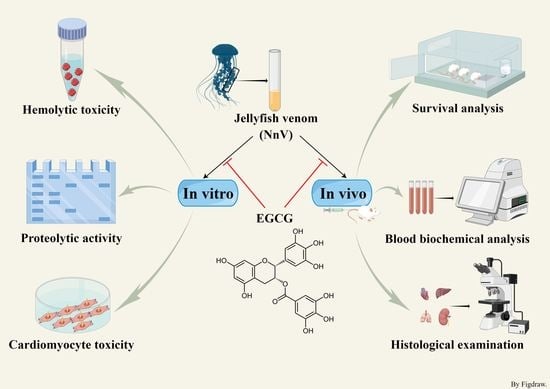
Protective Effects of Epigallocatechin-3-gallate (EGCG) against the Jellyfish Nemopilema nomurai Envenoming
Abstract
:1. Introduction
2. Results
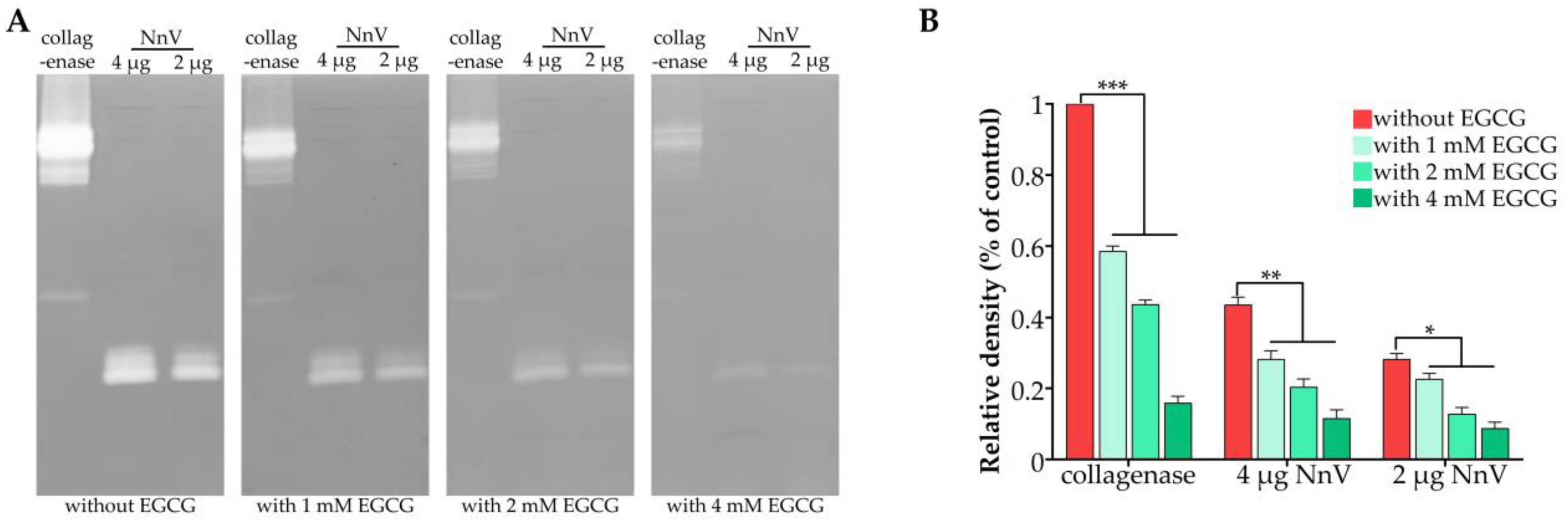
2.1. Effect of EGCG on the Proteolytic Activity of NnV
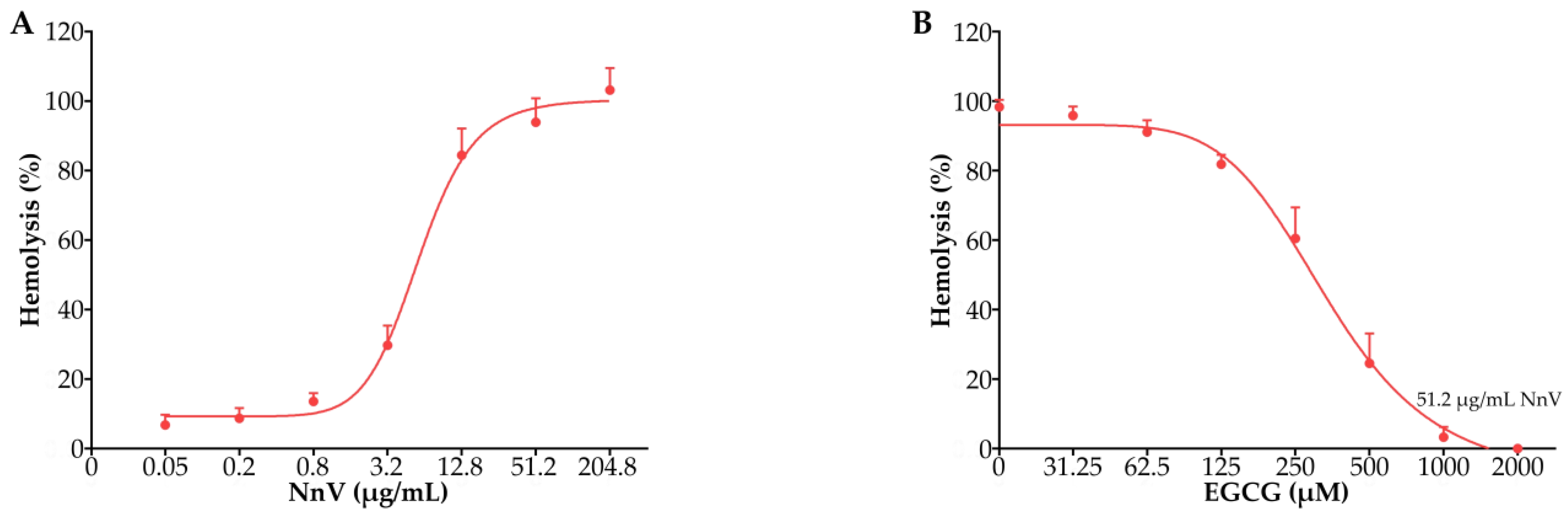
2.2. Effect of EGCG against the Hemolytic Toxicity of NnV
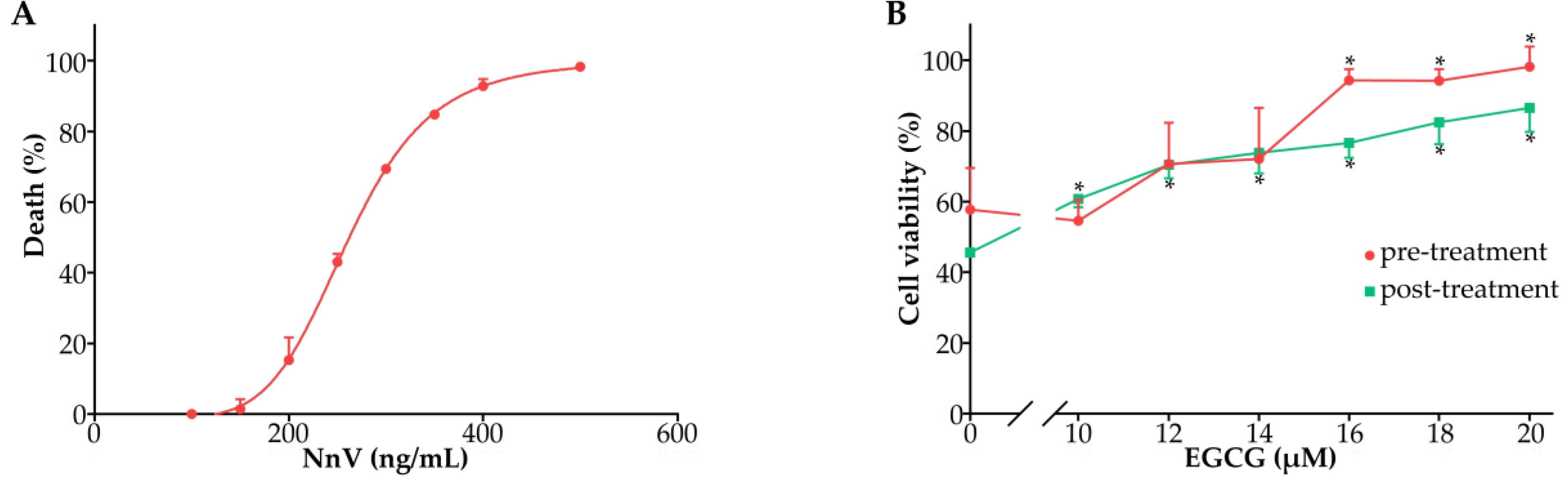
2.3. Effect of EGCG against the Cardiomyocyte Toxicity of NnV
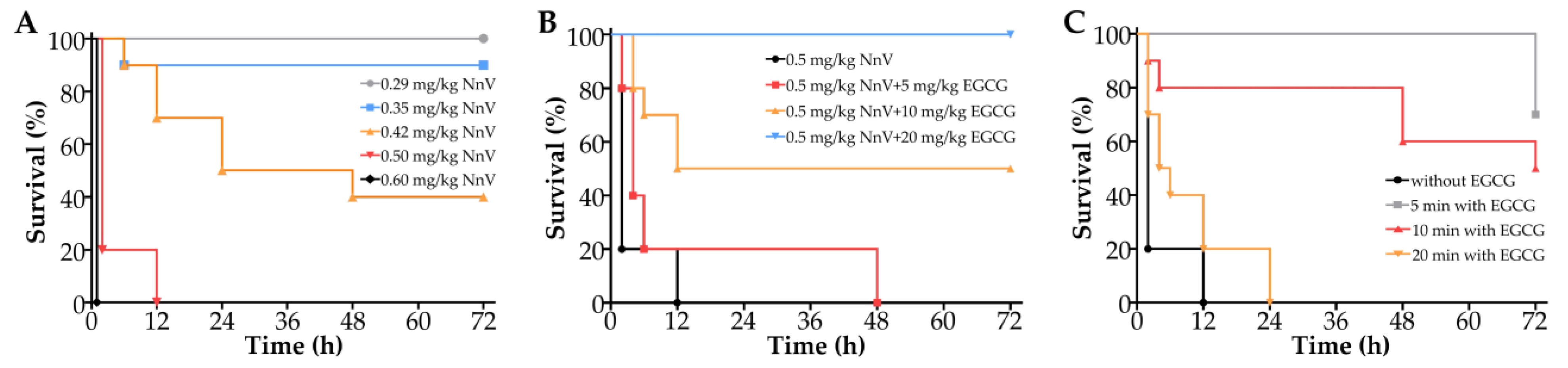
2.4. Effect of EGCG on the Survival Rate of Mice Envenomated by NnV
2.5. Effect of EGCG on the Changes in Serum Biochemical Indices Induced by NnV
2.6. Histopathological Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Reagents and Jellyfish Collection
5.2. Nematocyst Isolation
5.3. Venom Extraction
5.4. Animal Maintenance
5.5. Proteolytic Activity Assay
5.6. Hemolysis Test
5.7. Cell Culture and Cytotoxicity Test
5.8. Survival Analysis
5.9. Blood Biochemical Analysis
5.10. Histological Examination
5.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cunha, S.A.; Dinis-Oliveira, R.J. Raising Awareness on the Clinical and Forensic Aspects of Jellyfish Stings: A Worldwide Increasing Threat. IJERPH 2022, 19, 8430. [Google Scholar] [CrossRef] [PubMed]
- Morabito, R.; Condello, S.; Currò, M.; Marino, A.; Ientile, R.; La Spada, G. Oxidative Stress Induced by Crude Venom from the Jellyfish Pelagia Noctiluca in Neuronal-like Differentiated SH-SY5Y Cells. Toxicol. Vitr. 2012, 26, 694–699. [Google Scholar] [CrossRef] [PubMed]
- D’Ambra, I.; Lauritano, C. A Review of Toxins from Cnidaria. Mar. Drugs 2020, 18, 507. [Google Scholar] [CrossRef] [PubMed]
- Syazwan, W.M.; Rizman-Idid, M.; Low, L.B.; Then, A.Y.-H.; Chong, V.C. Assessment of Scyphozoan Diversity, Distribution and Blooms: Implications of Jellyfish Outbreaks to the Environment and Human Welfare in Malaysia. Reg. Stud. Mar. Sci. 2020, 39, 101444. [Google Scholar] [CrossRef]
- Sun, X.; Sun, X.; Zhu, L.; Li, X.; Sun, S. Seasonal and Spatial Variation in Abundance of the Copepod Calanus Sinicus: Effects of Decreasing Dissolved Oxygen and Small Jellyfish Bloom in Northern Yellow Sea, China, Nearshore Waters. Mar. Pollut. Bull. 2020, 161, 111653. [Google Scholar] [CrossRef]
- Yue, Y.; Yu, H.; Li, R.; Li, P. Topical Exposure to Nemopilema Nomurai Venom Triggers Oedematogenic Effects: Enzymatic Contribution and Identification of Venom Metalloproteinase. Toxins 2021, 13, 44. [Google Scholar] [CrossRef]
- Wang, Q. β Adrenergic Receptor/CAMP/PKA Signaling Contributes to the Intracellular Ca2+ Release by Tentacle Extract from the Jellyfish Cyanea Capillata. BMC Pharmacol. Toxicol. 2017, 18, 60. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Liu, D.; Keesing, J.K. Jellyfish Blooms in China: Dominant Species, Causes and Consequences. Mar. Pollut. Bull. 2010, 60, 954–963. [Google Scholar] [CrossRef]
- Pyo, M.-J.; Lee, H.; Bae, S.K.; Heo, Y.; Choudhary, I.; Yoon, W.D.; Kang, C.; Kim, E. Modulation of Jellyfish Nematocyst Discharges and Management of Human Skin Stings in Nemopilema Nomurai and Carybdea Mora. Toxicon 2016, 109, 26–32. [Google Scholar] [CrossRef]
- Kim, J.H.; Han, S.B.; Durey, A. Fatal Pulmonary Edema in a Child after Jellyfish Stings in Korea. Wilderness Environ. Med. 2018, 29, 527–530. [Google Scholar] [CrossRef] [Green Version]
- Remigante, A.; Costa, R.; Morabito, R.; La Spada, G.; Marino, A.; Dossena, S. Impact of Scyphozoan Venoms on Human Health and Current First Aid Options for Stings. Toxins 2018, 10, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.; Yue, Y.; Yin, X.; Li, R.; Yu, H.; Li, P. Identifying and Revealing the Geographical Variation in Nemopilema Nomurai Venom Metalloprotease and Phospholipase A2 Activities. Chemosphere 2021, 266, 129164. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Munawir, A.; Cha, M.; Sohn, E.-T.; Lee, H.; Kim, J.-S.; Yoon, W.D.; Lim, D.; Kim, E. Cytotoxicity and Hemolytic Activity of Jellyfish Nemopilema Nomurai (Scyphozoa: Rhizostomeae) Venom. Comp. Biochem. Physiol. C Toxicol. Pharm. 2009, 150, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, I.; Lee, H.; Pyo, M.-J.; Heo, Y.; Bae, S.K.; Kwon, Y.C.; Yoon, W.D.; Kang, C.; Kim, E. Proteomics Approach to Examine the Cardiotoxic Effects of Nemopilema Nomurai Jellyfish Venom. J. Proteom. 2015, 128, 123–131. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, J.; Torrens, E.; Segura-Puertas, L. Partial Purification and Characterization of a Novel Neurotoxin and Three Cytolysins from Box Jellyfish (Carybdea Marsupialis) Nematocyst Venom. Arch. Toxicol. 2006, 80, 163–168. [Google Scholar] [CrossRef]
- Li, A.; Yu, H.; Li, R.; Yue, Y.; Yu, C.; Geng, H.; Liu, S.; Xing, R.; Li, P. Jellyfish Nemopilema Nomurai Causes Myotoxicity through the Metalloprotease Component of Venom. Biomed. Pharmacother. 2022, 151, 113192. [Google Scholar] [CrossRef]
- Yue, Y.; Yu, H.; Li, R.; Xing, R.; Liu, S.; Li, K.; Wang, X.; Chen, X.; Li, P. Functional Elucidation of Nemopilema Nomurai and Cyanea Nozakii Nematocyst Venoms’ Lytic Activity Using Mass Spectrometry and Zymography. Toxins 2017, 9, 47. [Google Scholar] [CrossRef] [Green Version]
- Yue, Y.; Yu, H.; Li, R.; Xing, R.; Liu, S.; Li, K.; Wang, X.; Chen, X.; Li, P. Biochemical and Kinetic Evaluation of the Enzymatic Toxins from Two Stinging Scyphozoans Nemopilema Nomurai and Cyanea Nozakii. Toxicon 2017, 125, 1–12. [Google Scholar] [CrossRef]
- Chang, C.-W.; Hsieh, Y.-H.; Yang, W.-E.; Yang, S.-F.; Chen, Y.; Hu, D.-N. Epigallocatechingallate Inhibits Migration of Human Uveal Melanoma Cells via Downregulation of Matrix Metalloproteinase-2 Activity and ERK1/2 Pathway. Biomed. Res. Int. 2014, 2014, 141582. [Google Scholar] [CrossRef]
- Kim, J.E.; Shin, M.H.; Chung, J.H. Epigallocatechin-3-Gallate Prevents Heat Shock-Induced MMP-1 Expression by Inhibiting AP-1 Activity in Human Dermal Fibroblasts. Arch. Derm. Res 2013, 305, 595–602. [Google Scholar] [CrossRef]
- Srinivasa, V.; Sundaram, M.S.; Anusha, S.; Hemshekhar, M.; Chandra Nayaka, S.; Kemparaju, K.; Basappa; Girish, K.S.; Rangappa, K.S. Novel Apigenin Based Small Molecule That Targets Snake Venom Metalloproteases. PLoS ONE 2014, 9, e106364. [Google Scholar] [CrossRef] [Green Version]
- Pithayanukul, P.; Ruenraroengsak, P.; Bavovada, R.; Pakmanee, N.; Suttisri, R.; Saen-oon, S. Inhibition of Naja Kaouthia Venom Activities by Plant Polyphenols. J. Ethnopharmacol. 2005, 97, 527–533. [Google Scholar] [CrossRef]
- Hwang, D.H.; Lee, H.; Choudhary, I.; Kang, C.; Chae, J.; Kim, E. Protective Effect of Epigallocatechin-3-Gallate (EGCG) on Toxic Metalloproteinases-Mediated Skin Damage Induced by Scyphozoan Jellyfish Envenomation. Sci. Rep. 2020, 10, 18644. [Google Scholar] [CrossRef]
- Zhang, L.; He, Q.; Wang, Q.; Zhang, B.; Wang, B.; Xu, F.; Wang, T.; Xiao, L.; Zhang, L. Intracellular Ca(2+) Overload Induced by Extracellular Ca(2+) Entry Plays an Important Role in Acute Heart Dysfunction by Tentacle Extract from the Jellyfish Cyanea Capillata. Cardiovasc. Toxicol. 2014, 14, 260–274. [Google Scholar] [CrossRef]
- Li, R.; Yu, H.; Yue, Y.; Li, P. Combined Proteome and Toxicology Approach Reveals the Lethality of Venom Toxins from Jellyfish Cyanea Nozakii. J. Proteome Res. 2018, 17, 3904–3913. [Google Scholar] [CrossRef]
- Wang, T.; Wen, X.-J.; Mei, X.-B.; Wang, Q.-Q.; He, Q.; Zheng, J.-M.; Zhao, J.; Xiao, L.; Zhang, L.-M. Lipid Peroxidation Is Another Potential Mechanism besides Pore-Formation Underlying Hemolysis of Tentacle Extract from the Jellyfish Cyanea Capillata. Mar. Drugs 2013, 11, 67–80. [Google Scholar] [CrossRef] [Green Version]
- Leung, T.C.N.; Qu, Z.; Nong, W.; Hui, J.H.L.; Ngai, S.M. Proteomic Analysis of the Venom of Jellyfishes Rhopilema Esculentum and Sanderia Malayensis. Mar. Drugs 2020, 18, 655. [Google Scholar] [CrossRef]
- Choudhary, I.; Hwang, D.H.; Lee, H.; Yoon, W.D.; Chae, J.; Han, C.H.; Yum, S.; Kang, C.; Kim, E. Proteomic Analysis of Novel Components of Nemopilema Nomurai Jellyfish Venom: Deciphering the Mode of Action. Toxins 2019, 11, 153. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Ye, R.; Ma, C.; Wang, Y.; Wang, Y.; Chen, J.; Yang, J.; Hofer, J.; Zhu, Y.; Xiao, L.; et al. Toxicity Evaluation, Toxin Screening and Its Intervention of the Jellyfish Phacellophora Camtschatica Based on a Combined Transcriptome-Proteome Analysis. Ecotoxicol. Environ. Saf. 2022, 233, 113315. [Google Scholar] [CrossRef]
- Kang, C.; Jin, Y.B.; Kwak, J.; Jung, H.; Yoon, W.D.; Yoon, T.-J.; Kim, J.-S.; Kim, E. Protective Effect of Tetracycline against Dermal Toxicity Induced by Jellyfish Venom. PLoS ONE 2013, 8, e57658. [Google Scholar] [CrossRef]
- Lee, H.; Jung, E.; Kang, C.; Yoon, W.D.; Kim, J.-S.; Kim, E. Scyphozoan Jellyfish Venom Metalloproteinases and Their Role in the Cytotoxicity. Toxicon 2011, 58, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhong, L.; Zhou, B.; Chen, J.; Li, C. Interaction of Characteristic Structural Elements of Persimmon Tannin with Chinese Cobra PLA2. Toxicon 2013, 74, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Pellarín, M.G.; Albrecht, C.; Rojas, M.J.; Aguilar, J.J.; Konigheim, B.S.; Paraje, M.G.; Albesa, I.; Eraso, A.J. Inhibition of Cytotoxicity of Shiga Toxin of Escherichia Coli O157:H7 on Vero Cells by Prosopis Alba Griseb (Fabaceae) and Ziziphus Mistol Griseb (Rhamnaceae) Extracts. J. Food Prot. 2013, 76, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; de Villiers, W.J.; McClain, C.J.; Varilek, G.W. Green Tea Polyphenols Block Endotoxin-Induced Tumor Necrosis Factor-Production and Lethality in a Murine Model. J. Nutr. 1998, 128, 2334–2340. [Google Scholar] [CrossRef] [Green Version]
- Satoh, E.; Ishii, T.; Shimizu, Y.; Sawamura, S.I.; Nishimura, M. A Mechanism of the Thearubigin Fraction of Black Tea (Camellia Sinensis) Extract Protecting against the Effect of Tetanus Toxin. J. Toxicol. Sci. 2002, 27, 441–447. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Yu, H.; Yue, Y.; Liu, S.; Xing, R.; Chen, X.; Wang, X.; Li, P. In Depth Analysis of the in Vivo Toxicity of Venom from the Jellyfish Stomolophus Meleagris. Toxicon 2014, 92, 60–65. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Q.; Xiao, L.; Zhang, L. Intervention Effects of Five Cations and Their Correction on Hemolytic Activity of Tentacle Extract from the Jellyfish Cyanea Capillata. PeerJ 2017, 5, 19. [Google Scholar] [CrossRef] [Green Version]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, Q.; Zou, S.; Song, J.; Zhang, P.; Wang, F.; Huang, Y.; He, Q.; Zhang, L. Protective Effects of Epigallocatechin-3-gallate (EGCG) against the Jellyfish Nemopilema nomurai Envenoming. Toxins 2023, 15, 283. https://doi.org/10.3390/toxins15040283
Li J, Wang Q, Zou S, Song J, Zhang P, Wang F, Huang Y, He Q, Zhang L. Protective Effects of Epigallocatechin-3-gallate (EGCG) against the Jellyfish Nemopilema nomurai Envenoming. Toxins. 2023; 15(4):283. https://doi.org/10.3390/toxins15040283
Chicago/Turabian StyleLi, Jie, Qianqian Wang, Shuaijun Zou, Juxingsi Song, Peipei Zhang, Fan Wang, Yichao Huang, Qian He, and Liming Zhang. 2023. "Protective Effects of Epigallocatechin-3-gallate (EGCG) against the Jellyfish Nemopilema nomurai Envenoming" Toxins 15, no. 4: 283. https://doi.org/10.3390/toxins15040283