A. In Vitro Exposure of Penicillium Mycotoxins with or without a Modified Yeast Cell Wall Extract (MYCW) on Bovine Macrophages (BoMacs). Mycotoxin Res. 2015, 31, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Vartiainen, S.; Yiannikouris, A.; Apajalahti, J.; Moran, C.A. Comprehensive Evaluation of the Efficiency of Yeast Cell Wall Extract to Adsorb Ochratoxin A and Mitigate Accumulation of the Toxin in Broiler Chickens. Toxins 2020, 12, 37. [Google Scholar] [CrossRef] [Green Version]
- Yiannikouris, A.; Apajalahti, J.; Siikanen, O.; Dillon, G.P.; Moran, C.A. Saccharomyces Cerevisiae Cell Wall-Based Adsorbent Reduces Aflatoxin B1 Absorption in Rats. Toxins 2021, 13, 209. [Google Scholar] [CrossRef]
- Fumagalli, F.; Ottoboni, M.; Pinotti, L.; Cheli, F. Integrated Mycotoxin Management System in the Feed Supply Chain: Innovative Approaches. Toxins 2021, 13, 572. [Google Scholar] [CrossRef]
- Kunz, B.M.; Pförtner, L.; Weigel, S.; Rohn, S.; Lehmacher, A.; Maul, R. Growth and Toxin Production of Phomopsin A and Ochratoxin A Forming Fungi under Different Storage Conditions in a Pea (Pisum sativum) Model System. Mycotoxin Res. 2022, 38, 37–50. [Google Scholar] [CrossRef]
- Panasiuk, L.; Jedziniak, P.; Pietruszka, K.; Piatkowska, M.; Bocian, L. Frequency and Levels of Regulated and Emerging Mycotoxins in Silage in Poland. Mycotoxin Res. 2019, 35, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Zhao, L.; Gong, G.; Zhang, L.; Shi, L.; Dai, J.; Han, Y.; Wu, Y.; Khalil, M.M.; Sun, L. Invited Review: Remediation Strategies for Mycotoxin Control in Feed. J. Anim. Sci. Biotechnol. 2022, 13, 19. [Google Scholar] [CrossRef]
- Zhu, Y.; Hassan, Y.I.; Watts, C.; Zhou, T. Innovative Technologies for the Mitigation of Mycotoxins in Animal Feed and Ingredients—A Review of Recent Patents. Anim. Feed. Sci. Technol. 2016, 216, 19–29. [Google Scholar] [CrossRef]
- Brenaut, P.; Lefèvre, L.; Rau, A.; Laloë, D.; Pisoni, G.; Moroni, P.; Bevilacqua, C.; Martin, P. Contribution of Mammary Epithelial Cells to the Immune Response during Early Stages of a Bacterial Infection to Staphylococcus Aureus. Vet. Res. 2014, 45, 16. [Google Scholar] [CrossRef] [Green Version]
- Wellnitz, O.; Bruckmaier, R.M. Invited Review: The Role of the Blood–Milk Barrier and Its Manipulation for the Efficacy of the Mammary Immune Response and Milk Production. J. Dairy Sci. 2021, 104, 6376–6388. [Google Scholar] [CrossRef]
- ESFA. Scientific Opinion on the Risks for Public and Animal Health Related to the Presence of Citrinin in Food and Feed. EFSA J. 2012, 10, 2605. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the Risks to Human and Animal Health Related to the Presence of Beauvericin and Enniatins in Food and Feed. EFSA J. 2014, 12, 3802. [Google Scholar] [CrossRef]
- Gumus, R.; Ercan, N.; Imik, H. Determination of Ochratoxin A Levels in Mixed Feed and Feed Stuffs Used in Some Laying Hens and Ruminant Enterprises of Sivas City. Rev. Bras. Cienc. Avic. 2018, 20, 85–90. [Google Scholar] [CrossRef]
- Kelman, M.J.; Renaud, J.B.; Baines, D.; Yeung, K.K.-C.; Miller, J.D.; Sumarah, M.W. Mycotoxin Determination in Fungal Contaminated Canadian Silage Toxic to Dairy Cows and Goats. World Mycotoxin J. 2022, 15, 429–438. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Dvořáčková, M.; Kašparovský, T. Feedborne Mycotoxins Beauvericin and Enniatins and Livestock Animals. Toxins 2021, 13, 32. [Google Scholar] [CrossRef]
- Bertero, A.; Fossati, P.; Tedesco, D.E.A.; Caloni, F. Beauvericin and Enniatins: In Vitro Intestinal Effects. Toxins 2020, 12, 686. [Google Scholar] [CrossRef]
- Xu, R.; Karrow, N.A.; Shandilya, U.K.; Sun, L.; Kitazawa, H. In-Vitro Cell Culture for Efficient Assessment of Mycotoxin Exposure, Toxicity and Risk Mitigation. Toxins 2020, 12, 146. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Shandilya, U.K.; Yiannikouris, A.; Karrow, N.A. Ochratoxin A and Citrinin Differentially Modulate Bovine Mammary Epithelial Cell Permeability and Innate Immune Function. Toxins 2022, 14, 640. [Google Scholar] [CrossRef]
- Kemboi, D.C.; Antonissen, G.; Ochieng, P.E.; Croubels, S.; Okoth, S.; Kangethe, E.K.; Faas, J.; Lindahl, J.F.; Gathumbi, J.K. A Review of the Impact of Mycotoxins on Dairy Cattle Health: Challenges for Food Safety and Dairy Production in Sub-Saharan Africa. Toxins 2020, 12, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, D.; D’Ascanio, V.; Abbasciano, M.; Santovito, E.; Garbetta, A.; Logrieco, A.F.; Avantaggiato, G. Simultaneous Removal of Mycotoxins by a New Feed Additive Containing a Tri-Octahedral Smectite Mixed with Lignocellulose. Toxins 2022, 14, 393. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xingkai, X.; Cheng, W.; Lin, H. Dissolved Organic Matter and Inorganic N Jointly Regulate Greenhouse Gases Fluxes from Forest Soils with Different Moistures during a Freeze-Thaw Period. Soil Sci. Plant Nutr. 2020, 66, 163–176. [Google Scholar] [CrossRef]
- Zhang, F.C.; Zhong, H.X.; Zhou, X.M.; Han, S.A.; Wang, M.; Hao, J.Z.; Wu, X.Y.; Pan, M.Q. Photosynthesis of Grape Leaves with “OSC” Trellis and Cordon Based on Data Model Fitting. Photosyntetica 2021, 59, 160–170. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the Use and Interpretation of Adsorption Isotherm Models: A Review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Yiannikouris, A.; Poughon, L.; Cameleyre, X.; Dussap, C.-G.; François, J.; Bertin, G.; Jouany, J.-P. A Novel Technique to Evaluate Interactions between Saccharomyces cerevisiae Cell Wall and Mycotoxins: Application to Zearalenone. Biotechnol. Lett. 2003, 25, 783–789. [Google Scholar] [CrossRef]
- Venkatesh, N.; Keller, N.P. Mycotoxins in Conversation with Bacteria and Fungi. Front. Microbiol. 2019, 10, 403. [Google Scholar] [CrossRef]
- González-Jartín, J.M.; Ferreiroa, V.; Rodríguez-Cañás, I.; Alfonso, A.; Sainz, M.J.; Aguín, O.; Vieytes, M.R.; Gomes, A.; Ramos, I.; Botana, L.M. Occurrence of Mycotoxins and Mycotoxigenic Fungi in Silage from the North of Portugal at Feed-Out. Int. J. Food Microbiol. 2022, 365, 109556. [Google Scholar] [CrossRef]
- Yiannikouris, A.; André, G.; Poughon, L.; François, J.; Dussap, C.-G.; Jeminet, G.; Bertin, G.; Jouany, J.-P. Chemical and Conformational Study of the Interactions Involved in Mycotoxin Complexation with β-d-Glucans. Biomacromolecules 2006, 7, 1147–1155. [Google Scholar] [CrossRef]
- Jouany, J.-P.; Yiannikouris, A.; Bertin, G. How Yeast Cell Wall Components Can Alleviatemycotoxicosis in Animal Production and Improvethe Safety of Edible Animal Products. J. Anim. Feed Sci. 2005, 14, 171–190. [Google Scholar] [CrossRef] [Green Version]
- Bilal, M.; Rasheed, T.; Sosa-Hernández, J.E.; Raza, A.; Nabeel, F.; Iqbal, H.M.N. Biosorption: An Interplay between Marine Algae and Potentially Toxic Elements—A Review. Mar. Drugs 2018, 16, 65. [Google Scholar] [CrossRef] [Green Version]
- Avantaggiato, G.; Greco, D.; Damascelli, A.; Solfrizzo, M.; Visconti, A. Assessment of Multi-Mycotoxin Adsorption Efficacy of Grape Pomace. J. Agric. Food Chem. 2014, 62, 497–507. [Google Scholar] [CrossRef]
- Ying, Z.; Zhao, D.; Li, H.; Liu, X.; Zhang, J. Efficient Adsorption of Deoxynivalenol by Porous Carbon Prepared from Soybean Dreg. Toxins 2021, 13, 500. [Google Scholar] [CrossRef]
- Xu, R.; Shandilya, U.K.; Yiannikouris, A.; Karrow, N.A. Traditional and Emerging Fusarium Mycotoxins Disrupt Homeostasis of Bovine Mammary Cells by Altering Cell Permeability and Innate Immune Function. Anim. Nutr. 2022. [Google Scholar] [CrossRef]
- Grant, P.G.; Phillips, T.D. Isothermal Adsorption of Aflatoxin B1 on HSCAS Clay. J. Agric. Food Chem. 1998, 46, 599–605. [Google Scholar] [CrossRef]
- Pereyra, C.M.; Cavaglieri, L.R.; Chiacchiera, S.M.; Dalcero, A. The Corn Influence on the Adsorption Levels of Aflatoxin B1 and Zearalenone by Yeast Cell Wall. J. Appl. Microbiol. 2013, 114, 655–662. [Google Scholar] [CrossRef]
- Greco, D.; D’Ascanio, V.; Santovito, E.; Logrieco, A.F.; Avantaggiato, G. Comparative Efficacy of Agricultural By-Products in Sequestering Mycotoxins. J. Sci. Food Agric. 2019, 99, 1623–1634. [Google Scholar] [CrossRef]
- Yiannikouris, A.; Kettunen, H.; Apajalahti, J.; Pennala, E.; Moran, C.A. Comparison of the Sequestering Properties of Yeast Cell Wall Extract and Hydrated Sodium Calcium Aluminosilicate in Three in Vitro Models Accounting for the Animal Physiological Bioavailability of Zearalenone. Food Addit. Contam. Part A 2013, 30, 1641–1650. [Google Scholar] [CrossRef]
- Yiannikouris, A.; André, G.; Buléon, A.; Jeminet, G.; Canet, I.; François, J.; Bertin, G.; Jouany, J.-P. Comprehensive Conformational Study of Key Interactions Involved in Zearalenone Complexation with β-d-Glucans. Biomacromolecules 2004, 5, 2176–2185. [Google Scholar] [CrossRef]
- Basu, A.; Ali, S.S.; Hossain, S.S.; Asif, M. A Review of the Dynamic Mathematical Modeling of Heavy Metal Removal with the Biosorption Process. Processes 2022, 10, 1154. [Google Scholar] [CrossRef]
- Xiao, B.; Thomas, K.M. Competitive Adsorption of Aqueous Metal Ions on an Oxidized Nanoporous Activated Carbon. Langmuir 2004, 20, 4566–4578. [Google Scholar] [CrossRef] [PubMed]
- Hein, P.; Michel, M.C.; Leineweber, K.; Wieland, T.; Wettschureck, N.; Offermanns, S. Receptor and Binding Studies. In Practical Methods in Cardiovascular Research; Dhein, S., Mohr, F.W., Delmar, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 723–783. ISBN 978-3-540-40763-8. [Google Scholar]
- Kilbourn, M.R. 11C- and 18F-Radiotracers for In Vivo Imaging of the Dopamine System: Past, Present and Future. Biomedicines 2021, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Lo Dico, G.; Croubels, S.; Carcelén, V.; Haranczyk, M. Machine Learning-Aided Design of Composite Mycotoxin Detoxifier Material for Animal Feed. Sci. Rep. 2022, 12, 4838. [Google Scholar] [CrossRef]
- Tang, Z.; Wu, H.; Wen, Q.; Hu, L. Effect of Adsorbent Dosage to Adsorbate Concentration Ratio on the Adsorption of Cd(II) on Coal Gangue. In Proceedings of the 8th International Congress on Environmental Geotechnics, Hangzhou, China, 28 October–1 November 2018; Zhan, L., Chen, Y., Bouazza, A., Eds.; Springer: Singapore, 2019; Volume 1, pp. 428–435. [Google Scholar]
- Wu, H.; Wen, Q.; Hu, L.; Gong, M. Effect of Adsorbate Concentration to Adsorbent Dosage Ratio on the Sorption of Heavy Metals on Soils. J. Environ. Eng. 2018, 144, 04017094. [Google Scholar] [CrossRef]
- Albroomi, H.I.; Elsayed, M.A.; Baraka, A.; Abdelmaged, M.A. Batch and Fixed-Bed Adsorption of Tartrazine Azo-Dye onto Activated Carbon Prepared from Apricot Stones. Appl. Water Sci. 2017, 7, 2063–2074. [Google Scholar] [CrossRef] [Green Version]
- Kihal, A.; Rodriguez-Prado, M.; Godoy, C.; Cristofol, C.; Calsamiglia, S. In Vitro Assessment of the Capacity of Certain Mycotoxin Binders to Adsorb Some Amino Acids and Water-Soluble Vitamins. J. Dairy Sci. 2020, 103, 3125–3132. [Google Scholar] [CrossRef]
- Lv, M.; Mao, J.; Zhou, Y.; Zhou, R.; Zhou, J. Adsorption Performance and Mechanism of Mycotoxin on Montmorillonite Modified by Organosilicon Grafting. Arab. J. Chem. 2021, 14, 103314. [Google Scholar] [CrossRef]
- Xia, Y.; Zheng, M.-Q.; Holden, D.; Lin, S.; Kapinos, M.; Ropchan, J.; Gallezot, J.-D.; Huang, Y.; Carson, R.E. Measurement of Bmax and Kd with the Glycine Transporter 1 Radiotracer 18F-MK6577 Using a Novel Multi-Infusion Paradigm. J. Cereb. Blood Flow Metab. 2015, 35, 2001–2009. [Google Scholar] [CrossRef] [Green Version]
- Huynh, H.T.; Robitaille, G.; Turner, J.D. Establishment of Bovine Mammary Epithelial Cells (MAC-T): An in Vitro Model for Bovine Lactation. Exp. Cell Res. 1991, 197, 191–199. [Google Scholar] [CrossRef]
- Sabater-Vilar, M.; Malekinejad, H.; Selman, M.H.J.; van der Doelen, M.A.M.; Fink-Gremmels, J. In Vitro Assessment of Adsorbents Aiming to Prevent Deoxynivalenol and Zearalenone Mycotoxicoses. Mycopathologia 2007, 163, 81. [Google Scholar] [CrossRef] [Green Version]

Mycotoxin | Adsorbent | Hill | Langmuir | Freundlich | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | SSRES | Reduced χ2 | R2 | SSRES | Reduced χ2 | R2 | SSRES | Reduced χ2 | ||
| DON | YCW | 0.9154 | 0.446 | 0.030 | nc | 0.9154 | 0.446 | 0.028 | ||
| YCWE | 0.8429 | 1.196 | 0.080 | nc | 0.7792 | 1.680 | 0.105 | |||
| PYCW | nc | 0.3121 | 1.287 | 0.080 | 0.1685 | 1.556 | 0.097 | |||
| BEA | YCW | 0.9634 | 3.640 | 0.243 | 0.9577 | 4.211 | 0.263 | 0.8619 | 13.751 | 0.859 |
| YCWE | 0.8150 | 33.208 | 2.214 | nc | 0.7766 | 40.082 | 2.505 | |||
| PYCW | 0.8881 | 33.102 | 2.207 | 0.7142 | 84.584 | 5.286 | 0.5958 | 119.612 | 7.476 | |
| CIT | YCW | 0.7649 | 4.878 | 0.325 | 0.7404 | 5.387 | 0.337 | 0.7090 | 6.039 | 0.377 |
| YCWE | 0.9366 | 1.800 | 0.120 | 0.9307 | 1.967 | 0.123 | 0.9135 | 2.455 | 0.153 | |
| PYCW | nc | nc | 0.6361 | 15.133 | 0.946 | |||||
| OTA | YCW | nc | 0.7388 | 0.446 | 0.028 | 0.7835 | 0.370 | 0.023 | ||
| YCWE | 0.6874 | 0.973 | 0.065 | nc | 0.6874 | 0.973 | 0.061 | |||
| PYCW | nc | nc | 0.7953 | 1.742 | 0.109 | |||||
| Mycotoxin | Adsorbent | Best-Fitting Model | Parameters | Binding Potential | |
|---|---|---|---|---|---|
| DON | YCW | Hill | Vmax (mg/g) ± SE | 45.67 ± 11.174 | 0.258 |
| Kd (μg/mL) ± SE | 176.80 ± 33.58 | ||||
| n ± SE | 1.48 ± 0.749 | ||||
| YCWE | Hill | Vmax (mg/g) ± SE | 1.75 ±0.170 | 0.188 | |
| Kd (μg/mL) ± SE | 9.31 ± 0.632 | ||||
| n ± SE | 12.46 ± 1.258 | ||||
| PYCW | na | na | na | na | |
| BEA | YCW | Hill | Vmax (mg/g) ± SE | 5.91 ± 0.357 | 2.300 |
| Kd (μg/mL) ± SE | 2.57 ± 0.520 | ||||
| n ± SE | 1.30 ± 0.242 | ||||
| YCWE | Hill | Vmax (mg/g) ± SE | 7.69 ± 1.913 | 1.376 | |
| Kd (μg/mL) ± SE | 5.59 ± 2.615 | ||||
| n ± SE | 1.85 ± 0.979 | ||||
| PYCW | Hill | Vmax (mg/g) ± SE | 8.80 ± 0.775 | 2.465 | |
| Kd (μg/mL) ± SE | 3.57 ± 0.186 | ||||
| n ± SE | 9.33 ± 6.141 | ||||
| CIT | YCW | Hill | Vmax (mg/g) ± SE | 2.10 ± 0.309 | 0.230 |
| Kd (μg/mL) ± SE | 9.13 ± 4.982 | ||||
| n ± SE | 2.74 ± 2.387 | ||||
| YCWE | Hill | Vmax (mg/g) ± SE | 3.86 ± 0.897 | 0.175 | |
| Kd (μg/mL) ± SE | 22.11 ± 9.316 | ||||
| n ± SE | 1.41 ± 0.394 | ||||
| PYCW | Freundlich | Kf (μg/mL) ± SE | 1.61E-05 | na | |
| 1/n ± SE | 3.21 ± 1.6 | ||||
| OTA | YCW | Freundlich | Kf (μg/mL) ± SE | 0.18 ± 0.048 | na |
| 1/n ± SE | 0.48 ± 0.096 | ||||
| YCWE | Freundlich | Kf (μg/mL) ± SE | 0.01 ± 0.018 | na | |
| 1/n ± SE | 1.13 ± 0.403 | ||||
| PYCW | Freundlich | Kf (μg/mL) ± SE | 0.06 ± 0.046 | na | |
| 1/n ± SE | 1.11 ± 0.275 | ||||
| Mycotoxin | Overall Mean Adsorption Efficiency (%) (Mean ± SEM)1 | ||
|---|---|---|---|
| YCW | YCWE | PYCW | |
| DON | 18.45 ± 3.204 a | 21.72 ± 4.300 a | 33.46 ± 7.993 b |
| BEA | 73.37 ± 6.794 a | 61.00 ± 4.771 b | 70.15 ± 7.912 ab |
| CIT | 22.90 ± 1.151 a | 26.10 ± 1.661 ab | 35.04 ± 6.783 b |
| OTA | 32.28 ± 7.111 a | 23.55 ± 5.785 b | 36.86 ± 5.150 a |
| Adsorption Isotherm Model | Equation | Parameters | Reference |
|---|---|---|---|
| Hill’s | Qeq = VmaxCeqn/(Kdn + Ceqn) | Vmax = maximum mycotoxin uptake Kd = dissociation constant per site (ug/mL) related to adsorption affinity n = Hill cooperativity coefficient of the binding interaction; minimum number of binding sties | [47,53,57] |
| Langmuir | Qeq = VmaxKLCeq/(1 + KLCeq) | Vmax = maximum mycotoxin uptake KL = constant related to affinity of adsorption | |
| Freundlich | Qeq = KfCeq1/n | Kf = constant indicating capacity of the adsorbent for the mycotoxin n = adsorption intensity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Yiannikouris, A.; Shandilya, U.K.; Karrow, N.A. Comparative Assessment of Different Yeast Cell Wall-Based Mycotoxin Adsorbents Using a Model- and Bioassay-Based In Vitro Approach. Toxins 2023, 15, 104. https://doi.org/10.3390/toxins15020104
Xu R, Yiannikouris A, Shandilya UK, Karrow NA. Comparative Assessment of Different Yeast Cell Wall-Based Mycotoxin Adsorbents Using a Model- and Bioassay-Based In Vitro Approach. Toxins. 2023; 15(2):104. https://doi.org/10.3390/toxins15020104
Chicago/Turabian StyleXu, Ran, Alexandros Yiannikouris, Umesh K. Shandilya, and Niel A. Karrow. 2023. "Comparative Assessment of Different Yeast Cell Wall-Based Mycotoxin Adsorbents Using a Model- and Bioassay-Based In Vitro Approach" Toxins 15, no. 2: 104. https://doi.org/10.3390/toxins15020104





