Effect of Hydrogen Sulfide on Essential Functions of Polymorphonuclear Leukocytes
Abstract
1. Introduction
2. Results
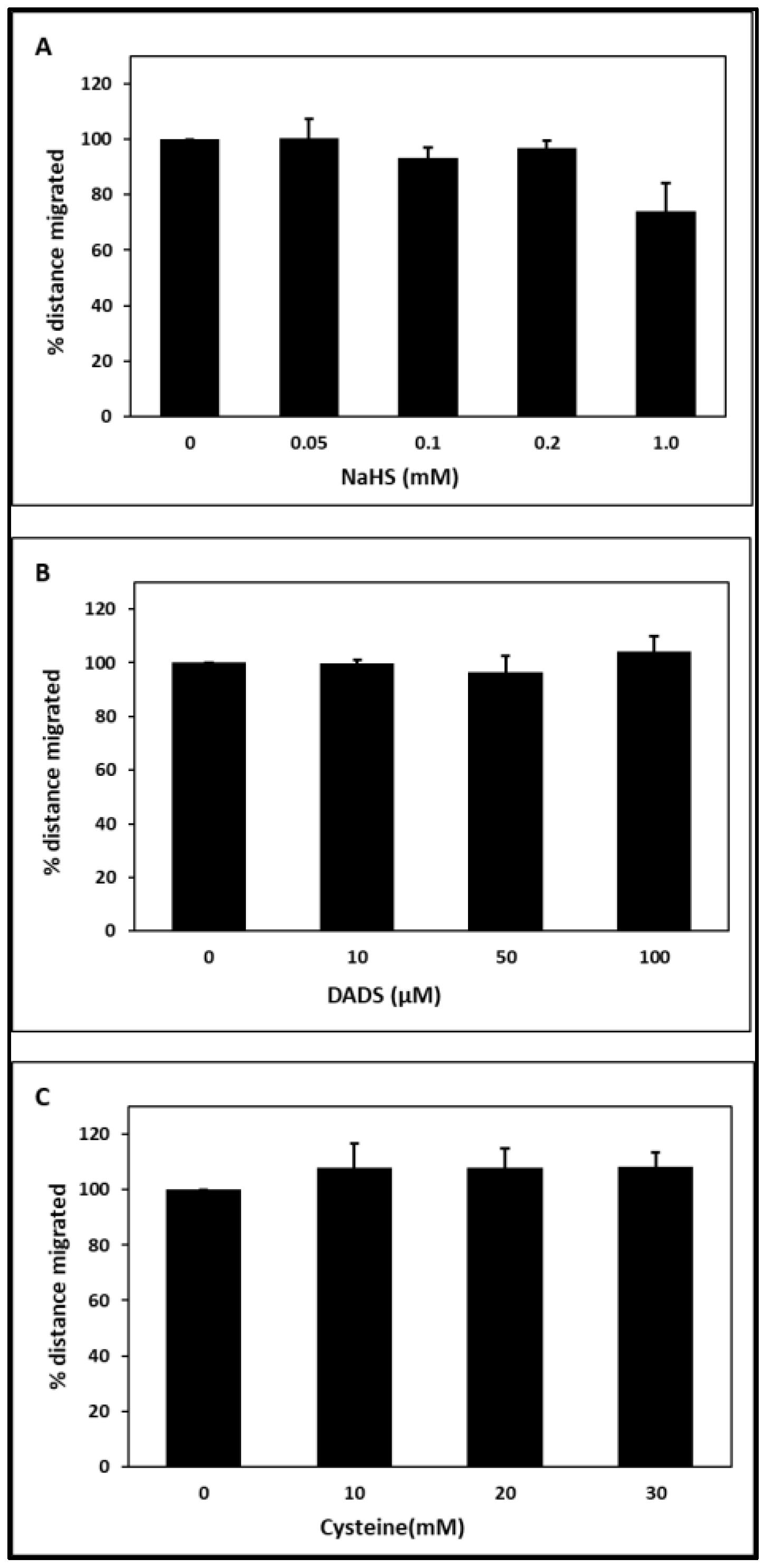
2.1. Chemotaxis
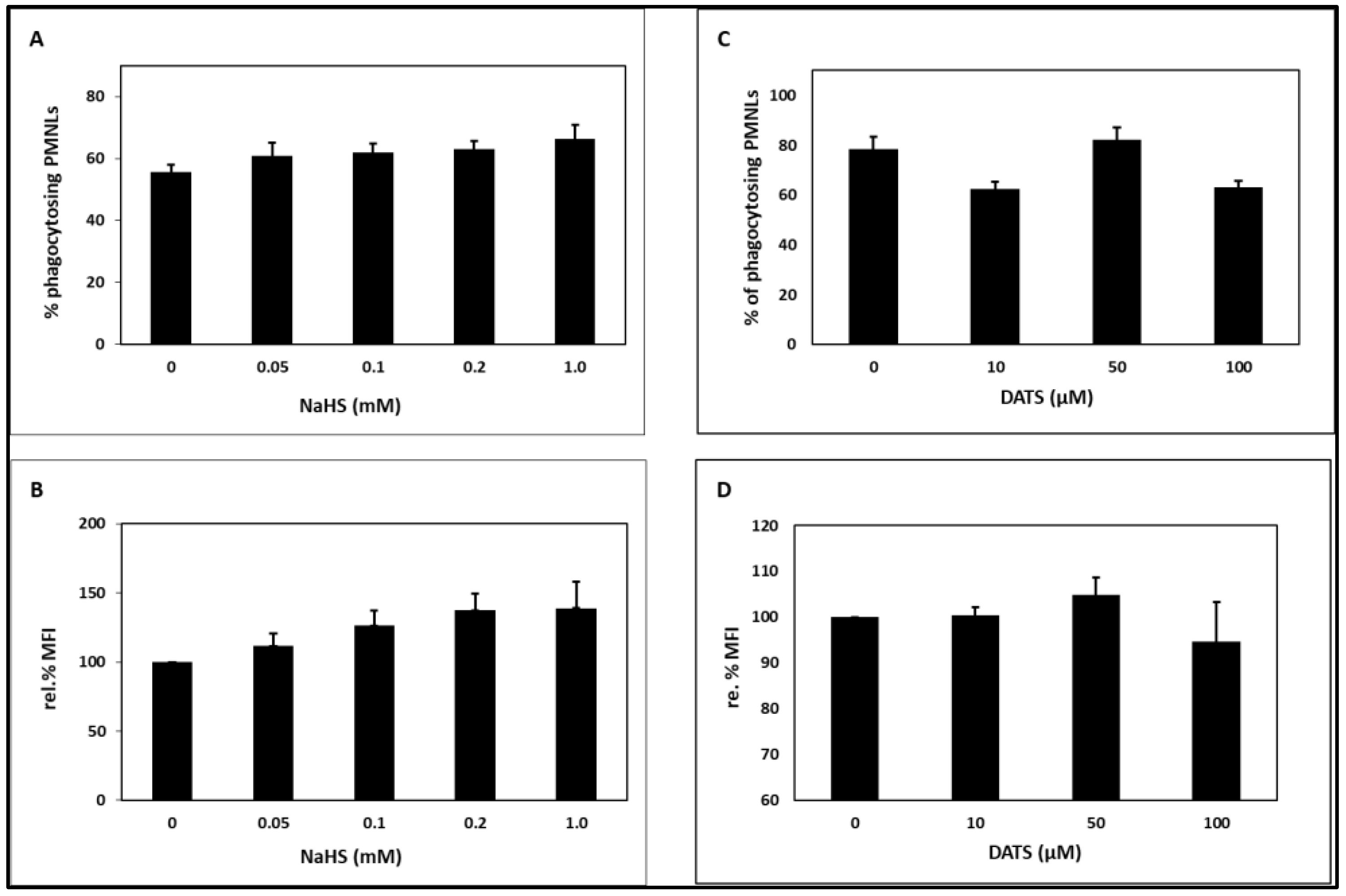
2.2. Phagocytosis
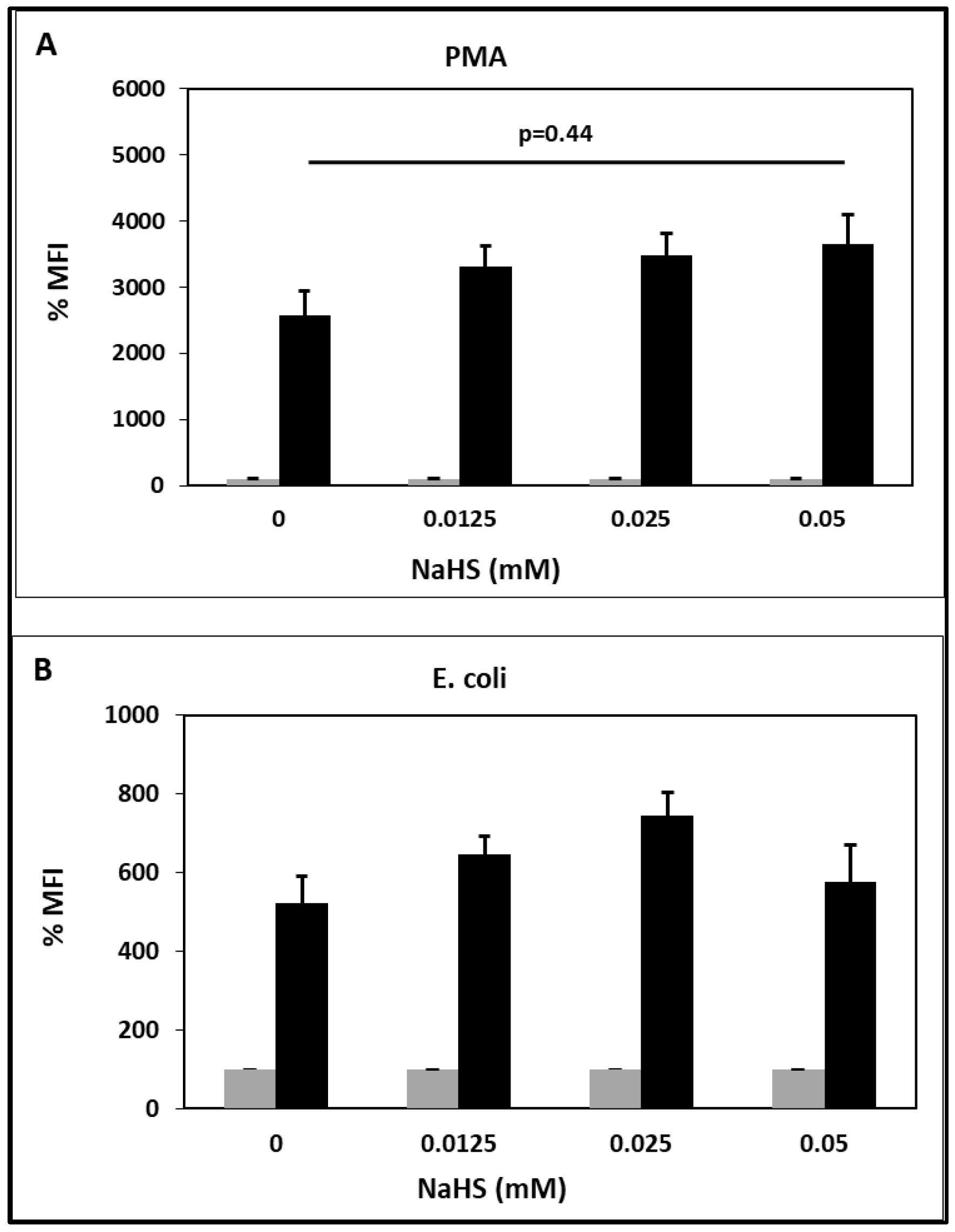
2.3. Oxidative Burst
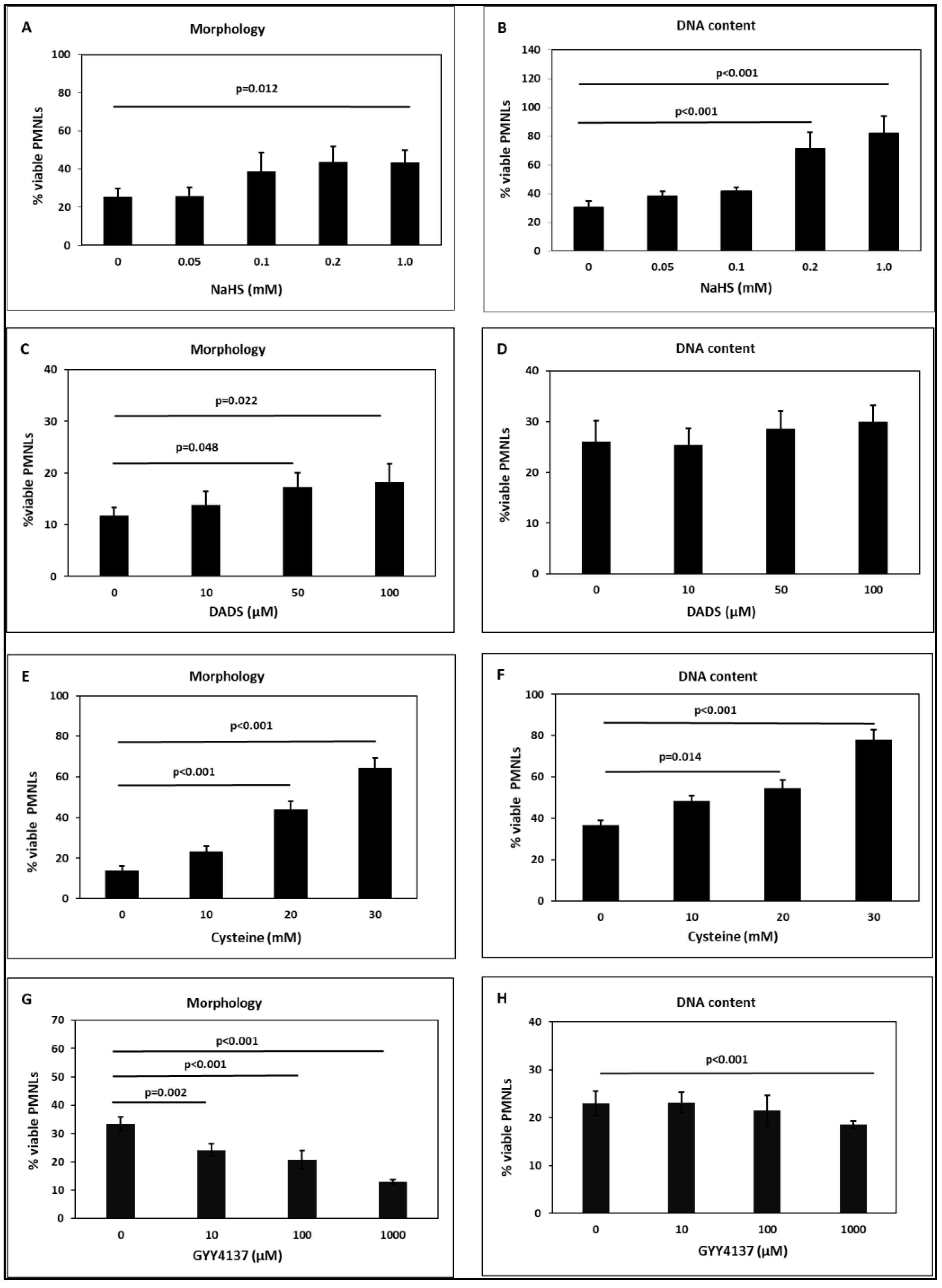
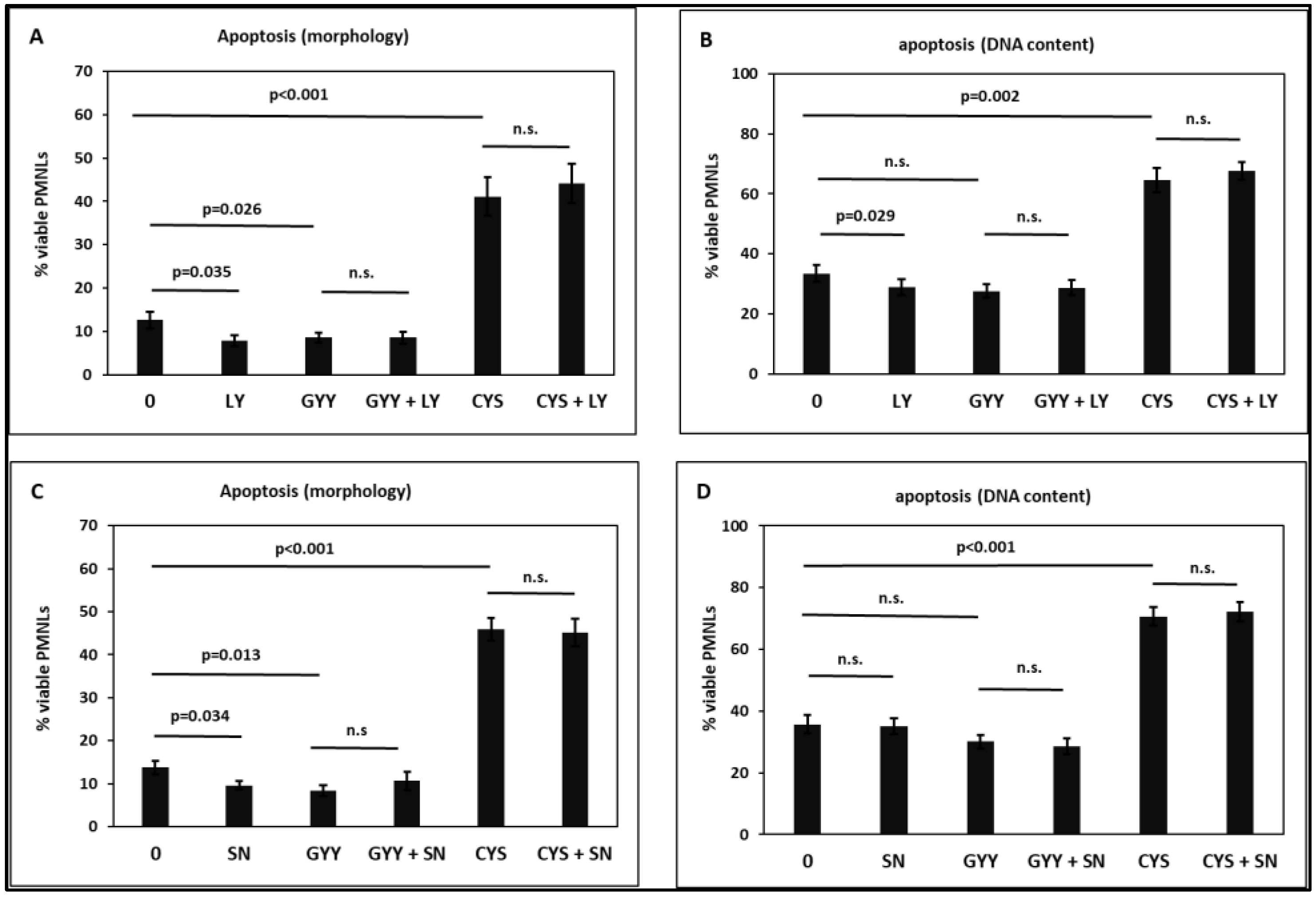
2.4. Apoptosis
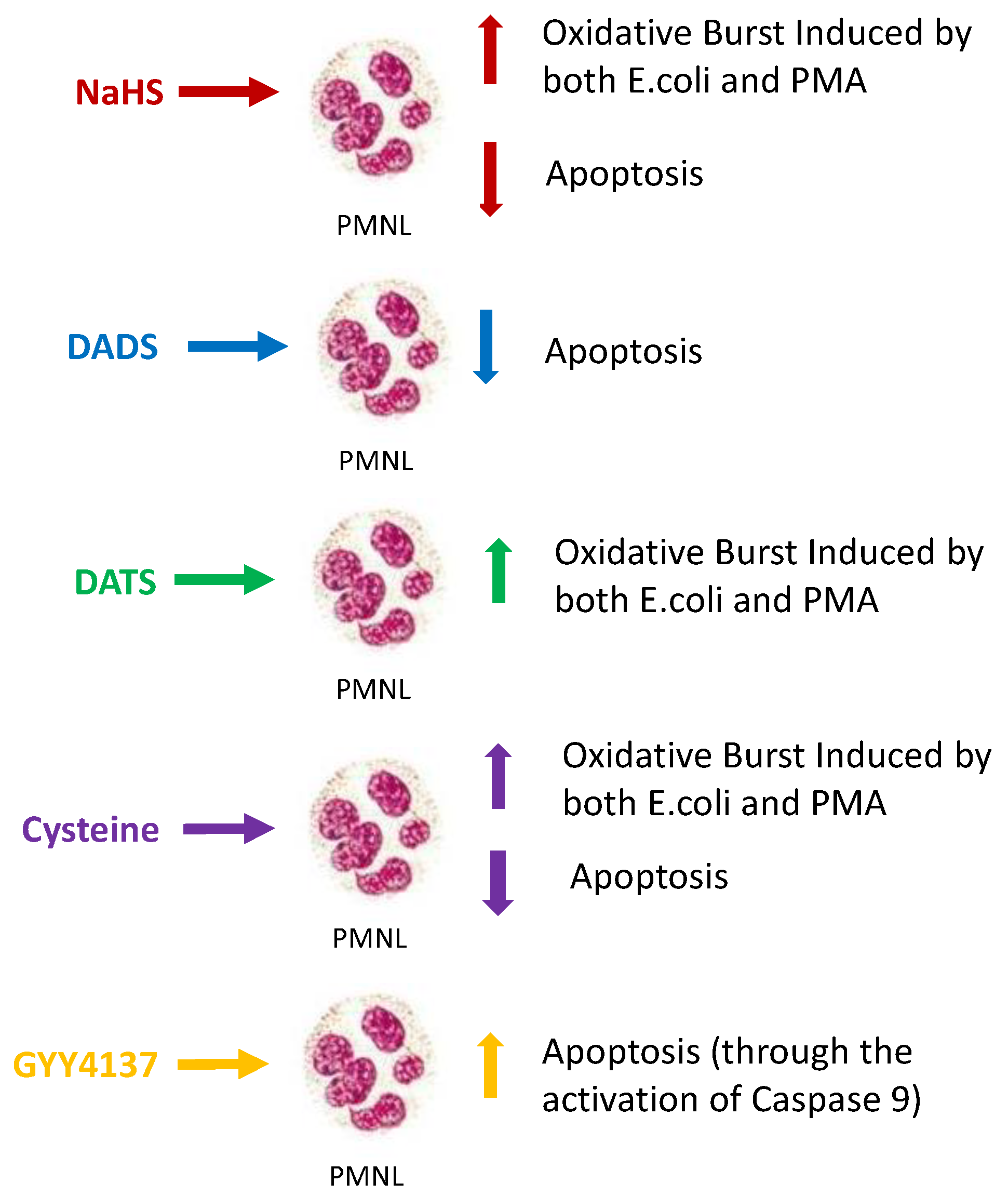
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Healthy Subjects
5.2. Material
5.3. Isolation of PMNLs
5.4. Chemotaxis
5.5. Phagocytosis
5.6. Oxidative Burst
5.7. Apoptosis
5.7.1. Incubations
5.7.2. Morphological Features
5.7.3. Analysis of the DNA Content by Flow Cytometry
5.7.4. Data Presentation
5.8. H2S-Releasing Substances
5.9. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, X.; Bian, J.S. The Role of Hydrogen Sulfide in Renal System. Front. Pharmacol. 2016, 7, 385. [Google Scholar] [CrossRef] [PubMed]
- Aminzadeh, M.A.; Vaziri, N.D. Downregulation of the renal and hepatic hydrogen sulfide (H2S)-producing enzymes and capacity in chronic kidney disease. Nephrol. Dial. Transplant. 2012, 27, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Feliers, D.; Lee, H.J.; Kasinath, B.S. Hydrogen Sulfide in Renal Physiology and Disease. Antioxid. Redox Signal. 2016, 25, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Ferraz, J.G.; Muscara, M.N. Hydrogen sulfide: An endogenous mediator of resolution of inflammation and injury. Antioxid. Redox Signal. 2012, 17, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; Lanza, D.; Sepe, I.; Raiola, I.; Capasso, R.; De Santo, N.G.; Ingrosso, D. Hydrogen sulfide, a toxic gas with cardiovascular properties in uremia: How harmful is it? Blood Purif. 2011, 31, 102–106. [Google Scholar] [CrossRef]
- Huerta de la Cruz, S.; Medina-Terol, G.J.; Tapia-Martinez, J.A.; Silva-Velasco, D.L.; Beltran-Ornelas, J.H.; Sanchez-Lopez, A.; Sancho, M.; Centurion, D. Hydrogen sulfide as a neuromodulator of the vascular tone. Eur. J. Pharmacol. 2023, 940, 175455. [Google Scholar] [CrossRef]
- Lin, Q.; Geng, B. The Role of Hydrogen Sulfide in Plaque Stability. Antioxidants 2022, 11, 2356. [Google Scholar] [CrossRef]
- Kimura, Y.; Goto, Y.; Kimura, H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid. Redox Signal. 2010, 12, 1–13. [Google Scholar] [CrossRef]
- Kimura, Y.; Kimura, H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004, 18, 1165–1167. [Google Scholar] [CrossRef]
- Askari, H.; Seifi, B.; Kadkhodaee, M.; Sanadgol, N.; Elshiekh, M.; Ranjbaran, M.; Ahghari, P. Protective effects of hydrogen sulfide on chronic kidney disease by reducing oxidative stress, inflammation and apoptosis. EXCLI J. 2018, 17, 14–23. [Google Scholar]
- Vanholder, R.; Argiles, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; Descamps-Latscha, B.; Henle, T.; et al. Uremic toxicity: Present state of the art. Int. J. Artif. Organs 2001, 24, 695–725. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G. Immune Dysfunction in Uremia 2020. Toxins 2020, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; Di Nunzio, A.; Amoresano, A.; Pane, F.; Fontanarosa, C.; Pucci, P.; Vigorito, C.; Cirillo, G.; Zacchia, M.; Trepiccione, F.; et al. Divergent behavior of hydrogen sulfide pools and of the sulfur metabolite lanthionine, a novel uremic toxin, in dialysis patients. Biochimie 2016, 126, 97–107. [Google Scholar] [CrossRef]
- Perna, A.F.; Zacchia, M.; Trepiccione, F.; Ingrosso, D. The Sulfur Metabolite Lanthionine: Evidence for a Role as a Novel Uremic Toxin. Toxins 2017, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.L.; Liao, C.H.; Wu, W.B.; Zheng, C.M.; Lu, K.C.; Ma, M.C. Uremic Toxin Indoxyl Sulfate Impairs Hydrogen Sulfide Formation in Renal Tubular Cells. Antioxidants 2022, 11, 361. [Google Scholar] [CrossRef] [PubMed]
- Kraus, L.M.; Kraus, A.P., Jr. Carbamoylation of amino acids and proteins in uremia. Kidney Int. Suppl. 2001, 78, S102–S107. [Google Scholar] [CrossRef]
- Basnakian, A.G.; Shah, S.V.; Ok, E.; Altunel, E.; Apostolov, E.O. Carbamylated LDL. Adv. Clin. Chem. 2010, 51, 25–52. [Google Scholar]
- Jaisson, S.; Pietrement, C.; Gillery, P. Carbamylation-derived products: Bioactive compounds and potential biomarkers in chronic renal failure and atherosclerosis. Clin. Chem. 2011, 57, 1499–1505. [Google Scholar] [CrossRef]
- Praschberger, M.; Hermann, M.; Laggner, C.; Jirovetz, L.; Exner, M.; Kapiotis, S.; Gmeiner, B.M.; Laggner, H. Carbamoylation abrogates the antioxidant potential of hydrogen sulfide. Biochimie 2013, 95, 2069–2075. [Google Scholar] [CrossRef]
- Bratton, D.L.; Henson, P.M. Neutrophil clearance: When the party is over, clean-up begins. Trends Immunol. 2011, 32, 350–357. [Google Scholar] [CrossRef]
- Filep, J.G.; El Kebir, D. Neutrophil apoptosis: A target for enhancing the resolution of inflammation. J. Cell. Biochem. 2009, 108, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Kristensson, M.; Poumlrn-Ares, M.I.; Grethe, S.; Smith, D.; Zheng, L.; Andersson, T. Fas-Induced Apoptosis of Human Neutrophils is Promoted by Phosphatidyl Inositol 3-Kinase but Suppressed by P38-MAPK Activity. Sci. World J. 2001, 1, 116. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Kristensson, M.; Porn-Ares, M.I.; Grethe, S.; Smith, D.; Zheng, L.; Andersson, T. p38 Mitogen-activated protein kinase and phosphatidylinositol 3-kinase activities have opposite effects on human neutrophil apoptosis. FASEB J. 2002, 16, 129–131. [Google Scholar] [CrossRef]
- Alvarado-Kristensson, M.; Melander, F.; Leandersson, K.; Ronnstrand, L.; Wernstedt, C.; Andersson, T. p38-MAPK signals survival by phosphorylation of caspase-8 and caspase-3 in human neutrophils. J. Exp. Med. 2004, 199, 449–458. [Google Scholar] [CrossRef]
- Heuertz, R.M.; Tricomi, S.M.; Ezekiel, U.R.; Webster, R.O. C-reactive protein inhibits chemotactic peptide-induced p38 mitogen-activated protein kinase activity and human neutrophil movement. J. Biol. Chem. 1999, 274, 17968–17974. [Google Scholar] [CrossRef]
- Hsu, M.J.; Lee, S.S.; Lee, S.T.; Lin, W.W. Signaling mechanisms of enhanced neutrophil phagocytosis and chemotaxis by the polysaccharide purified from Ganoderma lucidum. Br. J. Pharmacol. 2003, 139, 289–298. [Google Scholar] [CrossRef]
- Krump, E.; Sanghera, J.S.; Pelech, S.L.; Furuya, W.; Grinstein, S. Chemotactic peptide N-formyl-met-leu-phe activation of p38 mitogen-activated protein kinase (MAPK) and MAPK-activated protein kinase-2 in human neutrophils. J. Biol. Chem. 1997, 272, 937–944. [Google Scholar] [CrossRef]
- Yang, K.Y.; Arcaroli, J.; Kupfner, J.; Pitts, T.M.; Park, J.S.; Strasshiem, D.; Perng, R.P.; Abraham, E. Involvement of phosphatidylinositol 3-kinase gamma in neutrophil apoptosis. Cell. Signal. 2003, 15, 225–233. [Google Scholar] [CrossRef]
- Wang, R. The gasotransmitter role of hydrogen sulfide. Antioxid. Redox Signal. 2003, 5, 493–501. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, Q.; Li, X.; Wei, R.; Ge, T.; Zheng, X.; Li, B.; Liu, K.; Cui, R. Hydrogen sulfide: A new therapeutic target in vascular diseases. Front. Endocrinol. 2022, 13, 934231. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Yang, J.; Qi, Y.; Zhao, J.; Pang, Y.; Du, J.; Tang, C. H2S generated by heart in rat and its effects on cardiac function. Biochem. Biophys. Res. Commun. 2004, 313, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Tripatara, P.; Patel, N.S.; Collino, M.; Gallicchio, M.; Kieswich, J.; Castiglia, S.; Benetti, E.; Stewart, K.N.; Brown, P.A.; Yaqoob, M.M.; et al. Generation of endogenous hydrogen sulfide by cystathionine gamma-lyase limits renal ischemia/reperfusion injury and dysfunction. Lab. Investig. 2008, 88, 1038–1048. [Google Scholar] [CrossRef]
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Liu, X.; Geng, B.; Fang, L.; Tang, C. Hydrogen sulfide protects rat lung from ischemia-reperfusion injury. Life Sci. 2008, 82, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ahmad, S.; Cai, M.; Rennie, J.; Fujisawa, T.; Crispi, F.; Baily, J.; Miller, M.R.; Cudmore, M.; Hadoke, P.W.; et al. Dysregulation of hydrogen sulfide producing enzyme cystathionine gamma-lyase contributes to maternal hypertension and placental abnormalities in preeclampsia. Circulation 2013, 127, 2514–2522. [Google Scholar] [CrossRef]
- Distrutti, E.; Sediari, L.; Mencarelli, A.; Renga, B.; Orlandi, S.; Antonelli, E.; Roviezzo, F.; Morelli, A.; Cirino, G.; Wallace, J.L.; et al. Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. J. Pharmacol. Exp. Ther. 2006, 316, 325–335. [Google Scholar] [CrossRef]
- Greabu, M.; Totan, A.; Miricescu, D.; Radulescu, R.; Virlan, J.; Calenic, B. Hydrogen Sulfide, Oxidative Stress and Periodontal Diseases: A Concise Review. Antioxidants 2016, 5, 3. [Google Scholar] [CrossRef]
- Lu, M.; Liu, Y.H.; Goh, H.S.; Wang, J.J.; Yong, Q.C.; Wang, R.; Bian, J.S. Hydrogen sulfide inhibits plasma renin activity. J. Am. Soc. Nephrol. 2010, 21, 993–1002. [Google Scholar] [CrossRef]
- Banerjee, R.; Zou, C.G. Redox regulation and reaction mechanism of human cystathionine-beta-synthase: A PLP-dependent hemesensor protein. Arch. Biochem. Biophys. 2005, 433, 144–156. [Google Scholar] [CrossRef]
- Perna, A.F.; Ingrosso, D. Low hydrogen sulphide and chronic kidney disease: A dangerous liaison. Nephrol. Dial. Transplant. 2012, 27, 486–493. [Google Scholar] [CrossRef]
- Vigorito, C.; Anishchenko, E.; Mele, L.; Capolongo, G.; Trepiccione, F.; Zacchia, M.; Lombari, P.; Capasso, R.; Ingrosso, D.; Perna, A.F. Uremic Toxin Lanthionine Interferes with the Transsulfuration Pathway, Angiogenetic Signaling and Increases Intracellular Calcium. Int. J. Mol. Sci. 2019, 20, 2269. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhang, Z.; Huang, Z.; Duan, X.; Wang, Y.; Cao, J.; Li, L.; He, K.; Nice, E.C.; He, W.; et al. Protein persulfidation: Rewiring the hydrogen sulfide signaling in cell stress response. Biochem. Pharmacol. 2023, 209, 115444. [Google Scholar] [CrossRef]
- Gadalla, M.M.; Snyder, S.H. Hydrogen sulfide as a gasotransmitter. J. Neurochem. 2010, 113, 14–26. [Google Scholar] [CrossRef]
- Lo Faro, M.L.; Fox, B.; Whatmore, J.L.; Winyard, P.G.; Whiteman, M. Hydrogen sulfide and nitric oxide interactions in inflammation. Nitric Oxide 2014, 41, 38–47. [Google Scholar] [CrossRef]
- Bhatia, M. Role of hydrogen sulfide in the pathology of inflammation. Scientifica 2012, 2012, 159680. [Google Scholar] [CrossRef] [PubMed]
- Collin, M.; Anuar, F.B.; Murch, O.; Bhatia, M.; Moore, P.K.; Thiemermann, C. Inhibition of endogenous hydrogen sulfide formation reduces the organ injury caused by endotoxemia. Br. J. Pharmacol. 2005, 146, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Bhatia, M.; Zhu, Y.Z.; Zhu, Y.C.; Ramnath, R.D.; Wang, Z.J.; Anuar, F.B.; Whiteman, M.; Salto-Tellez, M.; Moore, P.K. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005, 19, 1196–1198. [Google Scholar] [CrossRef]
- Mok, Y.Y.; Atan, M.S.; Yoke Ping, C.; Zhong Jing, W.; Bhatia, M.; Moochhala, S.; Moore, P.K. Role of hydrogen sulphide in haemorrhagic shock in the rat: Protective effect of inhibitors of hydrogen sulphide biosynthesis. Br. J. Pharmacol. 2004, 143, 881–889. [Google Scholar] [CrossRef]
- Zhang, H.; Zhi, L.; Moochhala, S.; Moore, P.K.; Bhatia, M. Hydrogen sulfide acts as an inflammatory mediator in cecal ligation and puncture-induced sepsis in mice by upregulating the production of cytokines and chemokines via NF-kappaB. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2007, 292, L960–L971. [Google Scholar] [CrossRef]
- Gong, Q.H.; Wang, Q.A.; Pan, L.L.; Liu, X.H.; Xin, H.; Zhu, Y.Z. S-Propargyl-cysteine, a novel hydrogen sulfide-modulated agent, attenuates lipopolysaccharide-induced spatial learning and memory impairment: Involvement of TNF signaling and NF-kappa B pathway in rats. Brain Behav. Immun. 2011, 25, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, M.; Li, L.; Rose, P.; Tan, C.H.; Parkinson, D.B.; Moore, P.K. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid. Redox Signal. 2010, 12, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Zanardo, R.C.; Brancaleone, V.; Distrutti, E.; Fiorucci, S.; Cirino, G.; Wallace, J.L. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006, 20, 2118–2120. [Google Scholar] [CrossRef]
- Perna, A.F.; Sepe, I.; Lanza, D.; Capasso, R.; Zappavigna, S.; Capasso, G.; Caraglia, M.; Ingrosso, D. Hydrogen sulfide reduces cell adhesion and relevant inflammatory triggering by preventing ADAM17-dependent TNF-alpha activation. J. Cell. Biochem. 2013, 114, 1536–1548. [Google Scholar] [CrossRef]
- Whiteman, M.; Gooding, K.M.; Whatmore, J.L.; Ball, C.I.; Mawson, D.; Skinner, K.; Tooke, J.E.; Shore, A.C. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia 2010, 53, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; Luciano, M.G.; Ingrosso, D.; Pulzella, P.; Sepe, I.; Lanza, D.; Violetti, E.; Capasso, R.; Lombardi, C.; De Santo, N.G. Hydrogen sulphide-generating pathways in haemodialysis patients: A study on relevant metabolites and transcriptional regulation of genes encoding for key enzymes. Nephrol. Dial. Transplant. 2009, 24, 3756–3763. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, S.; Dong, S.; Wu, J.; Song, M.; Zhong, X.; Liu, Y. Sodium hydrosulfide attenuates hyperhomocysteinemia rat myocardial injury through cardiac mitochondrial protection. Mol. Cell. Biochem. 2015, 399, 189–200. [Google Scholar] [CrossRef]
- Xia, M.; Chen, L.; Muh, R.W.; Li, P.L.; Li, N. Production and actions of hydrogen sulfide, a novel gaseous bioactive substance, in the kidneys. J. Pharmacol. Exp. Ther. 2009, 329, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef]
- Perna, A.F.; Anishchenko, E.; Vigorito, C.; Zacchia, M.; Trepiccione, F.; D’Aniello, S.; Ingrosso, D. Zebrafish, a Novel Model System to Study Uremic Toxins: The Case for the Sulfur Amino Acid Lanthionine. Int. J. Mol. Sci. 2018, 19, 1323. [Google Scholar] [CrossRef]
- Furne, J.; Saeed, A.; Levitt, M.D. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 295, R1479–R1485. [Google Scholar] [CrossRef]
- Ishigami, M.; Hiraki, K.; Umemura, K.; Ogasawara, Y.; Ishii, K.; Kimura, H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid. Redox Signal. 2009, 11, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.; Folke, L.E. Hydrogen sulfide production in the periodontal environment. J. Periodontol. 1973, 44, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.; Testai, L.; Citi, V.; Marino, A.; Pugliesi, I.; Barresi, E.; Nesi, G.; Rapposelli, S.; Taliani, S.; Da Settimo, F.; et al. Arylthioamides as H2S Donors: L-Cysteine-Activated Releasing Properties and Vascular Effects in Vitro and in Vivo. ACS Med. Chem. Lett. 2013, 4, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Corvino, A.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Scognamiglio, A.; Caliendo, G.; Fiorino, F.; Severino, B. Trends in H2S-Donors Chemistry and Their Effects in Cardiovascular Diseases. Antioxidants 2021, 10, 429. [Google Scholar] [CrossRef]
- Rose, P.; Dymock, B.W.; Moore, P.K. GYY4137, a novel water-soluble, H2S-releasing molecule. Methods Enzymol. 2015, 554, 143–167. [Google Scholar]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.H.; Moore, P.K. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): New insights into the biology of hydrogen sulfide. Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef]
- Liang, D.; Wu, H.; Wong, M.W.; Huang, D. Diallyl Trisulfide Is a Fast H2S Donor, but Diallyl Disulfide Is a Slow One: The Reaction Pathways and Intermediates of Glutathione with Polysulfides. Org. Lett. 2015, 17, 4196–4199. [Google Scholar] [CrossRef]
- Szabo, C.; Papapetropoulos, A. International Union of Basic and Clinical Pharmacology. CII: Pharmacological Modulation of H2S Levels: H2S Donors and H2S Biosynthesis Inhibitors. Pharmacol. Rev. 2017, 69, 497–564. [Google Scholar] [CrossRef]
- Spiller, F.; Orrico, M.I.; Nascimento, D.C.; Czaikoski, P.G.; Souto, F.O.; Alves-Filho, J.C.; Freitas, A.; Carlos, D.; Montenegro, M.F.; Neto, A.F.; et al. Hydrogen sulfide improves neutrophil migration and survival in sepsis via K+ATP channel activation. Am. J. Respir. Crit. Care Med. 2010, 182, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Dal-Secco, D.; Cunha, T.M.; Freitas, A.; Alves-Filho, J.C.; Souto, F.O.; Fukada, S.Y.; Grespan, R.; Alencar, N.M.; Neto, A.F.; Rossi, M.A.; et al. Hydrogen sulfide augments neutrophil migration through enhancement of adhesion molecule expression and prevention of CXCR2 internalization: Role of ATP-sensitive potassium channels. J. Immunol. 2008, 181, 4287–4298. [Google Scholar] [CrossRef] [PubMed]
- Claesson, R.; Granlund-Edstedt, M.; Persson, S.; Carlsson, J. Activity of polymorphonuclear leukocytes in the presence of sulfide. Infect. Immun. 1989, 57, 2776–2781. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.D.; Rohn, T.T.; Quinn, M.T. Neutrophil priming in host defense: Role of oxidants as priming agents. Antioxid. Redox Signal. 2002, 4, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Sela, S.; Shurtz-Swirski, R.; Cohen-Mazor, M.; Mazor, R.; Chezar, J.; Shapiro, G.; Hassan, K.; Shkolnik, G.; Geron, R.; Kristal, B. Primed peripheral polymorphonuclear leukocyte: A culprit underlying chronic low-grade inflammation and systemic oxidative stress in chronic kidney disease. J. Am. Soc. Nephrol. 2005, 16, 2431–2438. [Google Scholar] [CrossRef]
- Feng, C.; Luo, Y.; Nian, Y.; Liu, D.; Yin, X.; Wu, J.; Di, J.; Zhang, R.; Zhang, J. Diallyl Disulfide Suppresses the Inflammation and Apoptosis Resistance Induced by DCA Through ROS and the NF-kappaB Signaling Pathway in Human Barrett’s Epithelial Cells. Inflammation 2017, 40, 818–831. [Google Scholar] [CrossRef]
- Faller, S.; Hausler, F.; Goeft, A.; von Itter, M.A.; Gyllenram, V.; Hoetzel, A.; Spassov, S.G. Hydrogen sulfide limits neutrophil transmigration, inflammation, and oxidative burst in lipopolysaccharide-induced acute lung injury. Sci. Rep. 2018, 8, 14676. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of Inflammation: What Controls Its Onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef]
- Luo, H.R.; Loison, F. Constitutive neutrophil apoptosis: Mechanisms and regulation. Am. J. Hematol. 2008, 83, 288–295. [Google Scholar] [CrossRef]
- Rinaldi, L.; Gobbi, G.; Pambianco, M.; Micheloni, C.; Mirandola, P.; Vitale, M. Hydrogen sulfide prevents apoptosis of human PMN via inhibition of p38 and caspase 3. Lab. Investig. 2006, 86, 391–397. [Google Scholar] [CrossRef]
- Shigemi, Z.; Furukawa, Y.; Hosokawa, K.; Minami, S.; Matsuhiro, J.; Nakata, S.; Watanabe, T.; Kagawa, H.; Nakagawa, K.; Takeda, H.; et al. Diallyl trisulfide induces apoptosis by suppressing NF-kappaB signaling through destabilization of TRAF6 in primary effusion lymphoma. Int. J. Oncol. 2016, 48, 293–304. [Google Scholar] [CrossRef]
- Wang, H.; Sun, N.; Li, X.; Li, K.; Tian, J.; Li, J. Diallyl trisulfide induces osteosarcoma cell apoptosis through reactive oxygen species-mediated downregulation of the PI3K/Akt pathway. Oncol. Rep. 2016, 35, 3648–3658. [Google Scholar] [CrossRef]
- Suangtamai, T.; Tanyong, D.I. Diallyl disulfide induces apoptosis and autophagy via mTOR pathway in myeloid leukemic cell line. Tumor Biol. 2016, 37, 10993–10999. [Google Scholar] [CrossRef]
- Luo, N.; Zhao, L.C.; Shi, Q.Q.; Feng, Z.Q.; Chen, D.L.; Li, J. Induction of Apoptosis in Human Leukemic Cell Lines by Diallyl Disulfide via Modulation of EGFR/ERK/PKM2 Signaling Pathways. Asian Pac. J. Cancer Prev. 2015, 16, 3509–3515. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Ji, X.X.; Lin, M.; Tan, H.; Tang, Y.; Wen, L.; Ma, Y.H.; Su, Q. Diallyl disulfide induces apoptosis in human leukemia HL-60 cells through activation of JNK mediated by reactive oxygen. Pharmazie 2010, 65, 693–698. [Google Scholar] [PubMed]
- Tsai, C.Y.; Wen, S.Y.; Shibu, M.A.; Yang, Y.C.; Peng, H.; Wang, B.; Wei, Y.M.; Chang, H.Y.; Lee, C.Y.; Huang, C.Y.; et al. Diallyl trisulfide protects against high glucose-induced cardiac apoptosis by stimulating the production of cystathionine gamma-lyase-derived hydrogen sulfide. Int. J. Cardiol. 2015, 195, 300–310. [Google Scholar] [CrossRef]
- Kuo, W.W.; Wang, W.J.; Tsai, C.Y.; Way, C.L.; Hsu, H.H.; Chen, L.M. Diallyl trisufide (DATS) suppresses high glucose-induced cardiomyocyte apoptosis by inhibiting JNK/NFkappaB signaling via attenuating ROS generation. Int. J. Cardiol. 2013, 168, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Fujinaka, Y.; Sipula, D.; Garcia-Ocana, A.; Vasavada, R.C. Characterization of mice doubly transgenic for parathyroid hormone-related protein and murine placental lactogen: A novel role for placental lactogen in pancreatic beta-cell survival. Diabetes 2004, 53, 3120–3130. [Google Scholar] [CrossRef]
- Kuritzkes, D.R.; Zhang, X.Y.; Lin, E.C. Use of phi(glp-lac) in studies of respiratory regulation of the Escherichia coli anaerobic sn-glycerol-3-phosphate dehydrogenase genes (glpAB). J. Bacteriol. 1984, 157, 591–598. [Google Scholar] [CrossRef]
- Cagnol, S.; Chambard, J.C. ERK and cell death: Mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. FEBS J. 2010, 277, 2–21. [Google Scholar] [CrossRef]
- Kilpatrick, L.E.; Sun, S.; Mackie, D.; Baik, F.; Li, H.; Korchak, H.M. Regulation of TNF mediated antiapoptotic signaling in human neutrophils: Role of delta-PKC and ERK1/2. J. Leukoc. Biol. 2006, 80, 1512–1521. [Google Scholar] [CrossRef]
- Klein, J.B.; Buridi, A.; Coxon, P.Y.; Rane, M.J.; Manning, T.; Kettritz, R.; McLeish, K.R. Role of extracellular signal-regulated kinase and phosphatidylinositol-3 kinase in chemoattractant and LPS delay of constitutive neutrophil apoptosis. Cell. Signal. 2001, 13, 335–343. [Google Scholar] [CrossRef]
- Webb, P.R.; Wang, K.Q.; Scheel-Toellner, D.; Pongracz, J.; Salmon, M.; Lord, J.M. Regulation of neutrophil apoptosis: A role for protein kinase C and phosphatidylinositol-3-kinase. Apoptosis 2000, 5, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Downward, J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell Biol. 1998, 10, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Marte, B.M.; Downward, J. PKB/Akt: Connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem. Sci. 1997, 22, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Guttridge, D.C.; Mayo, M.W.; Baldwin, A.S., Jr. NF-kappaB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol. Cell. Biol. 1999, 19, 5923–5929. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, M.; Winyard, P.G. Hydrogen sulfide and inflammation: The good, the bad, the ugly and the promising. Expert Rev. Clin. Pharmacol. 2011, 4, 13–32. [Google Scholar] [CrossRef]
- Sen, N.; Paul, B.D.; Gadalla, M.M.; Mustafa, A.K.; Sen, T.; Xu, R.; Kim, S.; Snyder, S.H. Hydrogen sulfide-linked sulfhydration of NF-kappaB mediates its antiapoptotic actions. Mol. Cell 2012, 45, 13–24. [Google Scholar] [CrossRef]
- Zhong, G.; Chen, F.; Cheng, Y.; Tang, C.; Du, J. The role of hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J. Hypertens. 2003, 21, 1879–1885. [Google Scholar] [CrossRef]
- Chen, Y.H.; Yao, W.Z.; Geng, B.; Ding, Y.L.; Lu, M.; Zhao, M.W.; Tang, C.S. Endogenous hydrogen sulfide in patients with COPD. Chest 2005, 128, 3205–3211. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Qin, J.; Chang, X.; Yang, Z.; Du, J. Hydrogen sulfide and carbon monoxide are in synergy with each other in the pathogenesis of recurrent febrile seizures. Cell. Mol. Neurobiol. 2006, 26, 101–107. [Google Scholar] [CrossRef] [PubMed]
- DeLeon, E.R.; Stoy, G.F.; Olson, K.R. Passive loss of hydrogen sulfide in biological experiments. Anal. Biochem. 2012, 421, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.S.; Pae, H.O.; Lee, B.S.; Kim, B.N.; Kim, J.M.; Kim, H.R.; Jeon, S.B.; Jeon, W.K.; Chae, H.J.; Chung, H.T. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic. Biol. Med. 2006, 41, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Al-Magableh, M.R.; Hart, J.L. Mechanism of vasorelaxation and role of endogenous hydrogen sulfide production in mouse aorta. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2011, 383, 403–413. [Google Scholar] [CrossRef]
- Whitfield, N.L.; Kreimier, E.L.; Verdial, F.C.; Skovgaard, N.; Olson, K.R. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 294, R1930–R1937. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Pattillo, C.B.; Pardue, S.; Bir, S.C.; Wang, R.; Kevil, C.G. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic. Biol. Med. 2011, 50, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Raupachova, J.; Ilic, D.; Werzowa, J.; Horl, W.H. Effect of leptin on polymorphonuclear leucocyte functions in healthy subjects and haemodialysis patients. Nephrol. Dial. Transplant. 2011, 26, 2271–2281. [Google Scholar] [CrossRef]
- Cohen, G.; Raupachova, J.; Wimmer, T.; Deicher, R.; Horl, W.H. The uraemic retention solute para-hydroxy-hippuric acid attenuates apoptosis of polymorphonuclear leukocytes from healthy subjects but not from haemodialysis patients. Nephrol. Dial. Transplant. 2008, 23, 2512–2519. [Google Scholar] [CrossRef]
- Ando, M.; Shibuya, A.; Tsuchiya, K.; Akiba, T.; Nitta, K. Reduced capacity of mononuclear cells to synthesize cytokines against an inflammatory stimulus in uremic patients. Nephron Clin. Pract. 2006, 104, c113–c119. [Google Scholar] [CrossRef]
- Gibbons, R.A.; Martinez, O.M.; Garovoy, M.R. Altered monocyte function in uremia. Clin. Immunol. Immunopathol. 1990, 56, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Mirandola, P.; Gobbi, G.; Sponzilli, I.; Pambianco, M.; Malinverno, C.; Cacchioli, A.; De Panfilis, G.; Vitale, M. Exogenous hydrogen sulfide induces functional inhibition and cell death of cytotoxic lymphocytes subsets. J. Cell. Physiol. 2007, 213, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Li, L.; Moore, P.K. Hydrogen sulfide-induces DNA damage and changes in apoptotic gene expression in human lung fibroblast cells. FASEB J. 2007, 21, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Ryazantseva, N.V.; Novitsky, V.V.; Starikova, E.G.; Kleptsova, L.A.; Jakushina, V.D.; Kaigorodova, E.V. Role of hydrogen sulfide in the regulation of cell apoptosis. Bull. Exp. Biol. Med. 2011, 151, 702–704. [Google Scholar] [CrossRef] [PubMed]
- Henderson, P.W.; Singh, S.P.; Belkin, D.; Nagineni, V.; Weinstein, A.L.; Weissich, J.; Spector, J.A. Hydrogen sulfide protects against ischemia-reperfusion injury in an in vitro model of cutaneous tissue transplantation. J. Surg. Res. 2010, 159, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Castro-Piedras, I.; Perez-Zoghbi, J.F. Hydrogen sulphide inhibits Ca2+ release through InsP3 receptors and relaxes airway smooth muscle. J. Physiol. 2013, 591, 5999–6015. [Google Scholar] [CrossRef]
- Yang, C.T.; Chen, L.; Chen, W.L.; Li, N.; Chen, M.J.; Li, X.; Zheng, X.; Zhao, Y.Z.; Wu, Y.X.; Xian, M.; et al. Hydrogen sulfide primes diabetic wound to close through inhibition of NETosis. Mol. Cell. Endocrinol. 2019, 480, 74–82. [Google Scholar] [CrossRef]
- Iciek, M.; Kwiecien, I.; Chwatko, G.; Sokolowska-Jezewicz, M.; Kowalczyk-Pachel, D.; Rokita, H. The effects of garlic-derived sulfur compounds on cell proliferation, caspase 3 activity, thiol levels and anaerobic sulfur metabolism in human hepatoblastoma HepG2 cells. Cell Biochem. Funct. 2012, 30, 198–204. [Google Scholar] [CrossRef]
- Choi, Y.H.; Park, H.S. Apoptosis induction of U937 human leukemia cells by diallyl trisulfide induces through generation of reactive oxygen species. J. Biomed. Sci. 2012, 19, 50. [Google Scholar] [CrossRef]
- Ma, H.B.; Huang, S.; Yin, X.R.; Zhang, Y.; Di, Z.L. Apoptotic pathway induced by diallyl trisulfide in pancreatic cancer cells. World J. Gastroenterol. 2014, 20, 193–203. [Google Scholar] [CrossRef]
- Hung, F.M.; Shang, H.S.; Tang, N.Y.; Lin, J.J.; Lu, K.W.; Lin, J.P.; Ko, Y.C.; Yu, C.C.; Wang, H.L.; Liao, J.C.; et al. Effects of diallyl trisulfide on induction of apoptotic death in murine leukemia WEHI-3 cells in vitro and alterations of the immune responses in normal and leukemic mice in vivo. Environ. Toxicol. 2015, 30, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, X.; Xu, L.; Wang, L.; Liu, J.; Ye, J.; Qiu, P.; Liu, Q. Apoptosis of rat hepatic stellate cells induced by diallyl trisulfide and proteomics profiling in vitro. Can. J. Physiol. Pharmacol. 2017, 95, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Chen, Q.; Cheng, Y.F.; Jin, H.H.; Kong, D.S.; Zhang, F.; Wu, L.; Shao, J.J.; Zheng, S.Z. Diallyl trisulfide attenuates ethanol-induced hepatic steatosis by inhibiting oxidative stress and apoptosis. Biomed. Pharmacother. 2016, 79, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, R.; Wei, B.; Chai, X.; Sun, D.; Guan, Y.; Liu, X.M. Diallyl disulfide inhibits proliferation and transdifferentiation of lung fibroblasts through induction of cyclooxygenase and synthesis of prostaglandin E2. Mol. Cell. Biochem. 2014, 393, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, A.; Jafari, D.; Kamarul, T.; Bagheri, A.; Sharifi, A.M. Evaluating the Protective Effects and Mechanisms of Diallyl Disulfide on Interlukin-1beta-Induced Oxidative Stress and Mitochondrial Apoptotic Signaling Pathways in Cultured Chondrocytes. J. Cell. Biochem. 2017, 118, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.J.; Kassie, F.; Mersch-Sundermann, V. The role of reactive oxygen species (ROS) production on diallyl disulfide (DADS) induced apoptosis and cell cycle arrest in human A549 lung carcinoma cells. Mutat. Res. 2005, 579, 115–124. [Google Scholar] [CrossRef]
- Hasan, M.A.; Ahn, W.G.; Song, D.K. N-acetyl-L-cysteine and cysteine increase intracellular calcium concentration in human neutrophils. Korean J. Physiol. Pharmacol. 2016, 20, 449–457. [Google Scholar] [CrossRef]
- Petrushanko, I.Y.; Melnikova, E.V.; Yurinskaya, M.M.; Vinokurov, M.G.; Suslikov, A.V.; Mitkevich, V.A.; Makarov, A.A. Influence of the Donor of Hydrogen Sulfide GYY4137 on the Activation of Human Neutrophils by E. coli Lipopolysaccharides. Mol. Biol. 2019, 53, 101–108. [Google Scholar] [CrossRef]
- Jiang, L.; Jiang, Q.; Yang, S.; Huang, S.; Han, X.; Duan, J.; Pan, S.; Zhao, M.; Guo, S. GYY4137 attenuates LPS-induced acute lung injury via heme oxygenase-1 modulation. Pulm. Pharmacol. Ther. 2019, 54, 77–86. [Google Scholar] [CrossRef]
- Lee, Z.W.; Zhou, J.; Chen, C.S.; Zhao, Y.; Tan, C.H.; Li, L.; Moore, P.K.; Deng, L.W. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS ONE 2011, 6, e21077. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 21 February 2023).
- Ogle, D.H.; Doll, J.C.; Wheeler, A.P.; Dinno, A. FSA: Simple Fisheries Stock Assessment Methods, R package version 0.9.4; FSA: Tokyo, Japan, 2023; Available online: https://CRAN.R-project.org/package=FSA (accessed on 21 February 2023).



| Substance | Concentration | Cells | Effect | Comment | Ref. |
|---|---|---|---|---|---|
| NaHS | 0.2–0.4 mM | Lymphocytes (CD8+, NK) | Apoptosis ↑ | Caspase independent, glutathione dependent | [112] |
| NaHS | 10–75 µM | Lung fibroblasts | Apoptosis ↑ | 12–48 h | [113] |
| NaHS | 10, 100, 300 µM | Neutrophils (mice) | (MIP-2 induced) chemotaxis ↑ | [72] | |
| NaHS | 10 mM | Jurkat | Apoptosis ↑ (caspase 9) | 15 min | [114] |
| NaHS | 10–1000 µM | HUVEC 3T3 fibroblasts | (IRI-induced) apoptosis ↓ | [115] | |
| NaHS | 0.23–3.66 mM | Neutrophils | Apoptosis ↓ | [80] | |
| NaHS | 100, 500, 1000 µM | Endothelial cells | Apoptosis ↑ | “100 µM safe in vitro concentration” | [54] |
| NaHS | 100, 200 µM | RAW 264.7 | LPS-stimulated PGE2- biosynthesis ↓ | ↑ (n.s.) at 1000 µM, “biphasic effect” | [52] |
| Na2S | 100 µM | SMC | Ca2+ release ↓ | [116] | |
| Na2S | 25 µM | Neutrophils | NETosis | PMA-induced | [117] |
| DATS | 100, 200 µM | HepG2 | H2O2 ↑ | not for DADS | [118] |
| DATS | 100 µM | HepG2 | Caspase 3 ↑ | not for DADS | [118] |
| DATS | 20 µM | U937 | Apoptosis ↑ | not in THP-1, HL-60, K562 cells | [119] |
| DATS | 1–10 µM | Cardiomyocytes | Glucose-induced apoptosis ↓ ROS ↓ | NFκB ↓ | [87] |
| DATS | 100 µM | Pancreatic cancer cells | Apoptosis ↑ | [120] | |
| DATS | 5, 10 µM | Cardiomyocytes | Glucose-induced apoptosis ↓ | [86] | |
| DATS | 1–5 µM | WEHI-3 | Apoptosis ↑ | [121] | |
| DATS | 12, 24 µM | Rat hepatic stellate cells | Apoptosis ↑ | [122] | |
| DATS | 10–100 µm | “several human cancer cells” | Apoptosis ↑ | [122] | |
| DATS | 20, 40 µM (5–120 µM) | Human osteosarcoma cells | Apoptosis ↑ ROS ↑ | [82] | |
| DATS | 0–50 µM | Primary effusion lymphoma cells | Apoptosis ↑ | [81] | |
| DATS | 1–5 µM | Ethanol-stimulated L02 | Apoptosis ↓ ROS ↓ | [123] | |
| DADS | 5–50 µM | Lung fibroblasts | Proliferation ↓ | [124] | |
| DADS | 5–300 µM | KG1α | Apoptosis ↑ | 24, 48, 72 h | [84] |
| DADS | 1–100 µM | C28I2 Chondrocytes | (IL1β-induced) ROS ↓ (mitochondrial) apoptosis ↓ | 2–24 h 25 µM after 24 h not cytotoxic | [125] |
| DADS | 1, 5, 10 µg/ml | BAR-T cells | Apoptosis ↑ (DCA-induced) ROS ↓ | 1, 3, 6, 12 h | [76] |
| DADS | 25–250 µM | A549 | Apoptosis ↑ ROS ↑ | [126] | |
| DADS | 10, 20 mg/l | HL-60 | Apoptosis ↑ ROS ↑ | [85] | |
| Cysteine | 10 mM | SMC | Ca2+ release ↓ | [116] | |
| Cysteine | 10–1000 µM | Neutrophils | [Ca2+]i ↑ | [127] | |
| GYY4137 | 1 mM | Hoxb8 neutrophils | ROS ↓ Endothelial transmigration ↓ | [77] | |
| GYY4137 | 200 µM | Neutrophils | (LPS stim.) ROS ↓ (LPS inhibited) apoptosis ↑ | [128] | |
| GYY4137 | 500 µM | RAW264.7cells | NF-κB activation (LPS) ↓ | [129] | |
| GYY4137 | 400 µM | MCF-7 | Apoptosis | Not for IMR90 cells | [130] |
| GYY4137 | 100–1000 µM | RAW 264.7 | LPS-stimulated PGE2-, NO2−-, TNFα-, IL-1β- biosynthesis ↓ | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farahat, S.; Kherkheulidze, S.; Nopp, S.; Kainz, A.; Borriello, M.; Perna, A.F.; Cohen, G. Effect of Hydrogen Sulfide on Essential Functions of Polymorphonuclear Leukocytes. Toxins 2023, 15, 198. https://doi.org/10.3390/toxins15030198
Farahat S, Kherkheulidze S, Nopp S, Kainz A, Borriello M, Perna AF, Cohen G. Effect of Hydrogen Sulfide on Essential Functions of Polymorphonuclear Leukocytes. Toxins. 2023; 15(3):198. https://doi.org/10.3390/toxins15030198
Chicago/Turabian StyleFarahat, Sarah, Salome Kherkheulidze, Stephan Nopp, Alexander Kainz, Margherita Borriello, Alessandra F. Perna, and Gerald Cohen. 2023. "Effect of Hydrogen Sulfide on Essential Functions of Polymorphonuclear Leukocytes" Toxins 15, no. 3: 198. https://doi.org/10.3390/toxins15030198
APA StyleFarahat, S., Kherkheulidze, S., Nopp, S., Kainz, A., Borriello, M., Perna, A. F., & Cohen, G. (2023). Effect of Hydrogen Sulfide on Essential Functions of Polymorphonuclear Leukocytes. Toxins, 15(3), 198. https://doi.org/10.3390/toxins15030198






