Antimicrobial Compounds from Skin Secretions of Species That Belong to the Bufonidae Family
Abstract
1. Introduction
2. Methodology
2.1. Literature Review
2.2. Chemical Structure of Compounds and Determination of Molecular Weight
3. Antimicrobial Activities of Compounds from Toads’ Cutaneous Secretions
3.1. Compounds with Antibacterial Activity
3.2. Compounds with Antifungal Activity
3.3. Compounds with Antiviral Activity
3.4. Compounds with Antiparasitic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AmphibiaWeb AmphibiaWeb<https://Amphibiaweb.Org> University of California, Berkeley, CA, USA. Available online: https://amphibiaweb.org/lists/Bufonidae.shtml (accessed on 1 November 2022).
- Frost D Amphibian Species of the World: An Online Reference (Version 6). Available online: https://amphibiansoftheworld.amnh.org/Amphibia/Anura/Bufonidae (accessed on 1 November 2022).
- Stewart, M.M. Biology of Amphibians. William E. Duellman, Linda Trueb. Q. Rev. Biol. 1994, 69, 670. [Google Scholar] [CrossRef]
- Jared, C.; Antoniazzi, M.M.; Jordão, A.E.C.; Silva, J.R.M.C.; Greven, H.; Rodrigues, M.T. Parotoid Macroglands in Toad (Rhinella Jimi): Their Structure and Functioning in Passive Defence. Toxicon 2009, 54, 197–207. [Google Scholar] [CrossRef]
- Regueira, E.; Dávila, C.; Sassone, A.G.; O’Donohoe, M.E.A.; Hermida, G.N. Post-Metamorphic Development of Skin Glands in a True Toad: Parotoids versus Dorsal Skin. J. Morphol. 2017, 278, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Regueira, E.; Dávila, C.; Hermida, G.N. Morphological Changes in Skin Glands During Development in Rhinella Arenarum (Anura: Bufonidae). Anat. Rec. 2016, 299, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Barnhart, K.; Forman, M.E.; Umile, T.P.; Kueneman, J.; McKenzie, V.; Salinas, I.; Minbiole, K.P.C.; Woodhams, D.C. Identification of Bufadienolides from the Boreal Toad, Anaxyrus Boreas, Active Against a Fungal Pathogen. Microb. Ecol. 2017, 74, 990–1000. [Google Scholar] [CrossRef]
- Clarke, B.T. The Natural History of Amphibian Skin Secretions, Their Normal Functioning and Potential Medical Applications. Biol. Rev. 1997, 72, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Li, F.J.; Hu, J.H.; Ren, X.; Zhou, C.M.; Liu, Q.; Zhang, Y.Q. Toad Venom: A Comprehensive Review of Chemical Constituents, Anticancer Activities, and Mechanisms. Arch. Pharm. 2021, 354, 2100060. [Google Scholar] [CrossRef]
- Mailho-Fontana, P.L.; Antoniazzi, M.M.; Toledo, L.F.; Verdade, V.K.; Sciani, J.M.; Barbaro, K.C.; Pimenta, D.C.; Rodrigues, M.T.; Jared, C. Passive and Active Defense in Toads: The Parotoid Macroglands in Rhinella Marina and Rhaebo Guttatus. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2014, 321, 65–77. [Google Scholar] [CrossRef]
- Rodriguez, C.; Ibáñez, R.; Mojica, L.; Ng, M.; Spadafora, C.; Durant-Archibold, A.A.; Gutiérrez, M. Bufadienolides from the Skin Secretions of the Neotropical Toad Rhinella Alata (Anura: Bufonidae): Antiprotozoal Activity against Trypanosoma Cruzi. Molecules 2021, 26, 4217. [Google Scholar] [CrossRef]
- Rodríguez, C.; Rollins-Smith, L.; Ibáñez, R.; Durant-Archibold, A.A.; Gutiérrez, M. Toxins and Pharmacologically Active Compounds from Species of the Family Bufonidae (Amphibia, Anura). J. Ethnopharmacol. 2017, 198, 235–254. [Google Scholar] [CrossRef]
- Rodriguez, C.; Ibáñez, R.; Rollins-Smith, L.A.; Gutiérrez, M.; Durant-Archibold, A.A. Antimicrobial Secretions of Toads (Anura, Bufonidae): Bioactive Extracts and Isolated Compounds against Human Pathogens. Antibiotics 2020, 9, 843. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.; Sun, S.; Zhang, H.; Ma, T.; Su, W.; Jiang, C. Identification of Cinobufagin and Resibufogenin as Inhibitors of Enterovirus 71 Infection. Chem. Res. Chin. Univ. 2014, 30, 953–958. [Google Scholar] [CrossRef]
- El-Seedi, H.; Yosri, N.; El-Aarag, B.; Mahmoud, S.; Zayed, A.; Du, M.; Saeed, A.; Musharraf, S.; El-Garawani, I.; Habib, M.; et al. Chemistry and the Potential Antiviral, Anticancer, and Anti-Inflammatory Activities of Cardiotonic Steroids Derived from Toads. Molecules 2022, 27, 6586. [Google Scholar] [CrossRef]
- Jin, Y.H.; Jeon, S.; Lee, J.; Kim, S.; Jang, M.S.; Park, C.M.; Song, J.H.; Kim, H.R.; Kwon, S. Broad Spectrum Antiviral Properties of Cardiotonic Steroids Used as Potential Therapeutics for Emerging Coronavirus Infections. Pharmaceutics 2021, 13, 1839. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Inagaki, Y.; Xu, H.; Wang, D.; Qi, F.; Kokudo, N.; Fang, D.; Tang, W. Anti-Hepatitis B Virus Activities of Cinobufacini and Its Active Components Bufalin and Cinobufagin in HepG2.2.15 Cells. Biol. Pharm. Bull. 2010, 33, 1728–1732. [Google Scholar] [CrossRef]
- Wong, R.W.; Lingwood, C.A.; Ostrowski, M.A.; Cabral, T.; Cochrane, A. Cardiac Glycoside/Aglycones Inhibit HIV-1 Gene Expression by a Mechanism Requiring MEK1/2-ERK1/2 Signaling. Sci. Rep. 2018, 8, 850. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, A.; Rathour, A.; Kaur, M. Bufadienolides and Their Medicinal Utility: A Review. Int. J. Pharm. Pharm. Sci. 2013, 5, 20–27. [Google Scholar]
- Baldo, E.C.F.; Anjolette, F.A.P.; Arantes, E.C.; Baldo, M.A. Toad poison and drug discovery. In Toxins and Drug Discovery; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Dos Filho, E.S.M.; Chaves, M.H.; Ferreira, P.M.P.; Pessoa, C.; Lima, D.J.B.; Maranhão, S.S.A.; de Jesus Rodrigues, D.; Vieira Júnior, G.M. Cytotoxicity Potential of Chemical Constituents Isolated and Derivatised from Rhinella Marina Venom. Toxicon 2021, 194, 37–43. [Google Scholar] [CrossRef]
- Shi, G.; Kang, X.; Dong, F.; Liu, Y.; Zhu, N.; Hu, Y.; Xu, H.; Lao, X.; Zheng, H. DRAMP 3.0: An Enhanced Comprehensive Data Repository of Antimicrobial Peptides. Nucleic Acids Res. 2022, 50, D488–D496. [Google Scholar] [CrossRef]
- Knight, G.M.; Glover, R.E.; McQuaid, C.F.; Olaru, I.D.; Gallandat, K.; Leclerc, Q.J.; Fuller, N.M.; Willcocks, S.J.; Hasan, R.; van Kleef, E.; et al. Antimicrobial Resistance and Covid-19: Intersections and Implications. Elife 2021, 10, e64139. [Google Scholar] [CrossRef]
- Pritchard, M.; Dankwa, E.A.; Hall, M.; Baillie, J.K.; Carson, G.; Docherty, A.; Donnelly, C.A.; Dunning, J.; Fraser, C.; Hardwick, H.; et al. ISARIC Clinical Data Report 4 October 2020 International Severe Acute Respiratory and Emerging Infections Consortium. medRxiv 2020. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic Prescribing in Patients with COVID-19: Rapid Review and Meta-Analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Raasch-Fernandes, L.D.; Bonaldo, S.M.; De Jesus Rodrigues, D.; Vieira-Junior, G.M.; Schwan-Estrada, K.R.F.; Da Silva, C.R.; Verçosa, A.G.A.; De Oliveira, D.L.; Debiasi, B.W. Induction of Phytoalexins and Proteins Related to Pathogenesis in Plants Treated with Extracts of Cutaneous Secretions of Southern Amazonian Bufonidae Amphibians. PLoS ONE 2019, 14, e0211020. [Google Scholar] [CrossRef]
- Tian, Z.; Liao, A.; Kang, J.; Gao, Y.; Lu, A.; Wang, Z.; Wang, Q. Toad Alkaloid for Pesticide Discovery: Dehydrobufotenine Derivatives as Novel Agents against Plant Virus and Fungi. J. Agric. Food Chem. 2021, 69, 9754–9763. [Google Scholar] [CrossRef]
- Tempone, A.G.; Pimenta, D.C.; Lebrun, I.; Sartorelli, P.; Taniwaki, N.N.; de Andrade, H.F.; Antoniazzi, M.M.; Jared, C. Antileishmanial and Antitrypanosomal Activity of Bufadienolides Isolated from the Toad Rhinella Jimi Parotoid Macrogland Secretion. Toxicon 2008, 52, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Cousins, K.R. Computer Review of ChemDraw Ultra 12.0. J. Am. Chem. Soc. 2011, 133, 8388. [Google Scholar] [CrossRef] [PubMed]
- Beavis, R.; Winsor, D.; Council, C. Peptide Mass Calculator. Available online: https://www.peptidesynthetics.co.uk/tools/ (accessed on 1 November 2022).
- Gordon, Y.J.; Romanowski, E.G.; McDermott, A.M. Mini Review: A Review of Antimicrobial Peptides and Their Therapeutic Potential as Anti-Infective Drugs. Curr. Eye Res. 2005, 30, 505–515. [Google Scholar] [CrossRef]
- de Medeiros, D.S.S.; Rego, T.B.; dos Santos, A.P.d.A.; Pontes, A.S.; Moreira-Dill, L.S.; Matos, N.B.; Zuliani, J.P.; Stábeli, R.G.; Teles, C.B.G.; Soares, A.M.; et al. Biochemical and Biological Profile of Parotoid Secretion of the Amazonian Rhinella Marina (Anura: Bufonidae). Biomed Res. Int. 2019, 2019, 4209743. [Google Scholar] [CrossRef] [PubMed]
- Zahari, M.; Darnis, D.; Hamid, T.H.T.A. Protein Profiles and Antimicrobial Activity of Common Sunda Toad, Duttaphrynus Melanostictus Paratoid Secretions. In Proceedings of the 3rd International Conference on Biological, Chemical & Environmental Sciences (BCES-2015), Kuala Lumpur, Malaysia, 21–22 September 2015. [Google Scholar]
- Sales, D.L.; Morais-Braga, M.F.B.; dos Santos, A.T.L.; Machado, A.J.T.; de Araujo Filho, J.A.; de Dias, D.Q.; da Cunha, F.A.B.; Saraiva, R.d.A.; Menezes, I.R.A.d.; Coutinho, H.D.M.; et al. Antibacterial, Modulatory Activity of Antibiotics and Toxicity from Rhinella Jimi (Stevaux, 2002) (Anura: Bufonidae) Glandular Secretions. Biomed. Pharmacother. 2017, 92, 554–561. [Google Scholar] [CrossRef]
- Dahham, S.S.; Hew, C.S.; Jaafar, I.; Gam, L.H. The Protein Profiling of Asian Giant Toad Skin Secretions and Their Antimicrobial Activity. Int. J. Pharm. Pharm. Sci. 2016, 8, 88–95. [Google Scholar]
- Thirupathi, K.; Shankar, C.; Chandrakala, G.; Krishna, I.; Venkaiah, Y. The Antifungal Activity of Skin Secretion and Its Extract of Indian Toad Bufo Melanostictus. Int. J. Environ. Ecol. Fam. urban Stud. 2019, 9, 7–12. [Google Scholar] [CrossRef]
- Riós-Orjuela, J.C.; Falcón-Espitia, N.; Arias-Escobar, A.; Espejo-Uribe, M.J.; Chamorro-Vargas, C.T. Conocimiento E Interacciones De La Comunidad Local Con La Herpetofauna En La Forestal De Quininí (Tibacuy-Cundinamarca, Colombia). J. Ethnobiol. Ethnomed. 2020, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Garraffo, H.M.; Gros, E.G. Biosynthesis of Bufadienolides in Toads. VI. Experiments with [1,2-3H]Cholesterol, [21-14C]Coprostanol, and 5β-[21-14C]Pregnanolone in the Toad Bufo Arenarum. Steroids 1986, 48, 251–257. [Google Scholar] [CrossRef]
- Krenn, L.; Kopp, B. Bufadienolides from Animal and Plant Sources. Phytochemistry 1998, 48, 1–29. [Google Scholar] [CrossRef]
- Cunha Filho, G.A.; Schwartz, C.A.; Resck, I.S.; Murta, M.M.; Lemos, S.S.; Castro, M.S.; Kyaw, C.; Pires, O.R.; Leite, J.R.S.; Bloch, C.; et al. Antimicrobial Activity of the Bufadienolides Marinobufagin and Telocinobufagin Isolated as Major Components from Skin Secretion of the Toad Bufo Rubescens. Toxicon 2005, 45, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Pearson, K.C.; Tarvin, R.D. A Review of Chemical Defense in Harlequin Toads (Bufonidae: Atelopus). Toxicon X 2022, 13, 100092. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.M.P.; Lima, D.J.B.; Debiasi, B.W.; Soares, B.M.; da Machado, K.C.; da Noronha, J.C.; Rodrigues, D.d.J.; Sinhorin, A.P.; Pessoa, C.; Vieira Júnior, G.M. Antiproliferative Activity of Rhinella Marina and Rhaebo Guttatus Venom Extracts from Southern Amazon. Toxicon 2013, 72, 43–51. [Google Scholar] [CrossRef]
- Perera Córdova, W.H.; Leitão, S.G.; Cunha-Filho, G.; Bosch, R.A.; Alonso, I.P.; Pereda-Miranda, R.; Gervou, R.; Touza, N.A.; Quintas, L.E.M.; Noël, F. Bufadienolides from Parotoid Gland Secretions of Cuban Toad Peltophryne Fustiger (Bufonidae): Inhibition of Human Kidney Na+/K+-ATPase Activity. Toxicon 2016, 110, 27–34. [Google Scholar] [CrossRef]
- Sun, T.; Zhan, B.; Gao, Y. A Novel Cathelicidin from Bufo Bufo Gargarizans Cantor Showed Specific Activity to Its Habitat Bacteria. Gene 2015, 571, 172–177. [Google Scholar] [CrossRef]
- Park, C.B.; Kim, M.S.; Kim, S.C. A Novel Antimicrobial Peptide from Bufo Bufo Gargarizans. Biochem. Biophys. Res. Commun. 1996, 218, 408–413. [Google Scholar] [CrossRef]
- Park, C.B.; Yi, K.S.; Matsuzaki, K.; Kim, M.S.; Kim, S.C. Structure-Activity Analysis of Buforin II, a Histone H2A-Derived Antimicrobial Peptide: The Proline Hinge Is Responsible for the Cell-Penetrating Ability of Buforin II. Proc. Natl. Acad. Sci. USA 2000, 97, 8245–8250. [Google Scholar] [CrossRef] [PubMed]
- Roshanak, S.; Shahidi, F.; Yazdi, F.T.; Javadmanesh, A.; Movaffagh, J. Evaluation of Antimicrobial Activity of Buforin I and Nisin and the Synergistic Effect of Their Combination as a Novel Antimicrobial Preservative. J. Food Prot. 2020, 83, 2018–2025. [Google Scholar] [CrossRef]
- Cho, J.H.; Sung, B.H.; Kim, S.C. Buforins: Histone H2A-Derived Antimicrobial Peptides from Toad Stomach. Biochim. Biophys. Acta-Biomembr. 2009, 1788, 1564–1569. [Google Scholar] [CrossRef]
- Kobayashi, S.; Takeshima, K.; Park, C.B.; Kim, S.C.; Matsuzaki, K. Interactions of the Novel Anfimicrobial Peptide Buforin 2 with Lipid Bilayers: Proline as a Translocation Promoting Factor. Biochemistry 2000, 39, 8648–8654. [Google Scholar] [CrossRef] [PubMed]
- Park, C.B.; Kim, H.S.; Kim, S.C. Mechanism of Action of the Antimicrobial Peptide Buforin II: Buforin II Kills Microorganisms by Penetrating the Cell Membrane and Inhibiting Cellular Functions. Biochem. Biophys. Res. Commun. 1998, 244, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Made Artika, I.; Pinontoan, S.; Kusrini, M.D. Antifungal Activity of Skin Secretion of Bleeding Toad Leptophryne Cruentata and Javan Tree Frog Rhacophorus Margaritifer. Am. J. Biochem. Biotechnol. 2015, 11, 5–10. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; de Andrade, J.P.; Soto-Vasquez, M.R.; Alvarado-García, P.A.A.; Palominos, C.; Fuentes-Retamal, S.; Mellado, M.; Correa, P.; Urra, F.A. The Parotoid Gland Secretion from Peruvian Toad Rhinella Horribilis (Wiegmann, 1833): Chemical Composition and Effect on the Proliferation and Migration of Lung Cancer Cells. Toxins 2020, 12, 608. [Google Scholar] [CrossRef]
- Scheele, B.C.; Pasmans, F.; Skerratt, L.F.; Berger, L.; Martel, A.; Beukema, W.; Acevedo, A.A.; Burrowes, P.A.; Carvalho, T.; Catenazzi, A.; et al. Amphibian Fungal Panzootic Causes Catastrophic and Ongoing Loss of Biodiversity. Science 2019, 363, 1459–1463. [Google Scholar] [CrossRef]
- De Souza Leal, É.; De Andrade Zanotto, P.M. Viral Diseases and Human Evolution. Mem. Inst. Oswaldo Cruz 2000, 95, 193–200. [Google Scholar] [CrossRef]
- Abate, C.; Carnamucio, F.; Giuffrè, O.; Foti, C. Metal-Based Compounds in Antiviral Therapy. Biomolecules 2022, 12, 933. [Google Scholar] [CrossRef]
- Oksenych, V.; Kainov, D.E. Broad-Spectrum Antivirals and Antiviral Drug Combinations. Viruses 2022, 14, 301. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Zhao, G.-H.; Zhang, J.; Shi, Q.-Y.; Guo, W.-X.; Tian, X.-L.; Qiu, J.-Z.; Yin, L.-Z.; Deng, X.-M.; Song, Y. Immunomodulatory Effects of Cinobufagin Isolated from Chan Su on Activation and Cytokines Secretion of Immunocyte in Vitro. J. Asian Nat. Prod. Res. 2011, 13, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Tan, C.K.; Hashimi, S.M.; Zulfiker, A.H.M.; Good, D.; Wei, M.Q. Toad Glandular Secretions and Skin Extractions as Anti-Inflammatory and Anticancer Agents. Evid.-Based Complement. Altern. Med. 2014, 2014, 312684. [Google Scholar] [CrossRef] [PubMed]
- Inada, A.; Nakanishi, T.; Nishino, H.; Ichiishi, E.; Mukainaka, T.; Okuda, M.; Tokuda, H. Inhibitory Effects of Bufadienolides on Epstein-Barr Virus Early Antigen Activation and on Growth of Mouse Skin and Mouse Pulmonary Tumors. Nat. Med. 1999, 53, 324–328. [Google Scholar]
- Kamano, Y.; Pettit, G.R.; Smith, C.R.; Satoh, N.; Nakayoshi, H. Rhinovirus Inhibition by Bufadienolides1a). Chem. Pharm. Bull. 1988, 36, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Vigerelli, H.; Sciani, J.M.; Jared, C.; Antoniazzi, M.M.; Caporale, G.M.M.; da Silva, A.d.C.R.; Pimenta, D.C. Bufotenine Is Able to Block Rabies Virus Infection in BHK-21 Cells. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 45. [Google Scholar] [CrossRef]
- CDC CDC—Parasites—About Parasites. Available online: https://www.cdc.gov/parasites/about.html (accessed on 1 November 2022).
- Fouque, F.; Knox, T. Special Programme for Research and Training in Tropical Diseases-Coordinated Multicountry Study to Determine the Burden and Causes of Residual Malaria across Different Regions. J. Infect. Dis. 2021, 223, S91–S98. [Google Scholar] [CrossRef]
- Geneva: World Health Organization World Malaria Report 2021. Angew. Chemie Int. Ed. 2021, 6, 951–952.
- Theel, E.S.; Pritt, B.S. Parasites. Microbiol. Spectr. 2016, 4, 411–466. [Google Scholar] [CrossRef]
- Pérez, J.L.; Carranza, C.; Mateos, F. Antiparasitic Drugs. Review of the Useful Drugs in the Treatment of Classic and Emergent Parasitic Diseases. Rev. Esp. Quimioter. 2009, 22, 93–105. [Google Scholar]
- Rojo-Medina, J.; Ruiz-Matus, C.; Salazar-Schettino, P.M.; González-Roldán, J.F. Enfermedad de Chagas En México. Gac. Mexico 2018, 154, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Sciani, J.M.; Angeli, C.B.; Antoniazzi, M.M.; Jared, C.; Pimenta, D.C. Differences and Similarities among Parotoid Macrogland Secretions in South American Toads: A Preliminary Biochemical Delineation. Sci. World J. 2013, 2013, 937407. [Google Scholar] [CrossRef]
- Banfi, F.F.; Krombauer, G.C.; da Fonseca, A.L.; Nunes, R.R.; Andrade, S.N.; de Rezende, M.A.; Chaves, M.H.; dos Santos Monção Filho, E.; Taranto, A.G.; de Jesus Rodrigues, D.; et al. Dehydrobufotenin Extracted from the Amazonian Toad Rhinella Marina (Anura: Bufonidae) as a Prototype Molecule for the Development of Antiplasmodial Drugs. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. In Pharmacopoeia of the People’s Republic of China; People’s Medical Publishing House, Co., Ltd.: Beijing, China, 2020; Volume 1, p. 402.
- Jiang, Z.; Vasil, A.I.; Hale, J.D.; Hancock, R.E.W.; Vasil, M.L.; Hodges, R.S. Effects of Net Charge and the Number of Positively Charged Residues on the Biological Activity of Amphipathic α-Helical Cationic Antimicrobial Peptides. Biopolymers 2008, 90, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; MacDonald, D.L.; Holroyd, K.J.; Thornsberry, C.; Wexler, H.; Zasloff, M. In Vitro Antibacterial Properties of Pexiganan, an Analog of Magainin. Antimicrob. Agents Chemother. 1999, 43, 369–383. [Google Scholar] [CrossRef]
- Krieg, R.; Jortzik, E.; Goetz, A.A.; Blandin, S.; Wittlin, S.; Elhabiri, M.; Rahbari, M.; Nuryyeva, S.; Voigt, K.; Dahse, H.M.; et al. Arylmethylamino Steroids as Antiparasitic Agents. Nat. Commun. 2017, 8, 14478. [Google Scholar] [CrossRef] [PubMed]
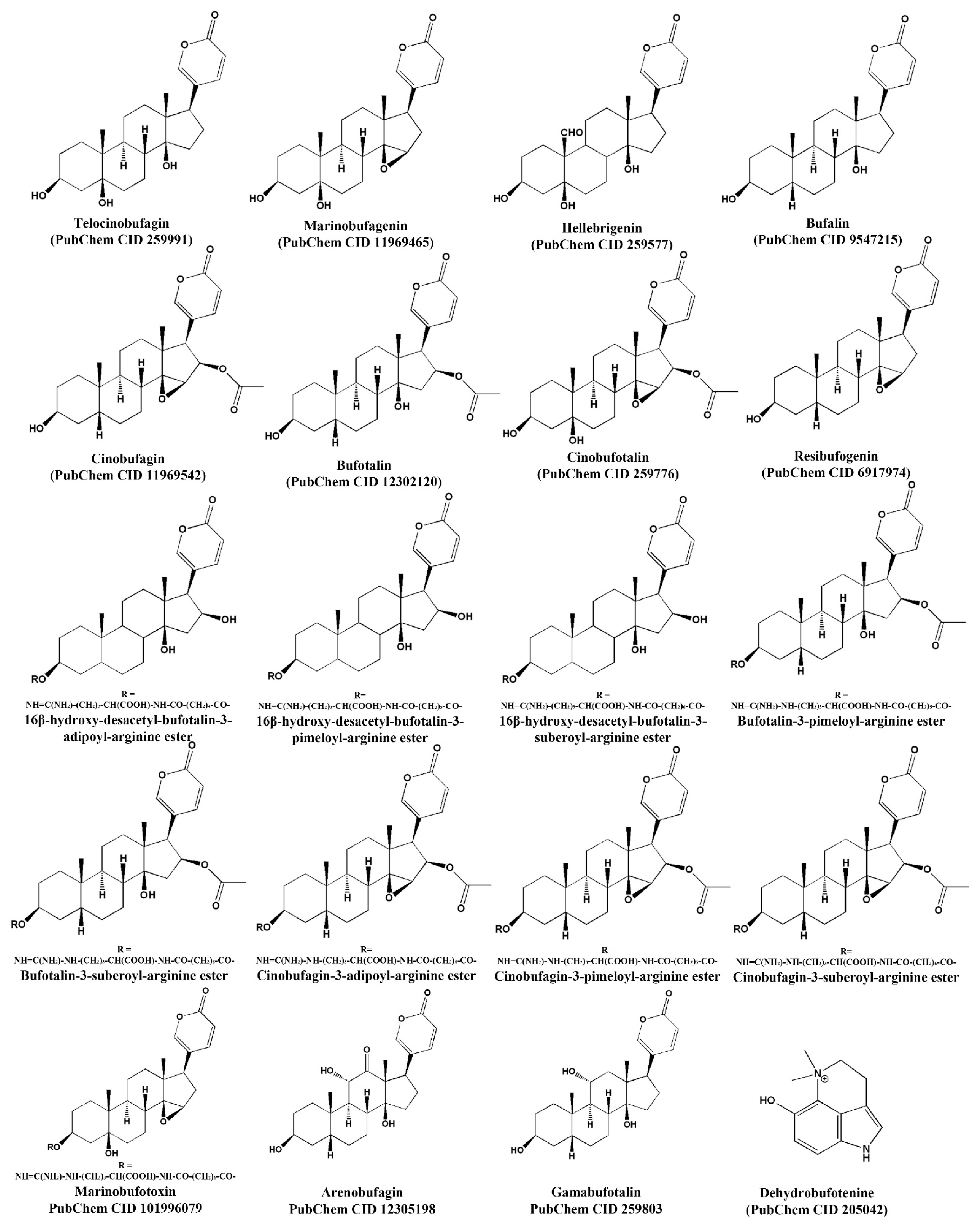
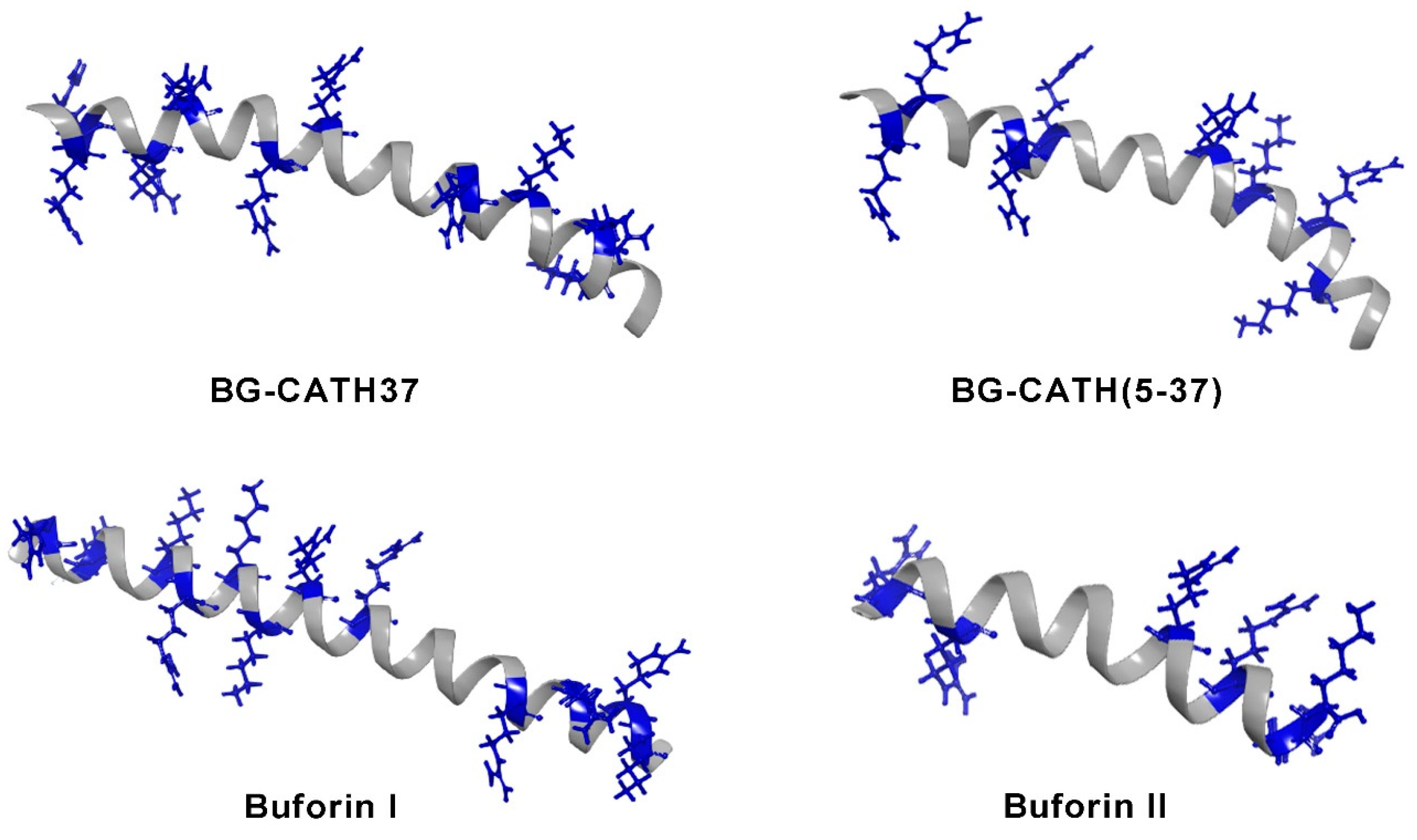
| Species | Component | Chem. Form. M. Weight (Theo./Exp.) | Activity Against | Ref. |
|---|---|---|---|---|
| Bufo rubescens | Telocinobufagin (bufadienolide) | 402.52 Da/402.1609 Da | E. coli ATCC 25922 (64 μg/mL), S. aureus ATCC 25213 (128 μg/mL). | [40] |
| Marinobufagin (bufadienolide) | 400.51 Da/400.1515 Da | E. coli ATCC 25922 (16 μg/mL), S. aureus ATCC 25213 (128 μg/mL). | [40] | |
| Bufo bufo gargarizans | BG-CATH37 (peptide) | Amino acid sequence: SSRRPCRGRSCGPRLRGGYTLIGRPVKNQNRPKYMWV 4299.25 Da | Streptococcus iniae (12.5 μg/mL), S. aureus (>200 μg/mL), Vibrio splendidus (6.25 μg/mL), Aeromonas hydrophila (40 μg/mL), P. aeruginosa (200 μg/mL), E. coli (>200 μg/mL). | [44] |
| BG-CATH(5-37) (peptide) | Amino acid sequence: PCRGRSCGPRLRGGYTLIGRPVKNQNRPKYMWV 3812.99 Da | S. iniae (12.5 μg/mL), S. aureus (>200 μg/mL), V. splendidus (3.125 μg/mL), Aeromonas hydrophila (12.5 μg/mL), P. aeruginosa (200 μg/mL), E. coli (>200 μg/mL). | [44] | |
| Buforin I (peptide) | Amino acid sequence: AGRGKQGGKVRAKAKTRSSRAGLQFPVGRVHRLLRKGNY 4363 Da/ MH+ 4309 Da | Bacillus subtilis (4 μg/mL), S. aureus (4 μg/mL), S. pneumoniae (4 μg/mL), Streptococcus mutans (8 μg/mL), S. typhimurium (4 μg/mL), P. putida (4 μg/mL), E. coli (8 μg/mL), Serratia spp. (8 μg/mL). | [45,47] | |
| Buforin II (peptide) | Amino acid sequence TRSSRAGLQFPVGRVHRLLRK 2434.9 Da/ MH+ 2432 Da | S. aureus (4 μg/mL), S. pneumoniae (4 μg/mL), B. subtilis (2 μg/mL), S. mutans (2 μg/mL), P. putida (2 μg/mL), E. coli (4 μg/mL), Serratia spp. (4 μg/mL), S. typhimurium (1 μg/mL). | [45] |
| Species | Molecule | Chem. Form. M. Weight | Activity Against | Ref. |
|---|---|---|---|---|
| Bufo arenarum | Dehydrobufotenine (alkaloid) | 203.26 Da | Alternaria solani (50 μg/mL). | [27] |
| Compound 6 (dehydrobufotenine analog) | 290.36 Da | Alternaria solani (50 μg/mL), Rhizoctonia solani (50 μg/mL), Botrytis cinereal (50 μg/mL), Cercospora arachidicola (50 μg/mL). | ||
| Compound 16c (dehydrobufotenine analog) | 424.45 Da | Alternaria solani (50 μg/mL), Rhizoctonia solani (50 μg/mL). | ||
| Compound 16d (dehydrobufotenine analog) | 454.48 Da | Sclerotinia sclerotiorum (50 μg/mL). Alternaria solani (50 μg/mL), Rhizoctonia solani (50 μg/mL), Botrytis cinereal (50 μg/mL), Cercospora arachidicola (50 μg/mL). | ||
| Compound 16h (dehydrobufotenine analog) | 459.29 Da | Sclerotinia sclerotiorum (50 μg/mL), Alternaria solani (50 μg/mL), Rhizoctonia solani (50 μg/mL), Botrytis cinereal (50 μg/mL), Fusarium graminearum (50 μg/mL). | ||
| Compound 16j (dehydrobufotenine analog) | 432.40 Da | Sclerotinia sclerotiorum (50 μg/mL), Alternaria solani (50 μg/mL), Botrytis cinereal (50 μg/mL), Cercospora arachidicola (50 μg/mL). | ||
| Compound 19 (dehydrobufotenine analog) | 293.38 Da | Alternaria solani (50 μg/mL), Cercospora arachidicola (50 μg/mL). | ||
| Anaxyrus boreas | Arenobufagin (bufadienolide) | M. weight (theo./Exp.) 416.51 Da/416.9 Da | B. dendrobatidis (12.9 μg/mL). | [7] |
| Gamabufotalin (bufadienolide) | M. weight (Theo./Exp.) 402.52 Da/403.4 Da | B. dendrobatidis (50 μg/mL). | ||
| Telocinobufagin (bufadienolide) | M. weight (Theo./Exp.) 402.52 Da/402.9 Da | B. dendrobatidis (14.3 μg/mL). |
| Species | Molecule | Chem. Form. M. Weight | Activity | Ref. |
|---|---|---|---|---|
| Bufo arenarum | Dehydrobufotenine (alkaloid) | 203.26 Da | At 500 μg/mL showed similar inactive, curative, and protective effects against TMV than the antiviral ribavirin. | [27] |
| Compound 12 (dehydrobufotenine analog) | 204.27 Da | At 500 μg/mL showed 5–7% higher inactivating, curative, and protective effects against TMV than the control ningnanmycin and 22–25% higher effects than ribavirin. | [27] | |
| Compound 17 (dehydrobufotenine analog) | 317.40 Da | At 500 μg/mL showed 5–6% higher inactivating, curative, and protective effects against TMV than the control ningnanmycin and 20–21% higher effects than the control ribavirin. | [27] | |
| Bufo rubescens | Telocinobufagin (bufadienolide) | 402.52 Da | IC50 0.027 μM (anti-MERS-CoV a), IC50 0.465 μM (anti-MERS-CoV b), IC50 0.071 μM (anti-SARS-CoV a), IC50 0.142 μM (anti-SARS-CoV2 a). | [16] |
| Bufo bufo gargarizans | Bufalin (bufadienolide) | 386.52 Da | IC50 0.018 μM (anti- MERS-CoV a), IC50 0.544 μM (anti-MERS-CoV b), IC50 0.017 μM (Anti-SARS-CoV a), IC50 0.019 μM (Anti-SARS-CoV2 a), IC90 15 nM (anti-HIV-1), 100% lethal activity at 10 mg/kg (in mice, IP). 20 nM inhibits the secretion of 14.42% of HBsAg and 30.95% of HBeAgc before three days of treatment, while at six days it inhibits the secretion of 11.36% of HBeAg c and 19.58% at 0.2 nM [16,17,18]. | [16,17,18] |
| Cinobufagin (bufadienolide) | 442.54 Da | IC50 0.017 μM (anti- MERS-CoV a), IC50 0.616 μM (anti-MERS-CoV b), IC50 0.061 μM (Anti-SARS-CoV a), IC50 0.072 μM (Anti-SARS-CoV2 a), IC50 10.94 nM (anti-enterovirus 71), IC90 40 nM (anti-HIV-1), 100% lethal activity at 10 mg/kg/day in a 2-day repeated dosage (in mice, IP). 1 nM inhibits the secretion of 8.28% of HBeAg c and 3.01% of HBcrAg d before three days of treatment, while at six days it inhibits the secretion of 7.01% of HBeAg c and 7.16% of HBcrAg d. | [14,16,17,18] | |
| Bufotalin (bufadienolide) | 444.56 Da | IC50 0.063 μM (anti- MERS-CoV a), IC50 1.630 μM (anti-MERS-CoV b), IC50 0.027 μM (Anti-SARS-CoV a), IC50 0.073 μM (Anti-SARS-CoV2 a). | [16] | |
| Cinobufotalin (bufadienolide) | 458.54 Da | IC50 0.231 μM (anti- MERS-CoV a), IC50 3.958 μM (anti-MERS-CoV b), IC50 0.429 μM (Anti-SARS-CoV a), IC50 0.399 μM (Anti-SARS-CoV2 a), 100% lethal activity in rats at 1 mg/kg (IV). | [16] | |
| Resibufogenin (bufadienolide) | 384.51 Da | IC50 1.612 μM (anti- MERS-CoV a), IC50 15.97 μM (anti-MERS-CoV b), IC50 1.364 μM (Anti-SARS-CoV a), IC50 1.606 μM (Anti-SARS-CoV2 a), IC50 218 nM (anti-enterovirus 71). | [14,16] |
| Species | Molecule | Chem. Form.M. Weight | Activity | Ref. |
|---|---|---|---|---|
| Rhinella jimi | Telocinobufagin (bufadienolide) | 402.52 Da | IC50 61.2 μg/mL (L. chagasi promastigotes). 150 μg/mL reduced 37.0% of amastigotes in macrophages. At 200 μg/mL, cytotoxicity was not observed. | [28] |
| Hellebrigenin (bufadienolide) | 416.51 Da | IC50 126.2 μg/mL (L. chagasi promastigotes). 150 μg/mL reduced 21.5% of amastigotes in macrophages. IC50 91.75 μg/mL (T. cruzi trypomastigotes) At 200 μg/mL, cytotoxicity was not observed. | ||
| Rhinella alata | Bufotalin (bufadienolide) | 444.57 Da | IC50 8.713 μg/mL (T. cruzi trypomastigotes), CC50 20.46 μg/mL, Vero cells. | [11] |
| 16β-hydroxy-desacetyl-bufotalin-3-pimeloyl-arginine ester (bufadienolide) | 700.4 Da | IC50 6.093 μg/mL (T. cruzi trypomastigotes), CC50 0.168 μg/mL, Vero cells. | ||
| Bufotalin-3-pimeloyl-arginine ester (bufadienolide) | 742.91 Da | IC50 3.41 μg/mL (T. cruzi trypomastigotes), CC50 0.126 μg/mL, Vero cells. | ||
| 16β-hydroxy-desacetyl-bufotalin-3-suberoyl-arginine ester (bufadienolide) | 714.9 Da | IC50 4.932 μg/mL (T. cruzi trypomastigotes), CC50 0.579 μg/mL, Vero cells. | ||
| Bufotalin-3-suberoyl-arginine ester (bufadienolide) | 756.94 Da C40H60N4O10 | IC50 3.079 μg/mL (T. cruzi trypomastigotes), CC50 0.267 μg/mL, Vero cells. | ||
| Cinobufagin-3-adipoyl-arginine ester (bufadienolide) | 726.87 Da | IC50 12.138 μg/mL (T. cruzi trypomastigotes), CC50 2.616 μg/mL, Vero cells. | ||
| Cinobufagin-3-pimeloyl-arginine ester (bufadienolide) | 740.9 Da | IC50 10.891 μg/mL (T. cruzi trypomastigotes), CC50 0.533 μg/mL, Vero cells. | ||
| Cinobufagin-3-suberoyl-arginine ester (bufadienolide) | 754.92 Da | IC50 14.343 μg/mL (T. cruzi trypomastigotes), CC50 0.188 μg/mL, Vero cells. | ||
| Cinobufagin (bufadienolide) | 442.55 Da C26H34O6 | IC50 12.833 μg/mL (T. cruzi trypomastigotes), CC50 23.897 μg/mL, Vero cells. | ||
| Rhinella marina | Marinobufotoxin (bufadienolide) | 712.89 Da | IC50 3.778 μg/mL (P. falciparum W2), LD50 6.337 μg/mL, WI-26VA4 cells. | [69] |
| Bufalin (bufadienolide) | 386.52 Da | IC50 1.329 μg/mL (P. falciparum W2), LD50 3.436 μg/mL, WI-26VA4 cells. | ||
| Dehydrobufotenine (Alkaloid) | 203.26 Da | IC50 3.884 μg/mL (P. falciparum W2), LD50 47.92 μg/mL, WI-26VA4 cells. | ||
| Marinobufagin (bufadienolide) | 400.51 Da | IC50 1.557 μg/mL (P. falciparum W2), LD50 1.217 μg/mL, WI-26VA4 cells. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibarra-Vega, R.; Galván-Hernández, A.R.; Salazar-Monge, H.; Zataraín-Palacios, R.; García-Villalvazo, P.E.; Zavalza-Galvez, D.I.; Valdez-Velazquez, L.L.; Jiménez-Vargas, J.M. Antimicrobial Compounds from Skin Secretions of Species That Belong to the Bufonidae Family. Toxins 2023, 15, 145. https://doi.org/10.3390/toxins15020145
Ibarra-Vega R, Galván-Hernández AR, Salazar-Monge H, Zataraín-Palacios R, García-Villalvazo PE, Zavalza-Galvez DI, Valdez-Velazquez LL, Jiménez-Vargas JM. Antimicrobial Compounds from Skin Secretions of Species That Belong to the Bufonidae Family. Toxins. 2023; 15(2):145. https://doi.org/10.3390/toxins15020145
Chicago/Turabian StyleIbarra-Vega, Rodrigo, Alan Roberto Galván-Hernández, Hermenegildo Salazar-Monge, Rocio Zataraín-Palacios, Patricia Elizabeth García-Villalvazo, Diana Itzel Zavalza-Galvez, Laura Leticia Valdez-Velazquez, and Juana María Jiménez-Vargas. 2023. "Antimicrobial Compounds from Skin Secretions of Species That Belong to the Bufonidae Family" Toxins 15, no. 2: 145. https://doi.org/10.3390/toxins15020145
APA StyleIbarra-Vega, R., Galván-Hernández, A. R., Salazar-Monge, H., Zataraín-Palacios, R., García-Villalvazo, P. E., Zavalza-Galvez, D. I., Valdez-Velazquez, L. L., & Jiménez-Vargas, J. M. (2023). Antimicrobial Compounds from Skin Secretions of Species That Belong to the Bufonidae Family. Toxins, 15(2), 145. https://doi.org/10.3390/toxins15020145





