Prospecting Local Treatments Used in Conjunction with Antivenom Administration Following Envenomation Caused by Animals: A Systematic Review
Abstract
1. Introduction
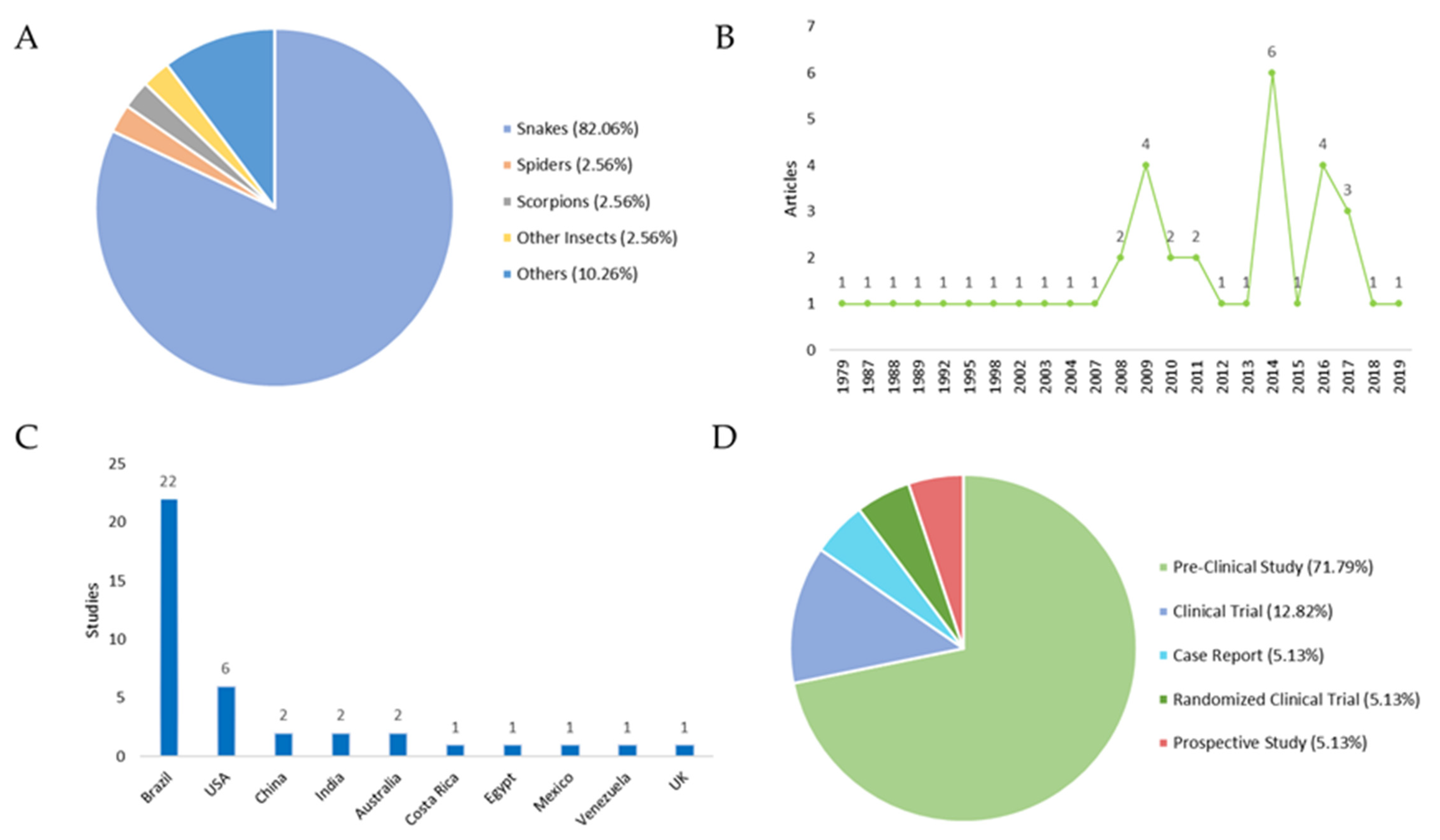
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bar-On, B. On the form and bio-mechanics of venom-injection elements. Acta Biomater. 2019, 85, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.D.S.; Queiroz, P.E.S.; Bastos, M.D.C.; Miranda, E.A.; Jesus, H.D.S.D.; Gatis, S.M.P. Tratamento da ferida por acidente ofídico: Caso clínico. Cuid. Enferm. 2016, 10, 172–179. [Google Scholar]
- Tavares, A.V.; Araújo, K.A.M.D.; Marques, M.R.D.V.; Leite, R. Epidemiology of the injury with venomous animals in the state of Rio Grande do Norte, Northeast of Brazil. Ciênc. Saúde Coletiva 2020, 25, 1967–1978. [Google Scholar] [CrossRef] [PubMed]
- Sachett, J.D.A.G.; Val, F.F.; Alcântara, J.A.; Cubas-Vega, N.; Montenegro, C.S.; da Silva, I.M.; de Souza, T.G.; Santana, M.F.; Ferreira, L.C.; Monteiro, W.M. Bothrops atrox Snakebite: How a Bad Decision May Lead to a Chronic Disability: A Case Report. Wilderness Environ. Med. 2020, 31, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Barreto, G.N.L.S.; de Oliveira, S.S.; dos Anjos, I.V.; Chalkidis, H.D.M.; Mourão, R.H.V.; Moura-Da-Silva, A.M.; Sano-Martins, I.S.; Gonçalves, L.R.D.C. Experimental Bothrops atrox envenomation: Efficacy of antivenom therapy and the combination of Bothrops antivenom with dexamethasone. PLoS Negl. Trop. Dis. 2017, 11, e0005458. [Google Scholar] [CrossRef]
- Tan, H.H.; Mong, R. Scorpion Stings Presenting to an Emergency Department in Singapore with Special Reference to Isometrus maculatus. Wilderness Environ. Med. 2013, 24, 42–47. [Google Scholar] [CrossRef]
- Ministério da Saúde do Brasil. Manual de Diagnóstico e Tratamento de Acidentes por Animais Peçonhentos; Fundação Nacional de Saúde: Brasília, Brazil, 2001. [Google Scholar]
- Wasserman, G.S. Wound care of spider and snake envenomations. Ann. Emerg. Med. 1988, 17, 1331–1335. [Google Scholar] [CrossRef]
- Shaikh, I.K.; Dixit, P.P.; Pawade, B.S.; Potnis-Lele, M.; Kurhe, B.P. Assessment of Cultivable Oral Bacterial Flora from Important Venomous Snakes of India and Their Antibiotic Susceptibilities. Curr. Microbiol. 2017, 74, 1278–1286. [Google Scholar] [CrossRef]
- Résière, D.; Olive, C.; Kallel, H.; Cabié, A.; Névière, R.; Mégarbane, B.; Gutiérrez, J.; Mehdaoui, H. Oral Microbiota of the Snake Bothrops lanceolatus in Martinique. Int. J. Environ. Res. Public Health 2018, 15, 2122. [Google Scholar] [CrossRef]
- Mendes, V.K.D.G.; Pereira, H.D.S.; Elias, I.C.; Soares, G.S.; Santos, M.; Talhari, C.; Cordeiro-Santos, M.; Monteiro, W.M.; Sachett, J.D.A.G. Secondary infection profile after snakebite treated at a tertiary referral center in the Brazilian Amazon. Rev. Soc. Bras. Med. Trop. 2022, 55, e0244-2021. [Google Scholar] [CrossRef]
- De Moura, V.M.; Mourão, R.H.V.; Dos-Santos, M.C. Acidentes ofídicos na Região Norte do Brasil e o uso de espécies vegetais como tratamento alternativo e complementar à soroterapia. Sci. Amazon 2015, 4, 73. [Google Scholar] [CrossRef]
- Félix-Silva, J.; Silva-Junior, A.A.; Zucolotto, S.M.; Fernandes-Pedrosa, M.D.F. Medicinal Plants for the Treatment of Local Tissue Damage Induced by Snake Venoms: An Overview from Traditional Use to Pharmacological Evidence. Evid.-Based Complement. Alternat. Med. 2017, 2017, 5748256. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, R.; Singh, P.; Yasir, M.; Hazarika, R.; Sugunan, S. A Review on Venom Enzymes Neutralizing Ability of Secondary Metabolites from Medicinal Plants. J. Pharmacopunct. 2017, 20, 173–178. [Google Scholar] [CrossRef]
- Borges, C.C.; Cavalcanti-Neto, A.J.; Boechat, A.L.; Francisco, C.H.; Arruda, M.R.E.; Dos-Santos, M.C. Eficácia da espécie vegetal Peltodon radicans (Labiatae, Lameaceae) na neutralização da atividade edematogênica e ineficácia do extrato vegetal Específico Pessoa na neutralização das principais atividades do veneno de Bothrops atrox. Rev. Univ. Amaz. 1996, 1, 97–113. [Google Scholar]
- Barbosa, A.M.; Villaverde, A.B.; Guimarães-Sousa, L.; Soares, A.M.; Zamuner, S.F.; Cogo, J.C.; Zamuner, S.R. Low-level laser therapy decreases local effects induced by myotoxins isolated from Bothrops jararacussu snake venom. J. Venom. Anim. Toxins Trop. Dis. 2010, 16, 470–479. [Google Scholar] [CrossRef]
- Dourado, D.M.; Fávero, S.; Baranauskas, V.; da Cruz-Höfling, M.A. Effects of the Ga-As laser irradiation on myonecrosis caused by Bothrops moojeni snake venom. Lasers Surg. Med. 2003, 33, 352–357. [Google Scholar] [CrossRef]
- Burgess, J.L.; Dart, R.C.; Egen, N.B.; Mayersohn, M. Effects of constriction bands on rattlesnake venom absorption: A pharmacokinetic study. Ann. Emerg. Med. 1992, 21, 1086–1093. [Google Scholar] [CrossRef]
- Sutherland, S.K.; Coulter, A.R.; Harris, R.D. Rationalisation of first-aid measures for elapid snakebite. Lancet 1979, 313, 183–186. [Google Scholar] [CrossRef]
- Howe, N.R.; Meisenheimer, J.L. Electric shock does not save snakebitten rats. Ann. Emerg. Med. 1988, 17, 254–256. [Google Scholar] [CrossRef]
- Nadur-Andrade, N.; Zamuner, S.R.; Toniolo, E.F.; de Lima, C.J.; Cogo, J.C.; Dale, C.S. Analgesic Effect of Light-Emitting Diode (LED) Therapy at Wavelengths of 635 and 945 nm on Bothrops moojeni Venom-Induced Hyperalgesia. Photochem. Photobiol. 2014, 90, 207–213. [Google Scholar] [CrossRef]
- Barbosa, A.M.; Villaverde, A.B.; Guimarães-Souza, L.; Ribeiro, W.; Cogo, J.C.; Zamuner, S.R. Effect of low-level laser therapy in the inflammatory response induced by Bothrops jararacussu snake venom. Toxicon 2008, 51, 1236–1244. [Google Scholar] [CrossRef]
- de Sousa, E.A.; Bittencourt, J.A.H.M.; de Oliveira, N.K.S.; Henriques, S.V.C.; Picanço, L.C.D.S.; Lobato, C.P.; Ribeiro, J.R.; Pereira, W.L.A.; Carvalho, J.C.T.; da Silva, J.O. Effects of a low-level semiconductor gallium arsenide laser on local pathological alterations induced by Bothrops moojeni snake venom. Photochem. Photobiol. Sci. 2013, 12, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Nadur-Andrade, N.; Barbosa, A.M.; Carlos, F.P.; Lima, C.J.; Cogo, J.C.; Zamuner, S.R. Effects of photobiostimulation on edema and hemorrhage induced by Bothrops moojeni venom. Lasers Med. Sci. 2012, 27, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Giaretta, V.M.D.A.; Santos, L.P.; Barbosa, A.M.; Hyslop, S.; Corrado, A.P.; Galhardo, M.S.; Nicolau, R.A.; Cogo, J.C. Low-intensity laser therapy improves tetanic contractions in mouse anterior tibialis muscle injected with Bothrops jararaca snake venom. Res. Biomed. Eng. 2016, 32, 153–160. [Google Scholar] [CrossRef]
- Souza, L.G.; Dale, C.; Nadur-Andrade, N.; Barbosa, A.M.; Cogo, J.; Zamuner, S. Low-level laser therapy reduces edema, leukocyte influx and hyperalgesia induced by Bothrops jararacussu snake venom. Clin. Exp. Med. Lett. 2011, 52, 97–102. [Google Scholar]
- Doin-Silva, R.; Baranauskas, V.; Rodrigues-Simioni, L.; Da Cruz-Höfling, M.A. The Ability of Low Level Laser Therapy to Prevent Muscle Tissue Damage Induced by Snake Venom. Photochem. Photobiol. 2009, 85, 63–69. [Google Scholar] [CrossRef]
- Nadur-Andrade, N.; Dale, C.S.; Oliveira, V.R.D.S.; Toniolo, E.F.; Feliciano, R.D.S.; da Silva, J.A., Jr.; Zamuner, S.R. Analgesic Effect of Photobiomodulation on Bothrops Moojeni Venom-Induced Hyperalgesia: A Mechanism Dependent on Neuronal Inhibition, Cytokines and Kinin Receptors Modulation. PLoS Negl. Trop. Dis. 2016, 10, e0004998. [Google Scholar] [CrossRef]
- Barbosa, A.M.; Villaverde, A.B.; Sousa, L.G.; Munin, E.; Fernandez, C.M.; Cogo, J.C.; Zamuner, S.R. Effect of Low-Level Laser Therapy in the Myonecrosis Induced by Bothrops jararacussu Snake Venom. Photomed. Laser Surg. 2009, 27, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Dourado, D.M.; Matias, R.; Barbosa-Ferreira, M.; da Silva, B.A.K.; de Araujo Isaias Muller, J.; Vieira, W.F.; da Cruz-Höfling, M.A. Effects of photobiomodulation therapy on Bothrops moojeni snake-envenomed gastrocnemius of mice using enzymatic biomarkers. Lasers Med. Sci. 2017, 32, 1357–1366. [Google Scholar] [CrossRef]
- Vieira, W.F.; Kenzo-Kagawa, B.; Cogo, J.C.; Baranauskas, V.; da Cruz-Höfling, M.A. Low-Level Laser Therapy (904 nm) Counteracts Motor Deficit of Mice Hind Limb following Skeletal Muscle Injury Caused by Snakebite-Mimicking Intramuscular Venom Injection. PLoS ONE 2016, 11, e0158980. [Google Scholar] [CrossRef]
- Dourado, D.M.; Fávero, S.; Matias, R.; Carvalho, P.D.T.C.; da Cruz-Höfling, M.A. Low-level Laser Therapy Promotes Vascular Endothelial Growth Factor Receptor-1 Expression in Endothelial and Nonendothelial Cells of Mice Gastrocnemius Exposed to Snake Venom. Photochem. Photobiol. 2011, 87, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Nadur-Andrade, N.; Dale, C.S.; dos Santos, A.S.; Soares, A.M.; de Lima, C.J.; Zamuner, S.R. Photobiostimulation reduces edema formation induced in mice by Lys-49 phospholipases A2 isolated from Bothrops moojeni venom. Photochem. Photobiol. Sci. 2014, 13, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Dourado, D.M.; Matias, R.; Almeida, M.F.; De Paula, K.R.; Vieira, R.P.; Oliveira, L.V.F.; Carvalho, P.T.C. The effects of low-level laser on muscle damage caused by Bothrops neuwiedi venom. J. Venom. Anim. Toxins Trop. Dis. 2008, 14, 423–434. [Google Scholar] [CrossRef]
- Salama, W.H.; Abdel-Aty, A.M.; Fahmy, A.S. Rosemary leaves extract: Anti-snake action against Egyptian Cerastes cerastes venom. J. Tradit. Complement. Med. 2018, 8, 465–475. [Google Scholar] [CrossRef]
- Gomes, J.A.D.S.; Félix-Silva, J.; Fernandes, J.M.; Amaral, J.G.; Lopes, N.P.; Egito, E.S.T.D.; da Silva-Júnior, A.A.; Zucolotto, S.M.; Fernandes-Pedrosa, M.D.F. Aqueous Leaf Extract of Jatropha mollissima (Pohl) Bail Decreases Local Effects Induced by Bothropic Venom. BioMed Res. Int. 2016, 2016, e6101742. [Google Scholar] [CrossRef]
- Tribuiani, N.; da Silva, A.M.; Ferraz, M.C.; Silva, M.G.; Bentes, A.P.G.; Graziano, T.S.; dos Santos, M.G.; Cogo, J.C.; Varanda, E.A.; Groppo, F.C.; et al. Vellozia flavicans Mart. ex Schult. hydroalcoholic extract inhibits the neuromuscular blockade induced by Bothrops jararacussu venom. BMC Complement. Altern. Med. 2014, 14, 48. [Google Scholar] [CrossRef]
- Sivaraman, T.; Sreedevi, N.S.; Meenatchisundaram, S.; Vadivelan, R. Antitoxin activity of aqueous extract of Cyclea peltata root against Naja naja venom. Indian J. Pharmacol. 2017, 49, 275–281. [Google Scholar] [CrossRef]
- Saturnino-Oliveira, J.; Santos, D.D.C.; Guimarães, A.G.; Santos Dias, A.; Tomaz, M.A.; Monteiro-Machado, M.; Estevam, C.S.; Lucca Júnior, W.D.; Maria, D.A.; Melo, P.A.; et al. Abarema cochliacarpos Extract Decreases the Inflammatory Process and Skeletal Muscle Injury Induced by Bothrops leucurus Venom. BioMed Res. Int. 2014, 2014, e820761. [Google Scholar] [CrossRef]
- Zeng, F.; Chen, C.; Chen, X.; Zhang, L.; Liu, M. Small Incisions Combined with Negative-Pressure Wound Therapy for Treatment of Protobothrops mucrosquamatus Bite Envenomation: A New Treatment Strategy. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 4495–4502. [Google Scholar] [CrossRef]
- De Oliveira, E.C.; Fernandes, C.P.; Sanchez, E.F.; Rocha, L.; Fuly, A.L. Inhibitory Effect of Plant Manilkara subsericea against Biological Activities of Lachesis muta Snake Venom. BioMed Res. Int. 2014, 2014, e408068. [Google Scholar] [CrossRef]
- Phillips, S.; Kohn, M.; Baker, D.; Leest, R.V.; Gomez, H.; McKinney, P.; McGoldrick, J.; Brent, J. Therapy of Brown Spider Envenomation: A Controlled Trial of Hyperbaric Oxygen, Dapsone, and Cyproheptadine. Ann. Emerg. Med. 1995, 25, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Golden, D.B.K.; Kelly, D.; Hamilton, R.G.; Craig, T.J. Venom immunotherapy reduces large local reactions to insect stings. J. Allergy Clin. Immunol. 2009, 123, 1371–1375. [Google Scholar] [CrossRef]
- Jorge, M.T.; Malaque, C.; Ribeiro, L.A.; Fan, H.W.; Cardoso, J.L.C.; Nishioka, S.A.; Sano-Martins, I.S.; França, F.O.S.; Kamiguti, A.S.; Theakston, R.D.G.; et al. Failure of chloramphenicol prophylaxis to reduce the frequency of abscess formation as a complication of envenoming by Bothrops snakes in Brazil: A double-blind randomized controlled trial. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Warrell, D.; Pe, T.; Swe, T.N.; Lwin, M.; Win, T. The efficacy of tourniquets as a first-aid measure for Russell’s viper bites in Burma. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 403–405. [Google Scholar] [CrossRef]
- Chippaux, J.-P.; Ramos-Cerrillo, B.; Stock, R.P. Study of the efficacy of the black stone on envenomation by snake bite in the murine model. Toxicon 2007, 49, 717–720. [Google Scholar] [CrossRef]
- Amaral, C.F.S.; Campolina, D.; Dias, M.B.; Bueno, C.M.; Rezende, N.A. Tourniquet ineffectiveness to reduce the severity of envenoming after Crotalus durissus snake bite in Belo Horizonte, Minas Gerais, Brazil. Toxicon 1998, 36, 805–808. [Google Scholar] [CrossRef]
- Korambayil, P.M.; Ambookan, P.V.; Abraham, S.V.; Ambalakat, A. A Multidisciplinary Approach with Hyperbaric Oxygen Therapy Improve Outcome in Snake Bite Injuries. Toxicol. Int. 2015, 22, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Aksel, G.; Güler, S.; Doğan, N.; Çorbacioğlu, Ş. A randomized trial comparing intravenous paracetamol, topical lidocaine, and ice application for treatment of pain associated with scorpion stings. Hum. Exp. Toxicol. 2015, 34, 662–667. [Google Scholar] [CrossRef]
- Exton, D.R.; Fenner, P.J.; Williamson, J.A. Cold packs: Effective topical analgesia in the treatment of painful stings by Physalia and other jellyfish. Med. J. Aust. 1989, 151, 625–626. [Google Scholar] [CrossRef]
- Nomura, J.T.; Sato, R.L.; Ahern, R.M.; Snow, J.L.; Kuwaye, T.T.; Yamamoto, L.G. A randomized paired comparison trial of cutaneous treatments for acute jellyfish (Carybdea alata) stings. Am. J. Emerg. Med. 2002, 20, 624–626. [Google Scholar] [CrossRef]
- Chaou, C.-H.; Chen, C.-K.; Chen, J.-C.; Chiu, T.-F.; Lin, C.-C. Comparisons of ice packs, hot water immersion, and analgesia injection for the treatment of centipede envenomations in Taiwan. Clin. Toxicol. 2009, 47, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Cazorla, D.; Loyo, J.; Lugo, L.; Acosta, M. Clinical, epidemiological and treatment aspects of five cases of sea urchin envenomation in Adicora, Paraguaná peninsula, Falcón state, Venezuela. Bol. Malariol. Salud Ambient. 2010, 50, 127–133. [Google Scholar]
- Aguayo-Albasini, J.L.; Flores-Pastor, B.; Soria-Aledo, V. GRADE System: Classification of Quality of Evidence and Strength of Recommendation. Cir. Esp. 2014, 92, 82–88. [Google Scholar] [CrossRef]
- Silva, L.M.G.; Zamuner, L.F.; David, A.C.; dos Santos, S.A.; Carvalho, P.D.T.C.D.; Zamuner, S.R. Photobiomodulation therapy on Bothrops snake venom-induced local pathological effects: A systematic review. Toxicon 2018, 152, 23–29. [Google Scholar] [CrossRef]
- Nelson, B.K. Snake Envenomation Incidence, Clinical Presentation and Management. Med. Toxicol. Advers. Drug Exp. 1989, 4, 17–31. [Google Scholar] [CrossRef]
- Lomonte, B.; Moreno, E.; Tarkowski, A.; Hanson, L.A.; Maccarana, M. Neutralizing interaction between heparins and myotoxin II, a lysine 49 phospholipase A2 from Bothrops asper snake venom. Identification of a heparin-binding and cytolytic toxin region by the use of synthetic peptides and molecular modeling. J. Biol. Chem. 1994, 269, 29867–29873. [Google Scholar] [CrossRef]
- Elwakil, T.F. An in-vivo experimental evaluation of He–Ne laser photostimulation in healing Achilles tendons. Lasers Med. Sci. 2007, 22, 53–59. [Google Scholar] [CrossRef]
- Feitosa, E.L.; Sampaio, V.S.; Salinas, J.L.; Queiroz, A.M.; da Silva, I.M.; Gomes, A.A.; Sachett, J.; Siqueira, A.M.; Ferreira, L.C.L.; dos Santos, M.C.; et al. Older Age and Time to Medical Assistance Are Associated with Severity and Mortality of Snakebites in the Brazilian Amazon: A Case-Control Study. PLoS ONE 2015, 10, e0132237. [Google Scholar] [CrossRef] [PubMed]
- Premmaneesakul, H.; Sithisarankul, P. Toxic jellyfish in Thailand. Int. Marit. Health 2019, 70, 22–26. [Google Scholar] [CrossRef]
- Carlson, J.; Golden, D.B.K. Large local reactions to insect envenomation. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 366–369. [Google Scholar] [CrossRef]
- Villas-Boas, I.M.; Bonfá, G.; Tambourgi, D.V. Venomous caterpillars: From inoculation apparatus to venom composition and envenomation. Toxicon 2018, 153, 39–52. [Google Scholar] [CrossRef]
- Fitzgerald, K.T.; Flood, A.A. Hymenoptera Stings. Clin. Tech. Small Anim. Pract. 2006, 21, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Hibel, J.A.; Clore, E.R. Prevention and Primary Care Treatment of Stings from Imported Fire Ants. Nurse Pract. 1992, 17, 65–66,68,71. [Google Scholar] [CrossRef]
- Varl, T.; Grenc, D.; Kostanjšek, R.; Brvar, M. Yellow sac spider (Cheiracanthium punctorium) bites in Slovenia: Case series and review. Wien. Klin. Wochenschr. 2017, 129, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.; Bassichis, B.; Itani, K. Brown Recluse Spider Bites to the Head: Three Cases and a Review. Ear Nose Throat J. 2004, 83, 465–470. [Google Scholar] [CrossRef]
- da Saúde, M.; de Vigilância em Saúde, S. Guia de Vigilância em Saúde, 5th ed.; Departamento de Articulação Estratégica de Vigilância em Saúde: Brasília, Brazil, 2021; ISBN 978-65-5993-102-6. [Google Scholar]
- Binder, L.S. Acute Arthropod Envenomation: Incidence, Clinical Features and Management. Med. Toxicol. Advers. Drug Exp. 1989, 4, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.A.; Keyler, D.E. Local envenoming by the Western hognose snake (Heterodon nasicus): A case report and review of medically significant Heterodon bites. Toxicon 2009, 54, 354–360. [Google Scholar] [CrossRef]
- Sachett, J.A.G.; da Silva, I.M.; Alves, E.C.; Oliveira, S.S.; Sampaio, V.S.; do Vale, F.F.; Romero, G.A.S.; dos Santos, M.C.; Marques, H.O.; Colombini, M.; et al. Poor efficacy of preemptive amoxicillin clavulanate for preventing secondary infection from Bothrops snakebites in the Brazilian Amazon: A randomized controlled clinical trial. PLoS Negl. Trop. Dis. 2017, 11, e0005745. [Google Scholar] [CrossRef]
- Gutierrez, J.; Lomonte, B.; Leon, G.; Rucavado, A.; Chaves, F.; Angulo, Y. Trends in Snakebite Envenomation Therapy: Scientific, Technological and Public Health Considerations. Curr. Pharm. Des. 2007, 13, 2935–2950. [Google Scholar] [CrossRef]
- Maciel Salazar, G.K.; Saturnino Cristino, J.; Vilhena Silva-Neto, A.; Seabra Farias, A.; Alcântara, J.A.; Azevedo Machado, V.; Murta, F.; Souza Sampaio, V.; Val, F.; Sachett, A.; et al. Snakebites in “Invisible Populations”: A cross-sectional survey in riverine populations in the remote western Brazilian Amazon. PLoS Negl. Trop. Dis. 2021, 15, e0009758. [Google Scholar] [CrossRef]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid.-Based Complement. Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.C. Laser (and LED) Therapy Is Phototherapy. Photomed. Laser Surg. 2005, 23, 78–80. [Google Scholar] [CrossRef]
- Frozanfar, A.; Ramezani, M.; Rahpeyma, A.; Khajehahmadi, S.; Arbab, H.R. The Effects of Low Level Laser Therapy on the Expression of Collagen Type I Gene and Proliferation of Human Gingival Fibroblasts (Hgf3-Pi 53): In vitro Study. Iran J. Basic Med. Sci. 2013, 16, 1071–1074. [Google Scholar] [PubMed]
- Silva, L.M.G.; da Silva, C.A.A.; da Silva, A.; Vieira, R.P.; Mesquita-Ferrari, R.A.; Cogo, J.C.; Zamuner, S.R. Photobiomodulation Protects and Promotes Differentiation of C2C12 Myoblast Cells Exposed to Snake Venom. PLoS ONE 2016, 11, e0152890. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, V.A.; Pisete, F.R.F.S.; Wagner, C.L.R.; Dalboni, M.A.; de Oliveira, A.P.L.; Cogo, J.C.; Zamuner, S.R. Photobiomodulation reduces cell death and cytokine production in C2C12 cells exposed to Bothrops venoms. Lasers Med. Sci. 2020, 35, 1047–1054. [Google Scholar] [CrossRef]
- Souza, N.H.C.; Ferrari, R.A.M.; Silva, D.F.T.; Nunes, F.D.; Bussadori, S.K.; Fernandes, K.P.S. Effect of low-level laser therapy on the modulation of the mitochondrial activity of macrophages. Braz. J. Phys. Ther. 2014, 18, 308–314. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, E.A.; Bittencourt, J.A.M.; De Oliveira, N.K.S. Influence of a low-level semiconductor gallium arsenate laser in experimental envenomation induced by Bothrops atrox snake venom. Am. J. Pharmacol. Toxicol. 2012, 7, 141–148. [Google Scholar] [CrossRef]
- dos Santos Fernandes, J.O.; de Gouveia, D.M.; David, A.C.; Nunez, S.C.; Zamuner, S.R.; Magalhães, D.S.F.; Navarro, R.S.; Cogo, J.C. The use of ozone therapy and photobiomodulation therapy to treat local effects of Bothrops jararacussu snake venom. Res. Biomed. Eng. 2021, 37, 773–783. [Google Scholar] [CrossRef]

| Title | Year | Country | Aim | Genus/Species | Ref. |
|---|---|---|---|---|---|
| SNAKES | |||||
| Effects of constriction bands on rattlesnake venom absorption: a pharmacokinetic study | 1992 | USA | Determine whether the use of a constriction band alters systemic absorption of rattlesnake venom in pigs and whether the use of constriction bands alters local swelling | Crotalus atrox | [18] |
| Rationalisation of first-aid measures for elapid snakebite | 1979 | Australia | Investigate the treatment for snakebite in monkeys (the anatomical similarities of their limbs to those of man allows the application of relevant first-aid measures to the envenomated limb) by radioimmunoassay monitoring of the distribution of whole-venom components and a neurotoxin in envenomated animals | Crotalus adamanteus | [19] |
| Electric shock does not save snake bitten rats | 1988 | USA | Verify the application of a series of high voltage, low magnification, direct current shocks directly to a snakebite site on a rat | Bothrops atrox | [20] |
| Analgesic effect of Light-Emitting Diode (LED) therapy at wavelengths of 635 and 945 nm on Bothrops moojeni venom-induced hyperalgesia | 2014 | Brazil | Evaluate the effectiveness of the light-emitting diode with 635 and 945 nm wavelengths in reducing inflammatory processes and hyperalgesia induced by Bothrops moojeni venom in mice | Bothrops moojeni | [21] |
| Effect of low-level laser therapy in the inflammatory response induced by Bothrops jararacussu snake venom | 2008 | Brazil | Report the effect of low power laser therapy on the formation of edema and leukocyte absorption caused by Bothrops jararacussu venom as an alternative treatment for Bothrops snakebites. | Bothrops jararacussu | [22] |
| Effects of a low-level semiconductor gallium arsenide laser on local pathological alterations induced by Bothrops moojeni snake venom | 2013 | Brazil | Investigate the effect of a low-power semiconductor gallium arsenide laser on local pathological changes induced by B. moojeni venom | Bothrops moojeni | [23] |
| Effects of photobiostimulation on edema and hemorrhage induced by Bothrops moojeni venom | 2012 | Brazil | Study the effectiveness of low-power laser and light-emitting diode irradiation alone or in combination with AV in the local reduction of edema and hemorrhage induced by Bothrops moojeni venom in mice | Bothrops moojeni | [24] |
| Low-intensity laser therapy improves tetanic contractions in mouse anterior tibialis muscle injected with Bothrops jararaca snake venom | 2016 | Brazil | Examine the influence of low-intensity laser therapy on the contractile activity of the mouse muscle injected with Bothrops jararaca venom | Bothrops jararaca | [25] |
| Low-level laser therapy decreases local effects induced by myotoxins isolated from Bothrops jararacussu snake venom | 2010 | Brazil | Analyze the effect of low-level laser therapy on the formation of edema, leukocyte influx, and myonecrosis caused by BthTX-I and BthTX-II, isolated from Bothrops jararacussu venom | Bothrops jararacussu | [16] |
| Low-level laser therapy reduces edema, leukocyte influx and hyperalgesia induced by Bothrops jararacussu snake venom | 2011 | Brazil | Investigate the effect of low-level laser therapy on inflammatory levels and hyperalgesia induced by B. jararacussu venom | Bothrops jararacussu | [26] |
| The ability of low level laser therapy to prevent muscle tissue damage induced by snake venom | 2009 | Brazil | Analyze the ation in the nerve-muscle by Bothrops jararacussu venom | Bothrops jararacussu | [27] |
| Analgesic effect of photobiomodulationon Bothrops moojeni venom induced hyperalgesia: a mechanism dependent on neuronal inhibition, cytokines and kinin receptors modulation | 2016 | Brazil | Evaluate the efficacy of photobiomodulation to reduce inflammatory hyperalgesia induced by Bothrops moojeni venom | Bothrops moojeni | [28] |
| Effect of low-level laser therapy in the myonecrosis induced by Bothrops jararacussu snake venom | 2009 | Brazil | Evaluate the ability of low-level laser therapy, alone or in combination with antivenom, to reduce venom-induced myonecrosis in rats | Bothrops jararacussu | [29] |
| Effects of photobiomodulation therapy on Bothrops moojeni snake-envenomed gastrocnemius of mice using enzymatic biomarkers | 2017 | Brazil | Evaluate the daily irradiation with neon helium laser and semiconductor laser of gallium arsenide at the site of intramuscular injection of venom in the gastrocnemius muscle of rats | Bothrops moojeni | [30] |
| Effects of the Ga-As laser irradiation on myonecrosis caused by Bothrops moojeni snake venom | 2003 | Brazil | Evaluate the effects of the gallium arsenide laser on gastrocnemius muscle in envenomated mice | Bothrops moojeni | [17] |
| Low-level laser therapy (904 nm) counteracts motor deficit of mice hind limb following skeletal muscle injury caused by snakebite-mimicking intramuscular venom injection | 2016 | Costa Rica | Investigate the motor function behavior of mice subjected to a Bothrops jararacussu snake venom injection and exposed to low-power laser therapy | Bothrops jararacussu | [31] |
| Low-level laser therapy promotes vascular endothelial growth factor receptor-1 expression in endothelial and nonendothelial cells of mice gastrocnemius exposed to snake venom | 2011 | Brazil | Evaluate the ability of low-power laser therapy to promote angiogenesis and myoregeneration in mice | Crotalinae and Bothrops moojeni | [32] |
| Photobiostimulation reduces edema formation induced in mice by Lys-49 phospholipases A2 isolated from Bothrops moojeni venom | 2014 | Brazil | Analyze the effect of a low-power laser (LED) after edema formation caused by snake venom in rats | Bothrops moojeni | [33] |
| The effects of low-level laser on muscle damage caused by Bothrops neuwiedi venom | 2008 | Brazil | Analyze the effects of low-power laser on myonecrosis induced by Bothrops neuwiedi venom in rats | Bothrops neuwiedi | [34] |
| Experimental Bothrops atrox envenomation: efficacy of antivenom therapy and the combination of Bothrops antivenom with dexamethasone | 2017 | Brazil | Test the effectiveness of Bothrops antivenom in treating signs, symptoms and toxic effects induced by B. atrox venom in mice | Bothrops atrox | [5] |
| Rosemary leaves extract: anti-snake action against egyptian Cerastes cerastes venom | 2018 | Egypt | Explore the neutralization capacity of the aqueous extract of leaves of Rosmarinus officinalis L. (RMAE) against Egyptian Cerastes cerastes (Cc) viper venom | Cerastes cerastes | [35] |
| Aqueous leaf extract of Jatropha mollissima (Pohl) bail decreases local effects induced by bothropic venom | 2016 | Brazil | Evaluate the effect of the aqueous extract of leaves of J. mollissima on the local effects induced by Bothrops venoms | Bothrops erythromelas and Bothrops jararaca | [36] |
| Vellozia flavicans Mart. Ex Schult. Hydroalcoholic extract inhibits the neuromuscular blockade induced by Bothrops jararacussu venom | 2014 | Brazil | Observe possible antimicrobial activities of V. flavicans against snake venom | Bothrops jararacussu | [37] |
| Antitoxin activity of aqueous extract of Cyclea peltata root against Naja naja venom | 2017 | India | Test the neutralization potential of Cyclea peltata root extract via ex vivo and in vivo approaches for Naja naja venom | Naja naja | [38] |
| Abarema cochliacarpos extract decreases the inflammatory process and skeletal muscle injury induced by Bothrops leucurus venom | 2014 | Brazil | Evaluate the anti-ophidic capacity of A. cochliacarpos extract and compare the activity of the extract with dexamethasone | Bothrops leucurus | [39] |
| Small incisions combined with negative-pressure wound therapy for treatment of Protobothrops Mucrosquamatus bite envenomation: a new treatment strategy | 2019 | China | Evaluate the effects of a new treatment strategy for envenomation, which consists of multiple small incisions and negative pressure wound therapy (NPWT) on the swelling of the injured limbs and the systemic inflammatory reaction | Protobothrops mucrosquamatus | [40] |
| Inhibitory effect of plant Manilkara subsericea against biological activities of Lachesis muta snake venom | 2014 | Brazil | Investigate in vitro and in vivo the ability of different ethanolic extracts of leaves and stems of M. subsericea and solvent-partitioned fractions to neutralize some biological activities induced by the L. muta venom | Lachesis muta | [41] |
| SPIDERS | |||||
| Therapy of brown spider envenomation: a controlled trial of hyperbaric oxygen, dapsone, and cyproheptadine | 1995 | USA | Determine whether hyperbaric oxygen, dapsone, or cyproheptadine decreases the severity of skin lesions resulting from an experimental Loxosceles envenomation | Laxosceles | [42] |
| Title | Year | Type of Study | Country | Aim | Animal | Ref. | GRADE Scale * |
|---|---|---|---|---|---|---|---|
| INSECTS | |||||||
| Venom immunotherapy reduces large local reactions to insect stings | 2009 | Clinical trial | USA | Determine the feasibility of performing a controlled trial to examine the efficacy of venom immunotherapy in reducing the size and duration of large local reactions | Hymenoptera | [43] | High |
| SNAKES | |||||||
| Failure of chloramphenicol prophylaxis to reduce the frequency of abscess formation as a complication of envenoming by Bothrops snakes in Brazil: a double-blind randomized controlled trial | 2004 | Randomize double-blind clinical trial | Brazil | Assess whether antibiotic therapy is effective in preventing infection as a complication of envenomation | Bothrops | [44] | High |
| The efficacy of tourniquets as a first-aid measure for Russell’s viper bites in Burma | 1987 | Clinical trial | UK | Evaluate the effectiveness of the tourniquet, which is commonly used as a first-aid measure in victims of Russell’s viper bites | Vipera russelli siamensis | [45] | Moderate |
| Study of the efficacy of the black stone on envenomation by snake bite in the murine model | 2007 | Case report | Mexico | Evaluate the effectiveness of black stone against snake venoms | Bitis arietans, Echis ocellatus and Naja nigricollis | [46] | Very low |
| Tourniquet ineffectiveness to reduce the severity of envenoming after Crotalus durissus snake bite in Belo Horizonte, Minas Gerais, Brazil | 1998 | Case report | Brazil | Describe whether tourniquet use reduces the severity of envenomations following a Crotalus durissus snakebite | Crotalus durissus | [47] | Very low |
| A multidisciplinary approach with hyperbaric oxygen therapy improves outcome in snake bite injuries | 2015 | Prospective study | India | Treatment of snakebite injuries in the extremities with various treatment modalities, including hyperbaric oxygen (HBO) therapy, surgical debridement, skin grafts, local or distant flaps to provide effective treatment from the perspective of plastic surgeons | Not specified | [48] | Low |
| SCORPIONS | |||||||
| A randomized trial comparing intravenous paracetamol, topical lidocaine, and ice application for treatment of pain associated with scorpion stings | 2014 | Clinical trial | USA | Compare the effectiveness of three treatment modalities in patients with pain caused by scorpion bites using visual analog scale (VAS) scores | Androctonus crassicauda | [49] | High |
| OTHERS | |||||||
| Cold packs: Effective tropical analgesia in the treatment of painful stings by Physalia and other jellyfish | 1989 | Clinical trial | Australia | Test whether application of cold packs is effective as topical analgesia in relieving mild to moderate pain in jellyfish stings | Physalia | [50] | High |
| A randomized paired comparison trial of cutaneous treatments for acute jellyfish (Carybdea alata) stings | 2002 | Randomized study | USA | Compare cutaneous treatments (heat, papain, and vinegar) for acute jellyfish (Carybdea alata) stings | Carybdea alata | [51] | Moderate |
| Comparisons of ice packs, hot water immersion, and analgesia injection for the treatment of centipede envenomations in Taiwan | 2009 | Clinical trial | China | Compare the effectiveness of ice packs and hot water immersion in treating centipede envenomation | Centipede | [52] | High |
| Clinical, epidemiological and treatment aspects of five cases of sea urchin poisoning in Adícora, Paraguaná Peninsula, Falcón State, Venezuela | 2010 | Descriptive and prospective study | Venezuela | Analyze clinical aspects, epidemiology and treatment of envenomation caused by sea urchins in Venezuela | Lytechinus variegatus and Echinometra lucunter | [53] | Low |
| Animal | Treatment | |
|---|---|---|
| Indicated | Contraindicated or Ineffective | |
| Snakes | Aqueous extract of rosemary in vivo [35], Jatropha mollissima Pohl leaf aqueous extract in vivo [36], Vellozia flavicans hydroalcoholic extract in vitro [37], root extracts of C. peltate [38], hydroethanol extract of Abarema cochliacarpos [39], Andrographis peniculata and Polygonum cuspidatum extract [41], low-level laser (reduction in inflammatory signs in vivo and myonecrosis in vitro and in vivo) [16,21,22,23,25,26,27,28,29,30,31,32,33,34,55], intraperitoneal dexamethasone in vivo [5], hyperbaric oxygen [48], and incisions combined with negative pressure in wound therapy [40] | Chloramphenicol is not effective for secundary infection [44], tourniquet is contraindicated for envenomation [47,56], and black stone application is not effective for envenomation [46] |
| Scorpion | Topical lidocaine and ice [49] | - |
| Others (jellyfish, centipedes and sea urchins) | Ice pack for centipede envenomations [52], and topical application of iodinated antiseptic solution, local anesthetic, analgesic, anti-inflammatory and antibiotic therapy for sea urchins [53] and jellyfish [50] | - |
| Treatment | Conclusion | Ref. |
|---|---|---|
| Application of venom immunotherapy in reducing the size and duration of large local reactions to insect stings. | The therapy significantly reduced the size and duration of the large local reactions, and the efficacy improved over a period of 2 to 4 years of treatment. | [43] |
| Venom of the fer-de-lance, Bothrops atrox, was injected subcutaneously into rats in a series of increasing doses. | Envenomated animals developed hemorrhagic ulcers at the injection sites, the size of which was strongly related to venom dose. | [20] |
| To assess the severity of skin lesions resulting from Loxosceles envenomation, New Zealand white rabbits were used. All groups received 20 micrograms of pooled L. deserta venom intradermally. The control group received 4 mL of a 5% ethanol solution by oral gavage every 12 h for 4 days. The HBO group received hyperbaric oxygen at 2.5 ATA for 65 min every 12 h for 2 days, plus 5% ethanol solution for 4 days. The dapsone group received dapsone 1.1 mg/kg in 5% ethanol by gavage every 12 h for 4 days. The cyproheptadine group received cyproheptadine. 125 mg/kg in 5% ethanol by gavage every 12 h for 4 days. | Total lesion size and ulcer size were followed for 10 days. The lesions were then excised, examined microscopically, and ranked by the severity of the histopathology. The groups did not differ significantly with respect to lesion size, ulcer size, or histopathologic ranking. Given the negative result in this study with adequate power to detect meaningful treatment benefits, hyperbaric oxygen, dapsone, or cyproheptadine are not recommended in the treatment of Loxosceles envenomations. | [42] |
| Using a crossover design, five pigs were studied with and without the use of a constriction band. First, 125l-Labeled Western Diamondback rattlesnake (Crotalus atrox) venom was injected subcutaneously into one foreleg. The protocol was repeated using the opposite foreleg six days later. The constriction band and leg circumference were measured serially. | The use of a constriction band was effective in reducing venom absorption while it was in place (reduced area under the venom concentration-time curve and maximum plasma venom concentration in the cuffed group), and constriction band removal did not result in a significant increase in maximum plasma venom concentration. Leg swelling was not affected by constriction band use. Because constriction band use delayed venom absorption without causing increased swelling, it may prove to be a useful first aid measure in human beings. | [18] |
| Monkeys envenomated with tiger snake (Notechis scutatus) venom were monitored by radioimmunoassay for both crude venom and a neurotoxin. | Venom movement can be effectively delayed for long periods by the application of a firm crepe bandage to the length of the bitten limb combined with immobilization by a splint. Pressure alone or immobilization alone did not delay venom movement. | [19] |
| Oral antibiotic prophylaxis with chloramphenicol in the prevention of infection in Bothrops snakebite envenomation. | Chloramphenicol for Bothrops snakebite victims with signs of local envenomation is not effective for preventing local infections. | [44] |
| Application of black stone after injection of intramuscular venom of Bitis arietans, Echis ocellatus and Naja nigricollis in rats. | Showed no effects on envenoming outcome. | [46] |
| Tourniquet use after Crotalus envenomations. | The data demonstrated the ineffectiveness of the use of the tourniquet to reduce the severity of envenomations by Crotalus. | [47] |
| Evaluation of the efficacy of light-emitting diode (LED) use in reducing inflammatory hyperalgesia induced by Bothrops moojeni venom in mice. | LED therapy is effective in reducing venom-induced hyperalgesia even after symptoms are present. | [21] |
| Evaluation of the effect of low-level laser therapy (LLLT) on edema formation and leukocyte influx caused by Bothrops jararacussu venom as an alternative treatment for Bothrops snakebites. | LLLT reduced edema and leukocyte influx, which suggests that LLLT should be considered as a potential therapy for the local effects of Bothrops envenomations. | [22] |
| Investigation of the effect of a low-intensity semiconductor gallium arsenide (GaAs) laser on local pathological alterations induced by B. moojeni venom. | Laser irradiation can help to reduce some local effects as it stimulates muscle fiber regeneration. | [23] |
| Evaluation of the effectiveness of LLLT and LED with and without antivenom for reducing local edema formation and hemorrhage induced by Bothrops moojeni venom in mice. | Laser and LED irradiation reduced venom-induced local effects in combination with antivenom. | [24] |
| The influence of LLLT on the contractile activity of the skeletal muscle of mice injected with Bothrops jararaca venom was examined, in laser with antivenom and only laser groups. | Antivenom and LLLT treatment groups improved muscle function after venom-induced damage. | [25] |
| Evaluation of the effect of LLLT, at a dose of 4.2 J/cm2, on edema formation, leukocyte influx and myonecrosis caused by Bothrops jararacussu venom. | LLLT significantly reduced edema formation, neutrophil accumulation, and myonecrosis induced by both myotoxins 24 h after envenomation. | [16] |
| Use of LLLT to reduce inflammatory signs in snakebite envenomations. | The group of mice treated with LLLT had a significant reduction in inflammatory signs. | [26] |
| Evaluation of the effects of neon helium laser (HeNe) at three energy densities on Bothrops jararacussu envenomations in rats. | HeNe laser irradiation, at a dosage of 3.5 J/ cm2, effectively reduces myonecrosis, and the blocking effect of neuromuscular transmission. | [27] |
| The efficacy of laser therapy, with energy of 2.2 J/cm2, to reduce hyperalgesia induced by Bothrops moojeni venom in mice was evaluated. | The use of photobiomodulation in the reduction of local pain induced by Bothrops venom was considered effective. | [28] |
| Investigation of the ability of LLLT alone or in combination with antivenom to reduce myonecrosis induced by Bothrops jararacussu venom. | Antivenom therapy alone was ineffective in reducing myonecrosis, but with LLLT it significantly reduced myonecrosis of the envenomated muscle. | [29] |
| The effectiveness of HeNe and GaAs lasers on local effects in rats injected with Bothrops moojeni venom was evaluated. | Histopathological analysis revealed increased muscle regeneration in groups of rats treated with both lasers; however, GaAs laser showed the best results. | [30] |
| The effects of GaAs lasers were evaluated in mice that had Bothrops moojeni venom injected intramuscularly. | GaAs irradiation significantly decreased the amount of myonecrosis in all tests performed. | [17] |
| Motor function was analyzed in mice submitted to injection of Bothrops jararacussu venom and exposed to LLLT at 3, 24, 48 and 72 h after envenomation | The motor function of the mice improved after the first laser application. | [31] |
| Evaluation of whether LLLT could accelerate angiogenesis and myoregeneration in mice injected with Bothrops moojeni venom. | In 3 days, LLLT increased angiogenesis, decreased neutrophils and increased proliferation of regenerating cells, i.e., improved revascularization. | [32] |
| The effect of LLLT and LED on edema formation in envenomated mice was analyzed. | Both LLLT and LED were similar in reducing edema formation, the effect being greater when photobiostimulation was combined with antivenom. | [33] |
| Evaluation of the effects of a low-intensity laser on myonecrosis caused by the insertion of Bothrops neuwiedi venom in the gastrocnemius muscle of rats. | Low-intensity laser reduced neutrophilic inflammation, as well as myofibrillar edema, hemorrhage, and myonecrosis, which suggests that laser therapy may be useful as an adjunctive therapy after Bothrops envenomations. | [34] |
| Antivenom administered intravenously and dexamethasone administered intraperitoneally were tested to analyze edema and muscle damage caused by Bothrops atrox envenomation. | The use of dexamethasone, associated with antivenom, reduced recovery time after edema and muscle injury. | [5] |
| Hyperbaric oxygen therapy (HBO) was performed during 90 min for 6 days in patients with cellulite and compartment syndrome. Patients with soft tissue necrosis underwent surgical debridement and soft tissue reconstruction. | Adjunctive HBO therapy has been found to be effective in treating snakebite injuries. The authors suggest further research. | [48] |
| To verify the venom inhibition using 30 µg of raw Egyptian viper venom in mice, different proportions of aqueous extract of rosemary leaves were injected for 30 min at 37 °C. | Rosemary leaf extract has a potential neutralizing action against local effects and lethality of Cerastes cerastes venom. Those who received the extract showed an increase in survival time when compared to the control. | [35] |
| Aqueous extract of Jatropha molissima Pohl leaves was injected in different doses in a group of five mice and, after 30 min, the animals received a subcutaneous injection of 25 µg of B. erythromelas and B. jararaca venoms. | J. mollissima has substances that can inhibit the toxins present in the venoms. However, further experiments are needed to define its adjuvant action in the treatment of local effects. | [36] |
| The capacity of the hydroalcoholic extract of Vellozia flavicans to neutralize the neuromuscular blockade in vitro caused by the venom of Bothrops jararacussu in mice and its antimicrobial capacity was also tested. | The hydroalcoholic extract of V. flavicans leaves was effective in neutralizing and decreasing in vitro the neuromuscular blockade caused by B. jararacussu. However, it has no significant antimicrobial activity against the tested bacteria. | [37] |
| The venom toxicity was evaluated in neutralization assays. Root extracts of Cyclea peltata were used to evaluate neutralization in ex vivo and in vivo tests using assays to determine acetylcholinesterase, protease, direct hemolysis, phospholipase activity and procoagulant activity. | The result of the ex vivo and in vivo analysis indicates that the root extract of C. peltata has significant compounds that can neutralize toxins from N. naja venom. | [38] |
| The treatment consisted of the application of hydroethanolic extract of Abarema cochliacarpos in mice by oral probe to evaluate the effects of Bothrops leucurus envenomation. | The crude extract of A. cochliacarpos was able to reduce the edematogenic effect and the hyperalgesic action of the venom. | [39] |
| Small incisions combined with negative pressure wound therapy (NPWT) for treatment of Protobothrops mucrosquamatus envenomations as a new treatment strategy. | Multiple small incisions combined with NPWT have been shown to be effective in controlling the release of inflammatory cytokines and accelerating the relief of the inflammatory reaction. | [40] |
| Via a survey of the literature, the authors found two species of plants (Andrographis peniculata and Polygonum cuspidatum) used topically in the healing of wounds after snakebite. | Upon review, the extracts proved to be effective as possible adjuvant treatments in the healing of snakebite wounds. | [41] |
| A randomized study carried out over a period of 1 year in patients with scorpion stings, who did not have systemic signs or symptoms. Patients were treated with intravenous paracetamol, topical lidocaine and the application of ice. | Topical lidocaine and ice were considered to be effective and safe treatments for scorpion stings with regard to pain in patients without systemic signs and symptoms. | [49] |
| Comparison of the effectiveness of ice packs and hot water immersion for the treatment of pain after centipede envenomation. Sixty centipede-envenomated patients were randomized into three groups and treated with ice packs, hot water immersion or analgesic injection. | Ice packs and soaking in hot water can reduce pain in centipede envenomations. Their effects seem to be equivalent to analgesics, but the study suggests that ice has the best cost effectiveness. | [52] |
| Treatment consisted of topical application of an iodinated antiseptic solution, local anesthetic, analgesic, intravenous anti-inflammatory, antibiotic therapy and tetanus therapy after sea urchin envenomation. | The patients evolved satisfactorily between 20 and 45 min after the beginning of the therapeutic treatment, and presented a low pain score. The wounds healed without edema, erythema, necrosis or bacterial growth. | [53] |
| Venom antigen levels were measured in blood samples obtained above and below a tourniquet, and before and after their release in human victims of Russell’s viper (Vipera russelli swnensis) envenomation, to discover whether the tourniquet retarded the proximal spread of venom from the site of the bite. | The efficacy of the tourniquets, which are commonly used, was studied in 37 cases by measuring venom antigen levels. In most cases, the tourniquet did not prevent proximal spread of venom. In 8/37 cases, however, venom antigen assays suggested but did not prove that venom absorption was being delayed by the tourniquet. | [45] |
| Application of cold packs as a topical analgesia is effective in relieving mild-to-moderate pain in jellyfish stings. | This study has shown that, when applied to Physalia (“bluebottle”) jellyfish stings, cold packs are effective as topical analgesia in the relief of mild-to-moderate skin pain. The application of ice also has been shown to be effective for topical analgesia in a number of other jellyfish stings, including those by Cyanea (“hair jellyfish”), Tamoya sp. (“Moreton Bay stinger” or “fire jelly”) and Carybdea rastoni (“jimble”) as well as by Physalia. | [50] |
| Treatment consisted of cutaneous treatments (heat, papain and vinegar) for acute jellyfish (Carybdea alata) stings. | This study suggests that the most efficacious initial treatment for C. alata jellyfish envenomation is hot-water immersion of the afflicted site. | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, É.S.; Oliveira, I.; Nascimento, T.P.; da Silva Neto, A.V.; Leal, B.A.S.; Araújo, F.Q.; Julião, B.F.V.; Souza, A.R.N.; Abrahim, A.W.; Macedo, B.B.O.; et al. Prospecting Local Treatments Used in Conjunction with Antivenom Administration Following Envenomation Caused by Animals: A Systematic Review. Toxins 2023, 15, 313. https://doi.org/10.3390/toxins15050313
Carvalho ÉS, Oliveira I, Nascimento TP, da Silva Neto AV, Leal BAS, Araújo FQ, Julião BFV, Souza ARN, Abrahim AW, Macedo BBO, et al. Prospecting Local Treatments Used in Conjunction with Antivenom Administration Following Envenomation Caused by Animals: A Systematic Review. Toxins. 2023; 15(5):313. https://doi.org/10.3390/toxins15050313
Chicago/Turabian StyleCarvalho, Érica S., Isadora Oliveira, Thaís P. Nascimento, Alexandre Vilhena da Silva Neto, Brenda A. S. Leal, Felipe Q. Araújo, Bruno F. V. Julião, Andrea R. N. Souza, Andreza W. Abrahim, Bruna B. O. Macedo, and et al. 2023. "Prospecting Local Treatments Used in Conjunction with Antivenom Administration Following Envenomation Caused by Animals: A Systematic Review" Toxins 15, no. 5: 313. https://doi.org/10.3390/toxins15050313
APA StyleCarvalho, É. S., Oliveira, I., Nascimento, T. P., da Silva Neto, A. V., Leal, B. A. S., Araújo, F. Q., Julião, B. F. V., Souza, A. R. N., Abrahim, A. W., Macedo, B. B. O., de Oliveira, J. T. S., Wen, F. H., Pucca, M. B., Monteiro, W. M., & Sachett, J. A. G. (2023). Prospecting Local Treatments Used in Conjunction with Antivenom Administration Following Envenomation Caused by Animals: A Systematic Review. Toxins, 15(5), 313. https://doi.org/10.3390/toxins15050313









